Abstract
Eleutheroside B or E, the main component of Acanthopanax, can relieve fatigue, enhance memory, and improve human cognition. Numerous studies have confirmed that high doses of acetylcholine significantly attenuate clinical symptoms and delay the progression of Alzheimer's disease. The present study replicated a rat model of aging induced by injecting quinolinic acid into the hippocampal CA1 region. These rats were intraperitoneally injected with low, medium and high doses of eleutheroside B or E (50, 100, 200 mg/kg), and rats injected with Huperzine A or PBS were used as controls. At 4 weeks after administration, behavioral tests showed that the escape latencies and errors in searching for the platform in a Morris water maze were dose-dependently reduced in rats treated with medium and high-dose eleutheroside B or E. Hematoxylin-eosin staining showed that the number of surviving hippocampal neurons was greater and pathological injury was milder in three eleutheroside B or E groups compared with model group. Hippocampal homogenates showed enhanced cholinesterase activity, and dose-dependent increases in acetylcholine content and decreases in choline content following eleutheroside B or E treatment, similar to those seen in the Huperzine A group. These findings indicate that eleutheroside B or E improves learning and memory in aged rats. These effects of eleutheroside B or E may be mediated by activation of cholinesterase or enhanced reuse of choline to accelerate the synthesis of acetylcholine in hippocampal neurons.
Keywords: neural regeneration, traditional Chinese medicine, eleutheroside B or E, quinolinic acid, aged rats, Huperzine A, learning and memory, hippocampus, acetylcholine, cholinesterase, choline, mechanism, grants-supported paper, neuroregeneration
Research Highlights
(1) Administration of high-dose acanthopanax significantly relieves the symptoms and shortens the course of Alzheimer's disease. However, little is known about eleutheroside B or E treatment for Alzheimer's disease or its related mechanisms.
(2) The results of this study demonstrate that eleutheroside B or E can improve learning and memory deficits in aged rats induced by quinolinic acid, possibly by activating cholinesterase or enhancing choline reuse to accelerate acetylcholine synthesis in hippocampal cells.
INTRODUCTION
Alzheimer's disease is a neurodegenerative disease associated with progressive cognitive and memory impairments[1,2], and has become one of the most severe geriatric diseases[3,4]. To date, there is no effective therapy for Alzheimer's disease[5]. Alzheimer's disease damages the hippocampus, amygdala, and cerebral cortex, which are regions critical for learning and memory. Substantial pathological data has confirmed atrophy of the hippocampus in Alzheimer's disease, including changes in both anatomy and physiology, such as dysfunction induced by reduced excitement of and transmission by cholinergic neurons, neuronal loss, increased neurofibrillary tangles, and increased extracellular amyloid deposition[2]. Numerous studies have confirmed that the excitement of cholinergic neurons is associated with learning and memory, and acetylcholine is an important neurotransmitter in this process[4].
Acetylcholine is synthesized by choline and acetyl coenzyme A by choline acetyltransferase, which can be broken down into choline and acetic acid by cholinesterase. Therefore, these substances are key indicators of neurotransmission changes in Alzheimer's disease. Based on these findings, a positive effect on memory function has been discovered in patients with age-related memory impairment and Alzheimer's disease following administration of precursors of acetylcholine such as choline and lecithin and administration of cholinesterase inhibitors (Huperzine A, rivastigmine, donepezil). Currently, these are the most promising drugs used to treat Alzheimer's disease through improved cognitive functioning and clinical scores[6]. However, clinical medication can only delay the progression and improve the symptoms of Alzheimer's disease, and their clinical application has been limited by weak efficacy and specificity and serious adverse effects[7]. Acanthopanax is present in the roots and stems of Araliaceaeas, Acanthopanax senticosus (Rupr.et Maxim) Harms, which contains multiple eleutheroside (B, C, D, E), polysaccharides and a variety of trace elements[8]. Acanthopanax has been used to enhance memory and slow aging[8].
A large number of experimental studies have reported that Acanthopanax extracts can improve sleep, reduce fatigue, elevate the efficiency of brain functioning, reduce errors, and stabilize central nerves[9]. Clinical reports show that high-dose Acanthopanax administration can significantly alleviate symptoms and delay the disease course of Alzheimer's disease patients. Eleutheroside B or E, the main ingredient of Acanthopanax, accounts for 45% and 12% of its total glycosides, respectively[9]. No studies have examined the treatment of Alzheimer's disease with eleutheroside B or E, or its related mechanisms of action[9]. Thus, in the present study, we investigated the effects of eleutheroside B or E on learning and memory and neurological functions in aged rats.
