Abstract
Adolescence is a critical period for neurodevelopment. Evidence from animal studies suggests that isolated rearing can exert negative effects on behavioral and brain development. The present study aimed to investigate the effects of adolescent social isolation on latent inhibition and brain-derived neurotrophic factor levels in the forebrain of adult rats. Male Wistar rats were randomly divided into adolescent isolation (isolated housing, 38–51 days of age) and social groups. Latent inhibition was tested at adulthood. Brain-derived neurotrophic factor levels were measured in the medial prefrontal cortex and nucleus accumbens by an enzyme-linked immunosorbent assay. Adolescent social isolation impaired latent inhibition and increased brain-derived neurotrophic factor levels in the medial prefrontal cortex of young adult rats. These data suggest that adolescent social isolation has a profound effect on cognitive function and neurotrophin levels in adult rats and may be used as an animal model of neurodevelopmental disorders.
Keywords: neural regeneration, adolescence, social isolation, latent inhibition, brain-derived neurotrophic factor, medial prefrontal cortex, nucleus accumbens, adult, cognitive function, neurodevelopmental disorders, grants-supported paper, neuroregeneration
Research Highlights
(1)This study investigated the effects of adolescent social isolation (postnatal days 38–51) on cognitive function in adult animals and the levels of brain-derived neurotrophic factor in the medial prefrontal cortex and nucleus accumbens.
(2) Adolescent social isolation impaired latent inhibition and increased brain-derived neurotrophic factor levels in the medial prefrontal cortex of adult rats.
(3) This study advances the use of isolation rearing as an animal model of psychiatric disease.
INTRODUCTION
Adolescence is a transitional period between childhood and adulthood, which can be characterized by behavioral, hormonal and neurochemical changes. For instance, adolescents differ from adults in behavioral measures of decision making, executive planning, working memory, and inhibitory control[1,2,3]. Adolescent rats demonstrate higher levels of social behavior than younger and older animals[4,5]. Maturation of neurotransmitter systems, such as the dopaminergic and glutamatergic systems, also occurs during this period[6,7].
Evidence from animal studies suggests that adolescence is a critical period when life experiences can exert positive and negative effects on brain development. For example, adolescent social stress or social isolation increases anxiety-like behaviors in both female and male adult rats[8,9]. Environmental enrichment in adolescence can reverse many of the deleterious effects of early life stressors[10]. We have previously reported that adolescent social isolation can induce delayed latent inhibition deficit, moderate hyperactivity toward a novel environment, reduced pain sensitivity, and delayed developmental effects on dopamine levels in the nucleus accumbens and the medial prefrontal cortex[11,12].
Brain-derived neurotrophic factor is a member of the neurotrophin family[13] and has a wide range of functions, such as supporting postmitotic neuronal survival, regulating axonal growth and promoting synaptic plasticity[14,15]. Brain-derived neurotrophic factor has also been implicated in the neurobiological mechanisms of schizophrenia[16,17,18] and depression[19,20,21]. Several recent studies reported that acute immobilization stress, inescapable tail-shock stress, and acute social defeat stress increases brain-derived neurotrophic factor mRNA expression in the medial prefrontal cortex, but not in the nucleus accumbens[22,23,24]. However, very few studies have explored the effects of isolation rearing on brain-derived neurotrophic factor levels. The few studies on this topic have presented inconsistent, if not contradictory, results[25,26], and the effects of adolescent social isolation on levels of brain-derived neurotrophic factor in the medial prefrontal cortex and nucleus accumbens are largely unknown.
The present study was designed to determine the developmental influence of adolescent social isolation on latent inhibition and brain-derived neurotrophic factor levels in the medial prefrontal cortex and nucleus accumbens of young adult rats.
RESULTS
Quantitative analysis of experimental animals
A total of 36 male Wistar rats were equally and randomly divided into adolescent isolation and social groups on postnatal day 38. The adolescent isolation group consisted of rats that were housed alone for 2 weeks during adolescence (postnatal days 38–51). Rats in the social group were housed under normal housing conditions. All 36 rats were included in the final analysis.
Effects of adolescent social isolation on latent inhibition in adult rats (Figure 1)
Figure 1.
