Abstract
The astrocyte is a critical regulator of neuronal survival after ischemic brain injury. Electroacupuncture may be an effective therapy for cerebral ischemia, as electroacupuncture frequency can affect the structural integrity of astrocytes. In this study, a rat model of middle cerebral artery occlusion established using the modified thread embolism method was treated with electroacupuncture of the bilateral Quchi (LI11) and Zusanli (ST36) at 15, 30, and 100 Hz frequencies. Behavioral testing, immunohistochemistry and electron microscopy were used to explore the effect of these electroacupuncture frequencies used on maintaining the structural integrity of ischemic brain tissue. Compared with the model and 100 Hz electroacupuncture groups, the 15 and 30 Hz electroacupuncture groups displayed decreased neurological deficit scores, as evaluated by the “Longa” method, significantly increased glial fibrillary acidic protein expression, and alleviated ultrastructural damage of astrocytes at the edge of the infarct. Our experimental findings indicate that 15 and 30 Hz electroacupuncture intervention can favorably maintain the structural integrity of astrocytes and play a protective role in cerebral ischemic injury. Astrocyte structural integrity may be the mechanism underlying acupuncture production of ischemic tolerance.
Keywords: neural regeneration, acupuncture and moxibustion, brain damage, electroacupuncture frequency, focal ischemia, glial fibrillary acidic protein, astrocytes, neuroprotection, cerebral ischemic tolerance, electron microscope, grants-supported paper, neuroregeneration
Research Highlights
(1) Astrocytes can aggravate ischemic brain damage and therefore determine the survival of ischemic neurons.
(2) Electroacupuncture is an effective treatment of cerebral ischemia, however, the optimal electroacupuncture frequency for the treatment of cerebral ischemia remains unclear.
(3) Electroacupuncture intervention at 15 and 30 Hz frequencies can maintain the structural integrity of astrocytes, showing a protective effect against cerebral ischemic injury. We speculate that maintenance of the structural integrity of astrocytes is one possible way that acupuncture produces ischemic tolerance.
INTRODUCTION
Astrocytes participate in central nervous system metabolism and provide nutritional support to maintain normal neuronal function. Additionally, astrocytes also play a crucial role in the maintenance of the blood-brain barrier and the activation of synapses[1,2,3,4]. Activated astrocytes contribute to the recovery of ischemic brain tissue. For example, astrocytes have been shown to maintain ionic homeostasis, scavenge free radicals, provide nutrition and growth factors, promote the formation of new blood vessels, and sustain the regeneration of synapse and nerve cells[5]. Glial fibrillary acidic protein is mainly present in astrocytes and is therefore regarded as a marker for mature astrocytes[6]. Glial fibrillary acidic protein is expressed in normal astrocytes, and this expression greatly increases in activated astrocytes. Therefore, the amount of glial fibrillary acidic protein reflects the level of activated astrocytes following brain damage[7,8,9].
Electroacupuncture has been shown to block the procession of necrosis and apoptosis in neuronal cells when cerebral ischemia-reperfusion occurs by resisting oxidative stress injury and protecting brain tissue[10]. Electroacupuncture improves or reverses neuronal impairment in ischemic brain tissue, and regulates energy supply and metabolism in the ischemic area, suggesting a neuroprotective effect[11]. Pulse frequency is one of the most important parameters in electroacupuncture intervention. Different frequencies of electroacupuncture could produce effects through a number of neurochemical mechanisms integrated by various central nervous system pathways[12,13,14,15], and would provide diverse effects in the clinical treatment of cerebral ischemia. High-frequency or low-frequency continuous wave stimulation is often used in the treatment of cerebral ischemia, along with low/high frequency dilatational wave stimulation. There is little clinical evidence or experimental data demonstrating which frequency is more suitable for the treatment of ischemic injury. Growing evidence demonstrates that astrocytes have a considerable role in initiating and aggravating ischemic brain injury, yet the survival of ischemic neurons mainly depends on the astrocytes[16].
In this study, a rat model of middle cerebral artery occlusion was performed using the modified thread embolism method. Rats were then treated with different frequencies of electroacupuncture. The protective mechanisms in brain ischemic tolerance produced by astrocytes following electroacupuncture were explored in a broader attempt to provide novel therapeutic strategies for treatment and prevention of ischemic injury.
RESULTS
Quantitative analysis of experimental animals
Middle cerebral artery occlusion was established in 60 rats, with success in 48 rats. These animals were randomly and equally divided into four groups (12 rats per group): the model group, and electroacupuncture groups at 15, 30, and 100 Hz (middle cerebral artery occlusion model + electroacupuncture at 15, 30, or 100 Hz, respectively). No animals in the model group were excluded, while one rat in the 15 Hz group, two rats in the 30 Hz group, and two rats in the 100 Hz group were excluded because of death. Overall, 36 rats were included in result analysis, with nine rats in each group.