RESULTS
Quantitative analysis of experimental animals
A total of 90 animals were equally and randomly assigned to nine groups. These groups were composed of model (quinolinic acid injection in the hippocampal CA1 region), sham-surgery (PBS injection in the hippocampal CA1 region), Huperzine A (model of aging in rats following intraperitoneal injection), and low-, medium- and high-dose eleutheroside B and eleutheroside E groups (model of aging in rats following intraperitoneal injection of 50, 100, or 200 mg/kg of each eleutheroside). Following model establishment, nine rats in the model, medium-dose of eleutheroside E, and high-dose eleutheroside B groups and eight in each of the high- and low-dose eleutheroside E groups survived. Therefore, 83 rats were included in the final analysis.
Learning and memory in aging-model rats following eleutheroside B or E administration
Escape latencies in the Morris water maze: Escape latencies were measured at 8:00 a.m. after animals received the administration on treatment day 34. After administration, escape latencies of each experimental group was significantly shorter than the model group. Escape latencies in the sham-surgery group were similar to those in the Huperzine A group, and significantly lower than those in the eleutheroside B or E groups (P < 0.05). Escape latencies were significantly shorter in the Huperzine A and medium-and high-dose eleutheroside B or E groups following administration compared with before administration (P < 0.05; Table 1).
Table 1.
Changes in escape latencies (second) in the Morris water maze test in aged rats before and after experimental administrations
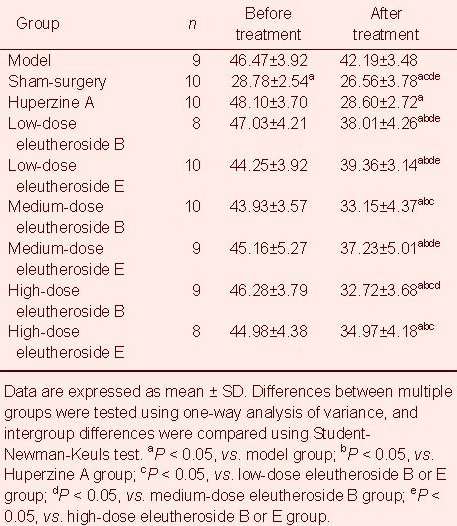
Error times in the Morris water maze: Water maze errors were significantly lower in the low-dose eleutheroside B or E groups compared with the medium- and high-dose eleutheroside B or E groups (P < 0.05). Moreover, these errors were significantly higher in the high-dose eleutheroside E group compared with the high-dose eleutheroside B group, but lower than in the other eleutheroside B or E groups (P < 0.05; Table 2).
Table 2.
Error times in the Morris water maze test in aged rats before and after experimental administrations
Hippocampal histological observations in rats treated with eleutheroside B or E
Hippocampal morphology was observed after determination of escape latencies at 8:00 a.m. on administration day 34. In the sham-surgery group, the right hippocampal CA1 exhibited a clear structure, with normal cell morphology, many neurons that were predominantly arranged in rows, cone-shaped cell bodies with intact appearances, large round or oval-shaped nuclei, evident nucleoli, and clear nuclear membranes. In the right hippocampal CA1 of rats in the model group, vacuolization was observed in the cytoplasm, Nissl bodies were reduced or absent, and nerve cells were swollen and unevenly distributed. In addition, in the model group, the intercellular space was wider, most cells were shrunken and deeply stained and wrinkled with blurred cell boundaries, nuceli were condensed and deeply stained, many nucleoli were absent, and the cells were arranged sparsely showing necrotic changes. The number of normal neurons was significantly lower in this group. In the high-dose eleutheroside B or E groups, the number of surviving neurons was significantly greater than in the low- and medium-dose eleutheroside B or E groups, and nerve cell arrangements were orderly. The staining intensity was lower than in sham-surgery group but higher than in the other groups. Moreover, swelling of the nerve cell bodies was significantly reduced, and a small amount of cytoplasmic vacuoles was observed, accompanied by Nissl bodies in good condition, enlarged and largely normal nuclei, clearly visible nucleoli, and significantly less damaged cells (Figure 1).
Figure 1.
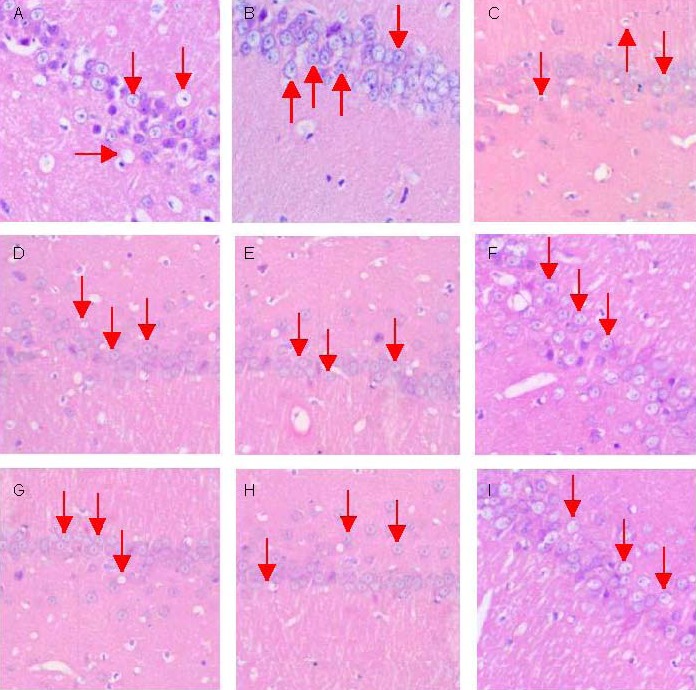
Morphology of nerve cells in the right hippocampal CA1 region in aged rats intraperitoneally injected with eleutheroside B or E (hematoxylin-eosin staining, × 400, inverted fluorescence microscope).