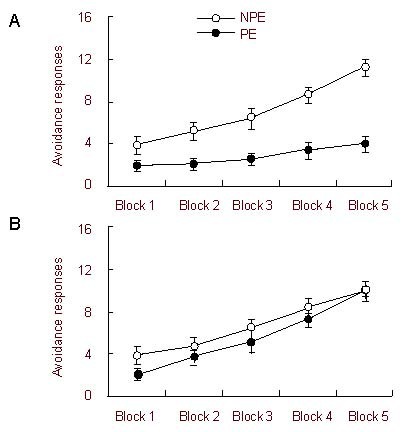
Effects of adolescent social isolation on latent inhibition in adult rats.
Compared to latent inhibition in the social group [(A) non-pre-exposure (NPE), n = 9; pre-exposure (PE), n = 9], latent inhibition was deficient in the adolescent isolation group [(B) NPE, n = 9; PE, n = 9] (2 × 2 × 5 analysis of variance). Latent inhibition was assessed using tone-conditioned stimulus pre-exposure in the two-way active avoidance test. Data are shown as mean ± SEM and five blocks of 10 trials were used.
The effect of blocks was significant [F(4, 128) = 144.08, P < 0.001], reflecting an overall increase in avoidance responses. In addition, the effect of the pre-exposure condition was significant [F(1, 32) = 15.40, P < 0.001]. However, the effect of the housing condition was only marginally significant [F(1, 32) = 3.41, P = 0.07]. The interaction between pre-exposure condition (pre-exposure and non-pre-exposure) and housing condition (isolation and social) was significant [F(1, 32) = 5.79, P < 0.05], as was the three-way interaction between all factors [F(4, 128) = 11.36, P < 0.001]. These results suggest a possible difference in the effect of pre-exposure between the adolescent isolation group and the social group.
Furthermore, the effect of blocks and the pre-exposure condition were both significant [F(4, 64) = 49.95, P < 0.001; F(1, 16) = 21.71, P < 0.001] for the social group. The interaction between these two factors was also significant [F(4, 64) = 14.78, P < 0.001]. Overall, these data suggest that the non-pre-exposure animals performed better than the pre-exposure animals, indicating a robust latent inhibition phenomenon in socially reared rats (Figure 1A). In contrast, the adolescent isolation group only had a significant effect of blocks [F(4, 64) = 96.79, P < 0.001]. The effect of the pre-exposure condition was not significant [F(1, 16) = 1.07, P = 0.32], nor was its interaction with blocks [F(4, 64) = 1.22, P = 0.31]. These results indicate that latent inhibition was deficient in adolescent isolated rats (Figure 1B).
Effects of adolescent social isolation on brain-derived neurotrophic factor protein levels in the forebrain of adult rats
Brain-derived neurotrophic factor levels in the medial prefrontal cortex and nucleus accumbens in the adolescent isolation and social groups are shown in Figure 2. Adolescent social isolation produced a significant increase in brain-derived neurotrophic factor levels in the medial prefrontal cortex [t(18) = 2.39, P < 0.05]. In contrast, there was no significant difference in brain-derived neurotrophic factor levels in the nucleus accumbens between the adolescent isolation and social groups [t(18) = 1.22, P = 0.24].
Figure 2.
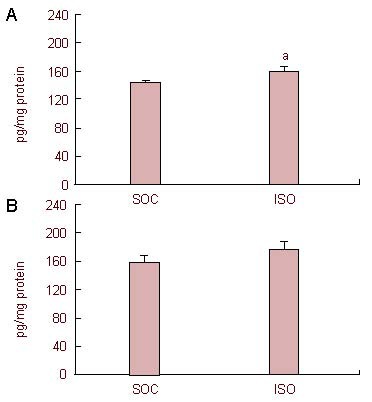
Effects of adolescent social isolation on brain-derived neurotrophic factor (BDNF) protein levels in the forebrain of adult rats.
BDNF levels by enzyme-linked immunosorbent assay, expressed as pg/mg total protein, in the medial prefrontal cortex (A) and nucleus accumbens (B) of social (SOC) and adolescent isolation (ISO) groups (mean ± SEM, n = 10 per group). aP < 0.05, vs. SOC group (independent t-test).