Neurological deficits were improved in middle cerebral artery occluded rats following electroacupuncture treatment
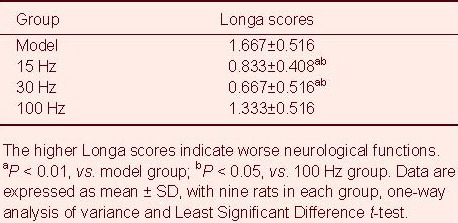
Rat neurological deficit scores were assessed at 5 days after middle cerebral artery occlusion (Table 1). Results showed that the neurological deficit scores in the 15 Hz group and 30 Hz group were lower than those in the model group and 100 Hz group (P < 0.01, P < 0.05). Experimental findings indicate that nerve function was improved by electroacupuncture.
Table 1.
Effect of electroacupuncture on neurological deficit scores in ischemic rats
Glial fibrillary acidic protein expression following electroacupuncture treatment in middle cerebral artery occluded rats
Glial fibrillary acidic protein positive cells were stained as brown yellow, yellow or light yellow, and staining was visible in cell bodies, nerve fibers and terminals. At the infarct edge (coronal plane at 3 mm posterior to bregma, sagittal plane at parietal cortex 3.5 mm lateral to the midline), glial fibrillary acidic protein positive cells and fibers were brown colored, with significant hypertrophy, protruding thickening and increasing number, showing the typical form seen on activation (Figure 1).
Figure 1.
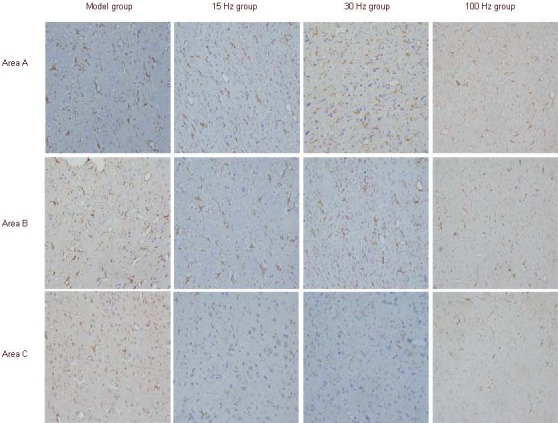
Effect of electroacupuncture on glial fibrillary acidic protein expression in brain tissue of ischemic rats (immunostaining, light microscopy, × 400).
Glial fibrillary acidic protein-positive cells were brown, yellow or pale yellow, and the cell body, nerve fibers and fiber terminals were all stained. Glial fibrillary acidic protein was greatly expressed in areas A and B and scarcely expressed in area C. Area A: Infarct edge; area B: ipsilateral parietal lobe cortex; area C: contralateral cortex.
After rats were subjected to electroacupuncture intervention at 15 and 30 Hz frequencies, glial fibrillary acidic protein immunoreactivity was observed in the cortex of the parietal lobe on the infarct side (coronal place at 3 mm posterior to bregma, sagittal plane at parietal cortex 4.5 mm lateral to the midline), with enlarged cell bodies and thickening fibers (Figure 1). In the cortex of the parietal lobe of the contralateral side (coronal plane at 3 mm posterior to bregma, sagittal plane at parietal cortex 3.5 mm lateral to the midline), there were fewer glial fibrillary acidic protein positive cells, sparse distribution, and decreased number (Figure 1). The extent of glial fibrillary acidic protein positive astrocytes, in the ipsilateral parietal lobe cortex was the same as the contralateral cortex in the model group and 100 Hz group (Figure 1).
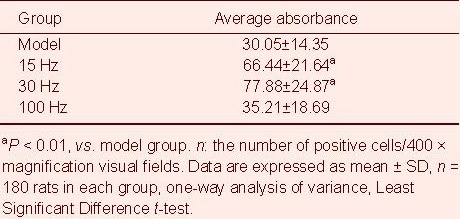
Compared with the model group, the number of positive astrocytes and the average absorbance value were increased at the infarct edge after intervention with 15 and 30 Hz electroacupuncture, but not 100Hz (P < 0.01; Tables 2, 3).
Table 2.
Effect of electroacupuncture on the number of glial fibrillary acidic protein positive cells in ischemic rats
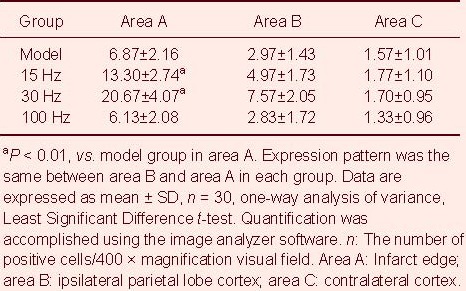
Table 3.