Arrows represent the shape and structure of the neurons.
(A) In model group neurons, a large number of cytoplasmic vacuoles were observed, and neurons were swollen, deeply stained and wrinkled, and showed neuronal necrosis; (B) In the sham-surgery group, neurons had a clear structure, were arranged in neat rows, and their cell bodies showed vertebral shape. Pathological changes were similar in the Huperzine A group (C), low-dose eleutheroside B (D), medium-dose eleutheroside B (E), low-dose eleutheroside E (G) and medium-dose eleutheroside E (H) groups.
In the high-dose eleutheroside B or E groups (F, I), the number of surviving neurons was significantly higher than in the other groups, the cells were arranged in rows, and cell transparency was lower than in the sham-surgery group, but higher than in the other groups (B–I). The neuronal cell bodies in this group only showed mild swelling, their nuclei were nearly normal, and their nucleolus was clearly visible.
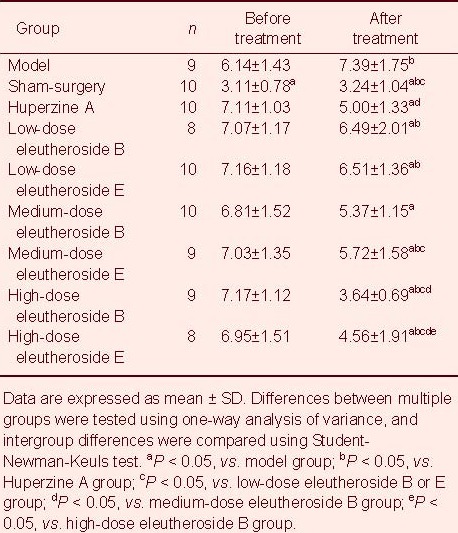
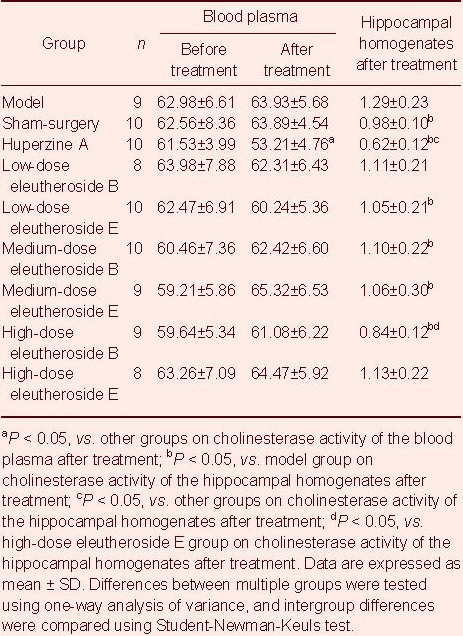
Cholinesterase activity in blood and hippocampal homogenates in rats treated with eleutheroside B or E
After administration, cholinesterase activity was significantly lower in the Huperzine A group compared with all other groups, and significantly lower in the eleutheroside B groups compared with the eleutheroside E groups (P < 0.05; Table 3).
Table 3.
Cholinesterase activity in blood (U/L) and hippocampal homogenates (U/L) before and after administration
Acetylcholine and choline content in blood plasma of rats injected with eleutheroside B or E (high performance liquid chromatography)
Cholinesterase determinations were conducted simultaneously to the determination of escape latencies at 8:00 a.m. on administration day 34. Results showed that acetylcholine content was significantly higher in the Huperzine A group compared with the other groups after administration (P < 0.05). Acetylcholine content was significantly higher in the high-dose eleutheroside E group compared with low- and medium-dose eleutheroside B or E groups, but significantly lower than in the high-dose eleutheroside B group (P < 0.05; Table 4).
Table 4.