DISCUSSION
The present study showed that adolescent social isolation impairs latent inhibition and increases brain-derived neurotrophic factor levels in the medial prefrontal cortex of young adult rats. The latent inhibition deficiency confirms our previous report that social isolation during puberty can affect latent inhibition in young adult rats[11].
Brain-derived neurotrophic factor expression can be affected by a variety of environmental factors and has been implicated in the etiology of multiple neuropsychiatric disorders[27]. The majority of studies exploring the effects of social isolation on brain-derived neurotrophic factor expression in the brain have focused on the hippocampus[28,29], although Scaccianoce et al[25] reported that 8-week social isolation does not affect brain-derived neurotrophic factor protein levels in the prefrontal cortex or striatum of adult Sprague-Dawley rats. In the present study, 2-week adolescent social isolation increased brain-derived neurotrophic factor levels in the medial prefrontal cortex, but not in the nucleus accumbens of young adult rats. We previously reported that 4-week adolescent social isolation increases the number of brain-derived neurotrophic factor-positive cells in the medial prefrontal cortex[30]. These conflicting results may be a consequence of methodological differences between the studies, including the duration of the isolation procedure and the age of the rats. As opposed to an 8-week isolation procedure from weaning to adulthood, this study used a 2-week social isolation procedure focusing on the adolescent period. Previous studies suggest that peri-adolescent stress produces several behavioral and neurochemical abnormalities in adult rats, including decreased levels of the D2 dopamine receptor in the prefrontal cortex, reduced dopamine turnover in the nucleus accumbens, and deficits in spatial learning and latent inhibition[11,31,32].
Authors have previously reported that similar adolescent social deprivation results in increased dopamine levels in the nucleus accumbens and decreased dopamine levels in the medial prefrontal cortex in adult rats[11]. A recent study found that 2-week adolescent social isolation is associated with a 3.3-fold increase in striatal D2 receptor expression in adult rats[33]. Taken together, these observations suggest that adolescent social isolation might disrupt the normal development of midbrain dopamine systems, and that the effects do not emerge until adulthood.
The nucleus accumbens and medial prefrontal cortex are two critical regions of the midbrain dopaminergic circuitry. The medial prefrontal cortex provides extensive input to the nucleus accumbens, and the perturbation of this region can modify dopamine function in the nucleus accumbens[34]. In rodents, dopaminergic innervation of the prefrontal cortex exhibits a dramatic increase at postnatal day 60, which is equivalent to early adulthood in humans[1]. The regulation of dopamine neuron development is mediated by neurotrophic factors, including brain-derived neurotrophic factor and glial-derived neurotrophic factor[35,36]. Brain-derived neurotrophic factor can promote the sprouting of dopaminergic axons and regulate the plasticity of dopaminergic neurons[37,38]. Brain-derived neurotrophic factor synthesized by dopaminergic neurons is responsible for the appearance of the dopamine D3 receptor during development and maintenance of its expression in adults[39]. Brain-derived neurotrophic factor can affect the control of emotions and sensorimotor gating via feedback loops in the circuitry that the nucleus accumbens forms with the prefrontal cortex, which can be found to be disturbed in some psychiatric disorders[40]. In the present study, increased brain-derived neurotrophic factor levels in the medial prefrontal cortex after adolescent social isolation may be related to hyperactivity of the midbrain dopaminergic system, and therefore may disrupt latent inhibition. Because the elevated levels of brain-derived neurotrophic factor in the prefrontal cortex of socially-isolated rats are expected to lead to synaptic remodeling, which may underlie the observed behavioral deficits, we will examine morphological changes in the prefrontal cortex in a future study.
In summary, adolescent social isolation induces a deficiency in latent inhibition and increases brain-derived neurotrophic factor levels in the medial prefrontal cortex of young adult rats. This study provides additional insight into brain and behavioral changes that occur following early neurodevelopmental manipulations.
MATERIALS AND METHODS
Design
This is a randomized, controlled animal study.
Time and setting
Experiments were performed in the Animal Laboratory of Peking University in China from May to August 2011.
Materials
A total of 36 male Wistar rats were obtained from the Academy of Chinese Military Medical Science at postnatal day 21 and were housed in groups of three per cage. The rats were kept under controlled environmental conditions (an ambient temperature of 22°C and a 12-hour light/dark cycle with lights on at 7:00 a.m.) with free access to food and water. All protocols were conducted in accordance with the Guidelines of Beijing Laboratory Animal Center and National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23).
Methods
Experimental procedure
The adolescent isolation group consisted of rats that were housed alone for 2 weeks during adolescence (postnatal days 38–51). Rats in the social group were housed under normal housing conditions. The isolated rats were returned to group rearing in random assignments for a 2-week re-socialization period (postnatal days 52–65). During this time, socially reared rats were also randomly re-assigned to new housing groups to ensure that any differences observed between isolation and socially reared rats were not caused by the re-socialization procedure. After 2 weeks of re-housed rearing, latent inhibition was examined in young adult rats at postnatal day 66. All rats were housed in the same room, allowing them to hear and smell the other rats in the study. All behavioral testing was performed during the light cycle between 7:00 a.m. and 7:00 p.m.
Latent inhibition test
Latent inhibition was evaluated using the conditioned avoidance response. The acquisition of a conditioned reaction in a two-way shuttle box was tested with or without pre-exposure to the conditioning stimulus. Rats in either group were randomly divided into pre-exposure and non-pre-exposure groups. By receiving pre- exposure to a stimulus, the animal learns to acquire a conditioned response. The conditioned stimulus was a 6-second tone (75 dB) produced by a buzzer that was centrally located on the ceiling of the box. The unconditioned stimulus was a 1 mA electric foot shock delivered through stainless steel rods. During the pre-exposure period, animals in the pre-exposure group were placed in the apparatus and a sound was delivered 90 times, for 6 seconds each time with a 14-second interval, over a period of 30 minutes. Animals in the non-pre-exposure group were placed in the same apparatus for 30 minutes without sound administration. Immediately after pre-exposure, the training session was started and consisted of five blocks of 10 trials; each trial was limited to 15 seconds. The rats were trained to escape to the other side of the shuttle box after the presentation of the conditioned stimulus (the foot shock). The conditioned stimulus lasted for 3 seconds. If the rat moved to the other side of the box within this time, the tone was turned off, and the foot shock was terminated. Otherwise, another foot shock was administered. If the animal failed to react, the trial continued for the remainder of the 15-second period. The inter-trial intervals were 10 seconds. The number of avoidance responses (with a response latency < 3 seconds) was recorded.
Tissue dissections
One day after the latent inhibition test, 10 rats from each group were randomly chosen for neurochemical assays. The rats were sacrificed by decapitation, and their brains were immediately removed and frozen in liquid nitrogen. The brains were cut into 50-μm thick sections at −20°C in a cryostat microtome. Bilateral tissue punches of the medial prefrontal cortex and nucleus accumbens were taken rapidly using a stainless steel cannula with an inner diameter of 0.6 mm, as described previously[8]. The tissue samples were stored immediately at −80°C for further use.
Enzyme-linked immunosorbent assay
Brain-derived neurotrophic factor protein content was measured by enzyme-linked immunosorbent assay according to the directions of the manufacturer using a DuoSet enzyme-linked immunosorbent assay development kit (R&D Systems, Minneapolis, MN, USA). Briefly, tissues were sonicated in extraction buffer (0.01 M Tris-HCL buffer, pH 7.4, containing 0.1 M NaCI, 1 mM ethylenediaminetetraacetic acid, 1 mM ethylene glycol bis(2-aminoethyl) tetraacetic acid, 2 mM phenylmethane sulfonyl fluoride, 50 mM leupeptin, 100 g/mL pepstatin and 100 g/mL aprotinin) and centrifuged at 4°C for 10 minutes at 13 000 r/min. Supernatants were collected, and total protein content was measured. Samples and standards were then run in duplicate, and absorbance was measured at 450 nm using a microplate reader (MULTISKAN MK3, Thermo Scientific, Rockford, USA). Data are presented as pg/mg total tissue protein[20,22].