Acetylcholine content (μg/mL) in blood plasma before and after experimental administrations
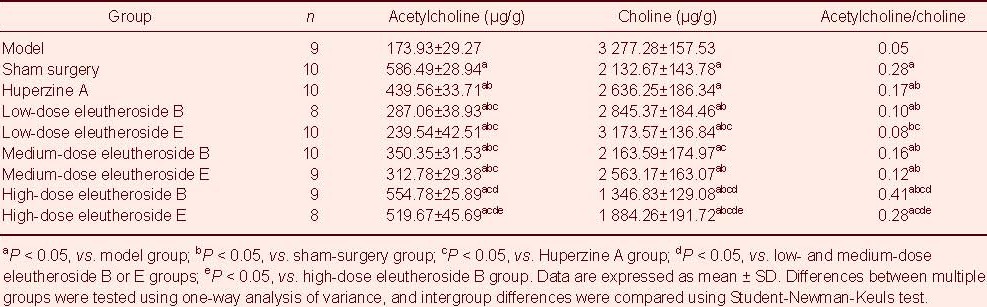
Acetylcholine and choline content in hippocampal homogenates of rats intraperitoneally injected with eleutheroside B or E
Acetylcholine and choline content in hippocampal homogenates was determined simultaneously with the determinations of acetylcholine in blood plasma at 8:00 a.m. on administration day 34. Acetylcholine content in hippocampal homogenates increased after treatment with increasing doses of eleutheroside. After administration, choline content in hippocampal homogenates decreased with increasing doses of eleutheroside B or E. Moreover, the ratio of acetylcholine to choline in hippocampal homogenates after treatment increased with increasing doses of eleutheroside (P < 0.05; Table 5).
Table 5.
Acetylcholine and choline content in hippocampal homogenates of aged rats injected with eleutheroside B or E
DISCUSSION
Eleutheroside B or E is the active compound found in Acanthopanax extracts, which mainly exists in the roots and stems of Araliaceae [Acanthopanax senticosus (Rupr.et Maxim.) Harms (Eleutherococcus senticosus Maxim].
These compounds are the most abundant in the bark and pulp, at 45% and 12% respectively[8]. Eleutherosides B and E are crystalline materials forming colorless needles, which has a variety of pharmacological effects, such as regulation of immunity, protection of brain tissues, and recovery from nerve injuries[9]. We have used Acanthopanax to attenuate aging in rats by injecting in combination with quinolinic acid into the hippocampus. Following this treatment, Acanthopanax significantly improved learning and memory in aged rats. Its mechanism is related to increased acetylcholine content and the regulation of enzyme expression (e.g., nitric oxide synthase, superoxide dismutase, and Na+-K+-ATP) in hippocampal homogenates. These effects can accelerate the synthesis of acetylcholine and enhance the function of cholinergic nerves in the hippocampus, alleviate damage to hippocampal neurons, orient growth and plasticizers of synapses, reduce metabolism of catecholamines, and maintain the excitability of neurons[8]. The basis for the present study was that the main ingredients of Acanthopanax are eleutherosides B and E.
At present, there are many reports regarding the clinical efficacy of Acanthopanax and its crude extracts to improve human memory. However, there has been no study of the effects of the eleutherosides B and E, including their effects on hippocampal neurotransmitters[10,11]. This study adopted a widely applied model of aged rats through central cholinergic neuron destruction following injection of quinolinic acid into the bilateral CA1 of the hippocampus[12,13,14]. Learning and memory in this group was nearly equivalent to the other groups before treatment and weaker than sham surgery group, which supports the use of this Alzheimer's disease model. Following treatment with eleutheroside B or E or Huperzine A, escape latencies, and errors in the Morris water maze were dose-dependently attenuated compared to before administration, with the effects of eleutheroside B stronger than those of eleutheroside E. This shows that eleutheroside B or E and Huperzine A can significantly improve learning and memory in animals aged by central cholinergic neuron destruction, and they can improve brain functions of brain and reduce error times[15,16]. The mechanisms underlying these effects may be complex.
Maintaining the function and structure of hippocampal neurons
Significantly more neurons survived in the hippocampal CA1 after high-dose eleutheroside B or E administration. Their arrangements were orderly and dense, and they exhibited mild swelling, intact Nissl bodies, normal nuclei, and clear nucleoli.
These results indicate that the mechanisms by which eleutheroside B or E improves learning and memory in aging-model rats are associated with maintaining the functions and structures of hippocampal nerve cells through molecular-level biological effects[17,18]. In the future, we will thoroughly examine these mechanisms.
Increasing neurotransmitters in the hippocampus associated with learning and memory
Pathological changes of Alzheimer's disease are manifested as degeneration and loss of cholinergic neurotransmission in the hippocampus and limbic system, decreased metabolism related to loss of neurotransmission (acetylcholine, 5-hydroxytryptamine, and norepinephrine), and loss of proteins and mRNA expression related to neurotransmitter receptors[19]. This study investigated the effect of eleutheroside B or E and Huperzine A on cholinesterase activity in hippocampal homogenates. Quinolinic acid or eleutheroside B or E injections into the hippocampus had no effect on cholinesterase in blood plasma, but cholinesterase activity in hippocampal homogenates significantly increased. Huperzine A significantly reduced cholinesterase activity in blood plasma and in the hippocampus, thus decreasing acetylcholine decomposition. This improved cholinergic neuron functioning and learning and memory, consistent with related reports. High-dose eleutheroside B or E also attenuated cholinesterase activity in hippocampal homogenates, which maintained normal levels of cholinesterase activity. This indicates that high-dose eleutheroside B or E may adjust cholinesterase activity by an indirect mechanism. These data do not definitively demonstrate that eleutheroside B or E improves learning and memory in a rat model of Alzheimer's disease[20].