Statistical analysis
Data are presented as mean ± SEM, and analyzed using SPSS 13.0 software (SPSS, Chicago, IL, USA). Active avoidance responses were analyzed using a 2 × 2 × 5 analysis of variance consisting of two between-subject factors, housing condition (adolescent isolation and social) and pre-exposure condition (non-pre-exposure and pre-exposure), and a within-subject factor, blocks (5 blocks of 10 trials). Brain-derived neurotrophic factor protein levels from different brain regions were analyzed by an independent t-test. A probability value of < 0.05 was considered statistically significant.
Footnotes
Funding: This work was supported by the National Natural Science Foundation of China, grant No. 31070910, 91132728; the Knowledge Innovation Program of the Chinese Academy of Sciences (KSCX2-EW-J-8) and the Key Laboratory of Mental Health, Institute of Psychology, Chinese Academy of Sciences.
Conflicts of interest: None declared.
Ethical approval: The study was approved by the Institutional Animal Care and Use Committee of Peking University in China.
(Reviewed by Patel B, Evans-Darby S, Qin YL, Yan LR)
(Edited by Li CH, Qiu Y, Song LP)
REFERENCES
- [1].Wahlstrom D, Collins P, White T, et al. Developmental changes in dopamine neurotransmission in adolescence: behavioral implications and issues in assessment. Brain Cogn. 2010;72(1):146–159. doi: 10.1016/j.bandc.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Nemati F, Kolb B. Motor cortex injury has different behavioral and anatomical effects in early and late adolescence. Behav Neurosci. 2010;124(5):612–622. doi: 10.1037/a0020911. [DOI] [PubMed] [Google Scholar]
- [3].Perna MK, Henderson YO, Bruner CL, et al. An analysis of nicotine conditioned place conditioning in early postweanling and adolescent rats neonatally treated with quinpirole. Behav Brain Res. 2011;220(1):254–261. doi: 10.1016/j.bbr.2011.02.004. [DOI] [PubMed] [Google Scholar]
- [4].Varlinskaya EI, Spear LP. Social interactions in adolescent and adult Sprague-Dawley rats: Impact of social deprivation and test context familiarity. Behav Brain Res. 2008;188(2):398–405. doi: 10.1016/j.bbr.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Varlinskaya EI, Spear LP, Spear NE. Social behavior and social motivation in adolescent rats: role of housing conditions and partner's activity. Physiol Behav. 1999;67(4):475–482. doi: 10.1016/s0031-9384(98)00285-6. [DOI] [PubMed] [Google Scholar]
- [6].Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24(4):417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- [7].Robinson DL, Zitzman DL, Smith KJ, et al. Fast dopamine release events in the nucleus accumbens of early adolescent rats. Neuroscience. 2011;176:296–307. doi: 10.1016/j.neuroscience.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lukkes JL, Mokin MV, Scholl JL, et al. Adult rats exposed to early-life social isolation exhibit increased anxiety and conditioned fear behavior, and altered hormonal stress responses. Horm Behav. 2009;55(1):248–256. doi: 10.1016/j.yhbeh.2008.10.014. [DOI] [PubMed] [Google Scholar]
- [9].McCormick CM, Smith C, Mathews IZ. Effects of chronic social stress in adolescence on anxiety and neuroendocrine response to mild stress in male and female rats. Behav Brain Res. 2008;187(2):228–238. doi: 10.1016/j.bbr.2007.09.005. [DOI] [PubMed] [Google Scholar]
- [10].Hellemans KG, Benge LC, Olmstead MC. Adolescent enrichment partially reverses the social isolation syndrome. Dev Brain Res. 2004;150(2):103–115. doi: 10.1016/j.devbrainres.2004.03.003. [DOI] [PubMed] [Google Scholar]
- [11].Shao F, Jin J, Meng Q, et al. Pubertal isolation alters latent inhibition and DA in nucleus accumbens of adult rats. Physiol Behav. 2009;98(3):251–257. doi: 10.1016/j.physbeh.2009.05.021. [DOI] [PubMed] [Google Scholar]
- [12].Meng Q, Li N, Han X, et al. Peri-adolescence isolation rearing alters social behavior and nociception in rats. Neurosci Lett. 2010;480(1):25–29. doi: 10.1016/j.neulet.2010.05.067. [DOI] [PubMed] [Google Scholar]
- [13].Hyman C, Hofer M, Barde YA, et al. BDNF is a neurotrophic factor for dopaminergic neurons of the substantia nigra. Nature. 1991;350(6315):230–232. doi: 10.1038/350230a0. [DOI] [PubMed] [Google Scholar]
- [14].Fumagalli F, Molteni R, Racagni G, et al. Stress during development: Impact on neuroplasticity and relevance to psychopathology. Prog Neurobiol. 2007;81(4):197–217. doi: 10.1016/j.pneurobio.2007.01.002. [DOI] [PubMed] [Google Scholar]
- [15].Pillai A, Mahadik SP. Increased truncated TrkB receptor expression and decreased BDNF/TrkB signaling in the frontal cortex of reeler mouse model of schizophrenia. Schizophr Res. 2008;100(1-3):325–333. doi: 10.1016/j.schres.2007.11.030. [DOI] [PubMed] [Google Scholar]
- [16].Hashimoto T, Bergen SE, Nguyen QL, et al. Relationship of BDNF and its receptor TrkB to altered inhibitory prefrontal circuitry in schizophrenia. J Neurosci. 2005;25(2):372–383. doi: 10.1523/JNEUROSCI.4035-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Weickert CS, Hyde TM, Lipska BK, et al. Reduced BDNF in prefrontal cortex of patients with schizophrenia. Mol Psychiatry. 2003;8:592–610. doi: 10.1038/sj.mp.4001308. [DOI] [PubMed] [Google Scholar]
- [18].Favalli G, Li J, Belmonte-de-Abreu P, et al. The role of BDNF in the pathophysiology and treatment of schizophrenia. J Psychiatr Res. 2012;46(1):1–11. doi: 10.1016/j.jpsychires.2011.09.022. [DOI] [PubMed] [Google Scholar]
- [19].Malkesman O, Weller A. Two different putative genetic animal models of childhood depression--A review. Prog Neurobiol. 2009;88(3):153–169. doi: 10.1016/j.pneurobio.2009.03.003. [DOI] [PubMed] [Google Scholar]
- [20].Schneider B, Prvulovic D, Oertel-Knöchel V, et al. Biomarkers for major depression and its delineation from neurodegenerative disorders. Prog Neurobiol. 2011;95(4):703–717. doi: 10.1016/j.pneurobio.2011.08.001. [DOI] [PubMed] [Google Scholar]
- [21].Klein AB, Santini MA, Aznar S, et al. Changes in 5-HT2A-mediated behavior and 5-HT2A- and 5-HT1A receptor binding and expression in conditional BDNF knock-out mice. Neuroscience. 2010;169(3):1007–1016. doi: 10.1016/j.neuroscience.2010.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Bland ST, Schmid MJ, Der-Avakian A, et al. Expression of c-fos and BDNF mRNA in subregions of the prefrontal cortex of male and female rats after acute uncontrollable stress. Brain Res. 2005;1051(1-2):90–99. doi: 10.1016/j.brainres.2005.05.065. [DOI] [PubMed] [Google Scholar]
- [23].Fanous S, Hammer RP, Jr, Nikulina EM. Short- and long-term effects of intermittent social defeat stress on BDNF expression in mesocorticolimbic brain regions. Neuroscience. 2010;167(3):598–607. doi: 10.1016/j.neuroscience.2010.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Lee Y, Duman RS, Marek GJ. The mGlu2/3 receptor agonist LY354740 suppresses immobilization stress-induced increase in rat prefrontal cortical BDNF mRNA expression. Neurosci Lett. 2006;398(3):328–332. doi: 10.1016/j.neulet.2006.01.021. [DOI] [PubMed] [Google Scholar]
- [25].Scaccianoce S, Del Bianco P, Paolone G, et al. Social isolation selectively reduces hippocampal BDNF without altering plasma corticosterone. Behav Brain Res. 2006;168(2):323–325. doi: 10.1016/j.bbr.2005.04.024. [DOI] [PubMed] [Google Scholar]