The determination of acetylcholine and choline content in blood plasma and hippocampal homogenates indicates that quinolinic acid injection into the hippocampus had minimal effects on their levels in blood plasma, but significantly reduced acetylcholine content in hippocampal homogenates. This indicates that quinolinic acid markedly damages cholinergic neurons of the hippocampus, and reduces cholinergic neuron function in a manner that affects learning and memory[13]. Determination of acetylcholine and choline activity in hippocampal homogenates showed that low-dose eleutheroside B or E significantly increased acetylcholine content and decrease choline content in the hippocampus. Indeed, the ratio of acetylcholine to choline was 2.47 and 1.65 times that in the Huperzine A group, 7.76 and 5.19 times that in the model group, and 1.5 and 1.0 times that in the sham-surgery group. This confirms a dose-dependent tendency. Choline is predominantly consumed to synthesize acetylcholine. According to the pathways of acetylcholine synthesis and metabolism, we conclude that there is no relationship between eleutheroside B or E and cholinesterase in improving learning and memory, but they are associated with the activation of acetylcholine synthetase (i.e., choline acetylase). This may promote choline transportation into nerve varicosities, accelerate acetylcoenzyme A release from mitochondria, or regulate hippocampal neurotransmitters through cAMP-response element binding protein expression and tau phosphorylation[21,22]. An understanding of this mechanism of action requires further investigation.
In summary, eleutheroside B or E effectively enhances learning and memory in aged rats, and the effects of high-dose eleutheroside B are stronger than those of high-dose eleutheroside E. Their mechanism of action does not appear to be related to cholinesterase activity in the hippocampus, even though they can increase acetylcholine content in the hippocampus and reduce choline content that may be associated with activation of cholinesterase. To date, the treatment of Alzheimer's disease is mainly based on the inhibition of cholinesterase activity and reduction of acetylcholine decomposition to enhance learning and memory abilities. This study provides a new target for the treatment of Alzheimer's disease.
MATERIALS AND METHODS
Design
A randomized, controlled, animal study.
Time and setting
The experiment was performed at the Hubei Province Key Laboratory of Biological Resources Protection and Utilization, Hubei Institute for Nationalities, and Laboratory of Molecular Biology, Chongqing Medical University, China from March to October 2011.
Materials
A total of 90 Wistar male rats of 10 months, weighing 400–550 g, were provided by the Experimental Animal Center of Chongqing (license No. 4111268) after screening according to behavioral experiments. They were maintained in standard laboratory conditions in a vivarium at 22 ± 2°C with 12-hour day/night cycle. They were allowed free access to food and water.
Eleutherosides B and E were purchased from Sigma Aldrich in the United States (No. 61226 and 70133; molecular formula C58H92O25 and C34H46O18, molecular weight 1188.59 and 742.72). The clinically used dose of eleutheroside B or E is 50 mg/kg, and we used eleutheroside B and E at doses 2 and 4 times the clinically used dose for the intervention in aged rats[8].
Methods
Establishment of the aged rat model
The experiment began after pretesting of the hippocampus CA1 area using three-dimensional localization puncture medicine. The temperature was controlled at 22 ± 2°C and the humidity at 55 ± 15%. Before the experiment, rats in each group were intraperitoneally injected with 3.5% chloral hydrate (400 mg/kg) and their heads were fixed by microelectrode three-dimensional brain position finders (WDT-11, Northwest Guangming Instrument Factory, 90015). An incision (2 cm) was made along the median line of calotte to expose the cranium. The periosteum was separated, and the cranium was penetrated with dentistry drills (Hongjin Laboratory Equipment Company, Shanghai, China). A stereotactic atlas of the rat brain (anterioposterior: 3.5 mm, mediolateral: 2.0 mm, dorsoventral: 2.7 mm) was used[23,24]. Drugs were injected perpendicularly into bilateral hippocampal CA1 regions with a microinjector (Ningbo Zhenhai Glass Instrument Factory, 0808-103, 5 μL), including 2 μL of PBS into the sham-surgery group, and 2 μL of a quinoline acid solution (Sigma-Aldrich Chemical Company, S10477-286) dissolved in PBS at 75 nmol/μL into the other groups. The injections were typically conducted within 5 minutes. The pin holes were filled with dental base acrylic resin powder and sutured. Three days later, penicillin (200 mg per day) was intraperitoneally injected and each rat was maintained separately for 1 week. The models were considered successfully established if the escape latency was nearly 40 seconds to the platform and errors were equal to or less than five times in the Morris water maze test[25].