- [26].Aguiar AS, Jr, Castro AA, Moreira EL, et al. Short bouts of mild-intensity physical exercise improve spatial learning and memory in aging rats: involvement of hippocampal plasticity via AKT, CREB and BDNF signaling. Mech Ageing Dev. 2011;132(11-12):560–567. doi: 10.1016/j.mad.2011.09.005. [DOI] [PubMed] [Google Scholar]
- [27].Duman RS. Role of neurotrophic factors in the etiology and treatment of mood disorders. Neuromolecular Med. 2004;5(1):11–25. doi: 10.1385/NMM:5:1:011. [DOI] [PubMed] [Google Scholar]
- [28].Manni L, Aloe L, Fiore M. Changes in cognition induced by social isolation in the mouse are restored by electro-acupuncture. Physiol Behav. 2009;98(5):537–542. doi: 10.1016/j.physbeh.2009.08.011. [DOI] [PubMed] [Google Scholar]
- [29].Weintraub A, Singaravelu J, Bhatnagar S. Enduring and sex-specific effects of adolescent social isolation in rats on adult stress reactivity. Brain Res. 2010;1343:83–92. doi: 10.1016/j.brainres.2010.04.068. [DOI] [PubMed] [Google Scholar]
- [30].Meng Q, Li N, Han X, et al. Effects of adolescent social isolation on the expression of BDNFs in the forebrain. Eur J Pharm. 2011;650(1):229–232. doi: 10.1016/j.ejphar.2010.09.061. [DOI] [PubMed] [Google Scholar]
- [31].Wright LD, Hébert KE, Perrot-Sinal TS. Periadolescent stress exposure exerts long-term effects on adult stress responding and expression of prefrontal dopamine receptors in male and female rats. Psychoneuroendocrinology. 2008;33(2):130–142. doi: 10.1016/j.psyneuen.2007.10.009. [DOI] [PubMed] [Google Scholar]
- [32].Lu L, Bao G, Chen H, et al. Modification of hippocampal neurogenesis and neuroplasticity by social environments. Exp Neurol. 2003;183(2):600–609. doi: 10.1016/s0014-4886(03)00248-6. [DOI] [PubMed] [Google Scholar]
- [33].King MV, Seeman P, Marsden CA, et al. Increased dopamine D2High receptors in rats reared in social isolation. Synapse. 2009;63(6):476–483. doi: 10.1002/syn.20624. [DOI] [PubMed] [Google Scholar]
- [34].Weiner I. The “two-headed” latent inhibition model of schizophrenia: modeling positive and negative symptoms and their treatment. Psychopharmacology (Berl) 2003;169(3-4):257–297. doi: 10.1007/s00213-002-1313-x. [DOI] [PubMed] [Google Scholar]
- [35].Andressoo JO, Saarma M. Signalling mechanisms underlying development and maintenance of dopamine neurons. Curr Opin Neurobiol. 2008;18(3):297–306. doi: 10.1016/j.conb.2008.07.005. [DOI] [PubMed] [Google Scholar]
- [36].Smidt MP, Smits SM, Burbach JP. Molecular mechanisms underlying midbrain dopamine neuron development and function. Eur J Pharm. 2003;480(1-3):75–88. doi: 10.1016/j.ejphar.2003.08.094. [DOI] [PubMed] [Google Scholar]
- [37].Oo TF, Marchionini DM, Yarygina O, et al. BDNF regulates early postnatal developmental cell death of dopamine neurons of the substantia nigra in vivo. Mol Cell Neurosci. 2009;41(4):440–447. doi: 10.1016/j.mcn.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Porritt MJ, Batchelor PE, Howells DW. Inhibiting BDNF expression by antisense oligonucleotide infusion causes loss of nigral dopaminergic neurons. Exp Neurol. 2005;192(1):226–234. doi: 10.1016/j.expneurol.2004.11.030. [DOI] [PubMed] [Google Scholar]
- [39].Guillin O, Griffon N, Bezard E, et al. BDNF controls dopamine D3 receptor expression: therapeutic implications in Parkinson's disease. Eur J Pharm. 2003;480(1-3):89–95. doi: 10.1016/j.ejphar.2003.08.096. [DOI] [PubMed] [Google Scholar]
- [40].Carlsson A. The current status of the dopamine hypothesis of schizophrenia. Neuropsychopharmacology. 1988;1(3):179–186. doi: 10.1016/0893-133x(88)90012-7. [DOI] [PubMed] [Google Scholar]