Eight days after model preparation and the learning and memory test, blood was collected from rat tails and drugs were administered starting the next day and continuing once per day between 8:00 a.m. and 9:00 a.m. The rats were weighed every 7 days and the dose adjusted according to their body mass. The model, sham-surgery, Huperzine A, and low-, medium- and high-dose eleutheroside B or E groups were intraperitoneally injected with 2 mL normal saline or Huperzine A (0.3 mg/kg; Pharmaceutical Co., Ltd., Henan Tailong, No. H11040156; positive control), or 50,100 or 200 mg/kg of eleutheroside B or E diluted with distilled water to 2 mL, respectively. These treatments continued daily for 34 days.
Morris water maze training and determination of learning and memory
Morris water maze training before administration was conducted during 3–7 days after model preparation, and the training after administration was conducted 29–33 days after administration. The training was conducted at 8:00 a.m. for 5 consecutive days. Briefly, before the experiment, clear water was poured into a Morris water maze (SMG-2, Institute of Pharmacology of Chinese Academy of Medical Sciences, 80 cm × 40 cm × 30 cm) to the indexed height and an appropriate amount of milk powder was added to murky the water. The water temperature was adjusted to 23–25°C. The water maze was divided into southeast, southwest, northwest, and northeast quadrants. The platform was fixed in the center of the southwest quadrant, which was 23 cm away from the center of the circle and 2 cm immersed underwater. Before training, animals were placed in the water maze (not onto the platform) to allow free swimming for 3 minutes, to provide for habituation to the environment of the water maze. Then, all animals were individually placed in the tank, facing the wall, along the middle of the four quadrants of the pool wall to let them seek the platform, on which they could climb up and stay on for 10 seconds. If the platform was not found within 60 seconds, the rat was guided to the platform and kept there for 10 seconds. They were then taken out from the tank to rest for 50 seconds, followed by the next training trial. Training was conducted four times daily for each rat[9,10]. On the 34th day (8:00 a.m.), the rats were placed into the tank along the middle tank wall of the four quadrants, an automated photography system recorded the time that the rats took to find the platform (place navigation test) and the error times (the number of errors in finding the platform). The maximum time allowed to escape was set as 60 seconds, and the recording stopped automatically after 60 seconds[11,12].
Sample collection
The samples were collected after the rats were anesthetized by intraperitoneal injection of 3.5% chloral hydrate (400 mg/kg). At 35 days after administration, the hippocampus was harvested and blood was collected, centrifuged at a low temperature in a freezing centrifuge (3K30, Sigma, 70376) and stored for subsequent use.
Pathological observation of the hippocampus (hematoxylin-eosin staining)
All rats were fasted for 12 hours and decapitated to collect blood after the final intraperitoneal injection of the drugs. The head was placed on ice, and the brain was harvested. The cerebral cortexes were stripped and the entire hippocampus was obtained. Hippocampal tissues were sliced continuously in coronal plane using an ultramicrotome (LKB-III, RM2245, Leica, Germany) for 3 sections (5–8 μm thick), which were stained with hematoxylin dye for 5–8 minutes. These sections were rinsed with tap water for 10–15 minutes and differentiated with hydrochloric acid-alcohol at room temperature for 30–45 seconds. They were stained with eosin dye for 30–45 seconds when they became blue. The sections were rinsed with tap water again, dehydrated with gradient ethanol, cleared with xylene, and mounted with neutral resin. Pathological changes of hippocampal CA1 region were then examined.
Sample extraction and preparation
According to a previously described method[13,26], blood samples were stored in heparinized tubes and centrifuged at 3 000 r/min for 10 minutes at a low temperature for the determination of cholinesterase activity and acetylcholine and choline levels. According to the proportion of 10 mL normal sodium added into the 1.0 g hippocampal tissues, the tissues were placed into an electric glass homogenate machine (DY89-I, Ningbo Chi Division device Institute, China) to prepare the homogenates by centrifugation. The supernatant was discarded and then 0.8 mL hippocampal homogenates were used for detection of acetylcholine and choline. The samples were stored in liquid nitrogen using serial numbers (below –40°C).
Determination of cholinesterase activity in hippocampal tissues
Enzyme linked immunosorbent assay was used for the determination of cholinesterase activity in the hippocampal homogenates using a cholinesterase kit (Y044,0492-2011, Beijing North Kangtai Clinical Reagent Co., Ltd., Beijing, China) based on the principle that S-Butyrylthiocholine iodide is decomposed into butanoic acid and thiocholine by cholinesterase. Thiocholine can then combine with 5,5’-dithiobis to form 5-mercapto-2-nitryl benzoic acid. Thus, cholinesterase activity can be determined by measuring the markup speed (410 nm) of 5-mercapto-2-nitryl benzoic acid[15]. To this end, 6 μL blood serum or hippocampal homogenates were used and R1 and R2 (4:1) were mixed (240 μL: 60 μL) and incubated at 37°C for 3 minutes. The concentration was determined with a 722 spectrophotometer (Shanghai Precision Instrument Co., Ltd., Shanghai, China) at a 340 nm dominant wavelength, 410 nm complementary wavelength, and 10 mm light path. Normal sodium was the reagent blank. The absorbance of each tube was continuously observed for 30 seconds to count the ΔA/min.
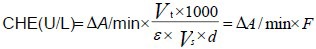
Δ/A/min: Rate of absorbency per minute; Vt: volume of response fluid (mL); 1 000: change factor; e: mole absorbency coefficient; Vs: specimen volume (mL); d: light path of colorimetric cylinder (10 mm).
Determination of acetylcholine and choline in hippocampal tissues
The concentration of acetylcholine and choline was determined by high performance liquid chromatography (LC-2010, Japan Shimadzu Corporation) in the hippocampal homogenates. According to a previously described method[11], eluted mobile phase was used to form a liquor with 0.005% (mass fraction) ProClin, 50 mM NaH2PO3, and 0.5 mM Na2 EDTA, and the pH was adjusted to 8.50 with NaOH. The flow rate was 130 μL/min. The potential of the reference electrode was set to +500 mV, sensitivity to 5 nAFS, and temperature of the chromatographic analysis to 25°C. AchC1 35.3 mg and ChC1 27.9 mg (U.S. BAS) were resolved separately in 100 mL ethanoic acid standard diluent, 0.05 M (containing 0.005% ProClin). Of this diluent, 1 mL was used to prepare standard reserve fluid (40 μM). Then, a series of standard mixture fluids of 10, 40, 100, 400 nM and 2 μM were prepared, filtered with a 0.22 μm filter membrane, and stored as samples (10 μL).
Statistical analysis
All experimental data are expressed as mean ± SD and analyzed using SPSS 11.5 software (SPSS, Chicago, IL, USA). One-way variance of analysis was used for multiple group comparisons and Student-Newman-Keuls test to contrast the groups. P < 0.05 was considered statistically significant.
Acknowledgments:
We appreciate the assistance from the staff and administration of the Pharmacology Teaching and Research Office of the Medical College of Chongqing Medical University and the Key Laboratory of Biologic Resources Protection and Utilization of Hubei Province. We also thank the staff of the Department of Clinical Laboratories of Enshi Central Hospital of Hubei Province for their assistance with the data measurements.
Footnotes
Conflicts of interest: None declared.
Funding: The study was supported by the Foundation from Department of Education of Hubei Province, No. D20111903.
Ethical approval: This study was approved by the Animal Ethics Committee of Hubei Institute for Nationalities, China.
(Reviewd by Murnane K, Raye W, Hou YP, Zhang GR)
(Edited by Wang J, Su LL, Li CH, Song LP)
REFERENCES
- [1].Wu XQ. Nanjing: Nanjing Yike Daxue, China; 2011. The researchon toxin-removing, blood-activatin and phlegm-removing methodin protecting of cerebral microvascular endothelial cells. [Google Scholar]
- [2].Wang Y, Tie XX, Yao CY, et al. Effect of recombinant human erythropoietin on the expression of TNF-α in cerebral ischemia-reperfusion mice. Zhongguo Yike Daue Xuebao. 2012;41(5):396–398. [Google Scholar]
- [3].Yasuda T, Shiozaki H. Esophageal reconstruction using a pedicled jejunum with microvascular augmentation. Ann Thorac Cardiovasc Surg. 2011;17(2):103–109. doi: 10.5761/atcs.ra.10.01648. [DOI] [PubMed] [Google Scholar]
- [4].Liu J, Shi WJ, Zhang SN. Experimental use of pulmonary flap with alloy stent for the reconstruction of trachea and esophagus. Zhonghua Yi Xue Za Zhi. 2011;91(1):65–68. [PubMed] [Google Scholar]
- [5].Wang Y, Yu YJ, Qin XF, et al. The effect of recombinant human erythropoietin on the expression of VEGF in hippocampus of cerebral ischemia-reperfusion mouse. Jiepou Kexue Jinzhan. 2012;18(3):285–288. [Google Scholar]
- [6].Juul SE, Mc Pherson RJ, Bammler TK, et al. Recombinant erythropoietin is neuro protective in a novel mouse oxidative injury model. Dev Neurosci. 2008;30(4):231–242. doi: 10.1159/000110348. [DOI] [PubMed] [Google Scholar]
- [7].Yan L, Huang DB. Effects of astragalan on neurotransmitters and expression of c-fos Mrna in hippocampus after ischemic brain injury in rats. Zhongguo Bingli Shengli Zazhi. 2012;28(2):263–268. [Google Scholar]
- [8].Huang DB, Liu XH. Effects of acanthopanax senticosus injection on learning and memory impairment and monoamine neurotransmitter of hippocampus in aging-model rats. Hubei Minzu Xueyuan Xuebao: Yixue Ban. 2008;25(3):1–4. [Google Scholar]
- [9].Diao B, Tang Y, Zhu YL, et al. Effects of Acanthopanacis senticosi polysaccharides on expressions of c-fos and p53 genes in hippocampal neurons injured by H2O2 in rats. Zhongguo Linchuang Shenjing Waike Zazhi. 2010;15(6):347–349. [Google Scholar]
- [10].Wang Li, Yu XF, Qu SC, et al. Effects of CASI on myocardial ischemia-reperfusion arrhythmia in rats. Zhongguo Zhong Yao Za Zhi. 2007;32(20):2174–2177. [PubMed] [Google Scholar]
- [11].Huang LG, Wang D, Ge ZL. Effects of acanthopanax senticosus injection on the brain tissue, beta-endorphin in rats with intracerebral hemorrhage. Zhongguo Laonianxue Zazhi. 2006;26(9):1232–1234. [Google Scholar]
- [12].Zhang Y, Liu BZ, Pei Y, et al. Pharmacological action of acanthopanax senticosus. Shenyang Yaoke Daxue Xuebao. 2002;19(2):143–145. [Google Scholar]
- [13].Yang JZ, Li JB, Zhang DM, et al. HPLC method adopted to measure the contents of eleutheroside B or E in different places. Zhongyiyao. 2005;28(8):669–673. [Google Scholar]
- [14].St George-Hyslop PH, Petit A. Molecular biology and genetics of Alzheimer's disease. C R Biol. 2005;328(2):1194–1130. doi: 10.1016/j.crvi.2004.10.013. [DOI] [PubMed] [Google Scholar]
- [15].Suzanne M, Monte DE. Review of insulin and insulin-like growth factor expression, signaling, and malfunction in the central nervous system: relevance to Alzheimer's disease. J Alzheimers Dis. 2005;7(1):123–126. doi: 10.3233/jad-2005-7106. [DOI] [PubMed] [Google Scholar]
- [16].Zhang DM, Liu HY, Xie L, et al. Effect of baicalin and berberine on transport of nimodipine on primary cultured, rat brain microvascular endothelial cells. Acta Pharmacol Sin. 2007;28(4):573–578. doi: 10.1111/j.1745-7254.2007.00521.x. [DOI] [PubMed] [Google Scholar]
- [17].Jin R, Jiang XY, Ma X, et al. Effect of gamma-hydroxybutyric acid receptor on focal cerebral ischemia-reperfusion injury in rats. Yao Xue Xue Bao. 2007;42(8):838–842. [PubMed] [Google Scholar]
- [18].Li H, Deng CQ, Chen BY, et al. Effects of Panax Notoginseng saponins on expression of caspase after focal cerebral ischemia-reperfusion in rats. Zhongguo Yaolixue Tongbao. 2006;22(2):189–193. [Google Scholar]
- [19].Ou Y, Yuan XD, Cai YN, et al. Ultrastructure and electrophysiology of astrocytes differentiated from adult adipose-derived stromal cells. Chin Med J (Engl) 2011;124(17):2656–2660. [PubMed] [Google Scholar]
- [20].Oh SY, Choi SJ, Kim KH, et al. Autophagy-related proteins,LC3 and Beclin-1, in placentas from pregnancies complicated by preeclampsia. Reprod Sci. 2008;15(9):912–920. doi: 10.1177/1933719108319159. [DOI] [PubMed] [Google Scholar]
- [21].Maiuri MC, Zalckvar E, Kimchi A, et al. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol. 2007;8(9):741–752. doi: 10.1038/nrm2239. [DOI] [PubMed] [Google Scholar]
- [22].Casteilla L, Planat-Benard V, Laharrague P, et al. Adipose-derived stromal cells: their identity and uses in clinical trials, an update. World J Stem Cells. 2011;3(4):25–33. doi: 10.4252/wjsc.v3.i4.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Yan L, Huang DB, Liu JH, et al. Effects of astragalus polysaccharide on expression of HSP70, PKB and P53 in rat cerebral cortex with cerebral ischemia and reperfusion. Zhongguo Bingli Shengli Zazhi. 2012;28(2):263–268. [Google Scholar]
- [24].Liu B, Wu MH, Dong J, et al. Neuronal differentiation of adipose tissue-derived stromal cells. Zhongguo Zuzhi Gongcheng Yanjiu yu Linchuang Kangfu. 2010;14(1):15–18. [Google Scholar]
- [25].Girolami EI, Bouhy D, Haber M, et al. Differential expression and potential role of SOCS1 and SOCS3 in Wallerian degeneration in injured peripheral nerve. Exp Neurol. 2010;223(1):173–182. doi: 10.1016/j.expneurol.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Drăghici S, Khatri P, Tarca AL, et al. A systems biology approach for pathway level analysis. Genome Res. 2007;17(10):1537–1545. doi: 10.1101/gr.6202607. [DOI] [PMC free article] [PubMed] [Google Scholar]