Effect of electroacupuncture on expression of glial fibrillary acid protein at the infarct edge in ischemic rats
Change in astrocyte ultrastructure in rats following electroacupuncture
The ratio of nuclei to endoplasm was reduced at the infarct edge in the model group, while organelles, such as lysosomes and mitochondria, were increased. Astrocytes in the 15 Hz group were rich in heterochromatin. Furthermore, the ratio of nuclei to endoplasm was increased and the number of organelles, such as rough endoplasmic reticulum and mitochondria, were increased. The ratio of nuclei to endoplasm was reduced in the 100 Hz group, but heterochromatin was visible and organelles, such as rough endoplasmic reticulum and lysosomes, were increased. The ultrastructural features of astrocytes in the parietal lobe cortex and the contralateral cortex were normal (Figure 2).
Figure 2.
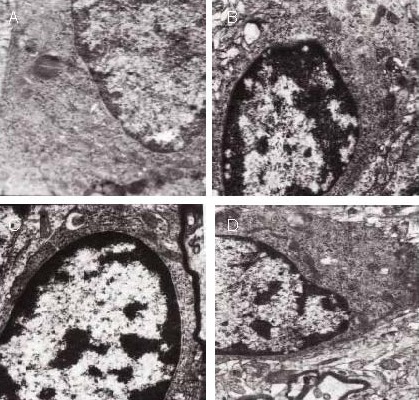
Effect of electroacupuncture on the ultrastructure of astrocyte nuclei at the infarct edge in ischemic rats (uranyl acetate and lead citrate double staining, transmission electron microscopy, × 12 000).
Compared with the model and 100 Hz groups (A, D), in the 15 and 30 Hz groups (B, C), the ratio of nuclei to endoplasm was increased, as was the number of organelles. The ratio of nuclei to endoplasm and the number of organelles was decreased in the 100 Hz group.
In the model group, presynaptic structure was fuzzy and sparse at the infarct edge. In the 15 Hz group, the presynaptic and postsynaptic structures were clearly visible, with the presynaptic density being thicker. The synaptic structure was similar between the 30 Hz and 15 Hz groups, and between the 100 Hz and model groups. The synaptic structure in the ipsilateral parietal lobe cortex and contralateral cortex were analogous to the 15 Hz group (Figure 3).
Figure 3.
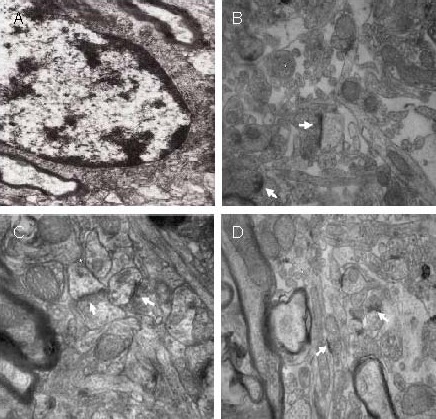
Effect of electroacupuncture on the ultrastructure of astrocyte synapses at the infarct edge in ischemic rats (uranyl acetate and lead citrate double staining, transmission electron microscopy).
Compared with the model group (A; × 12 000) and 100 Hz group (D; × 15 000), in the 15 and 30 Hz groups (B, C; × 15 000), the presynaptic and postsynaptic structures were clearly visible, with the presynaptic density being thicker. Arrows indicate the synapse.
Furthermore, neurons were severely degenerated in the model group at the infarct edge, but mildly degenerated in the 15 Hz group. Normal morphology was seen in the 30 Hz group, and moderate degeneration was seen in the 100 Hz group. Neurons in the ipsilateral parietal lobe cortex and contralateral cortex were analogous to the 30 Hz group (Figure 4).
Figure 4.
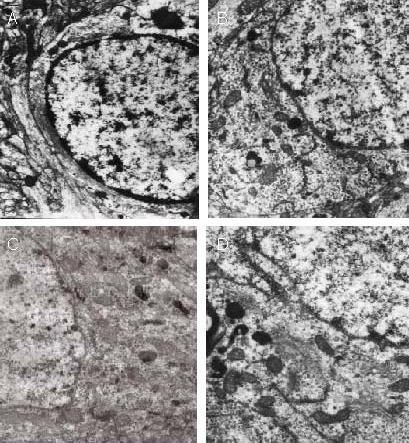
Effect of electroacupuncture on the ultrastructure of neurons at the infarct edge in ischemic rats (uranyl acetate and lead citrate staining, transmission electron microscopy, × 10 000).
Compared with the model and 100 Hz groups (A, D), in the 15 and 30 Hz groups (B, C), neurons were minimally damaged. Neuronal degeneration was determined by the morphology and number of organelles in the cytoplasm, changes in specific organelles (such as lysosomes) and morphological changes in the nuclei. Mild degeneration: only organelles exhibited morphological changes, specific organelles increased, and the nucleus was not changed. Severe regeneration: nuclear chromatin aggregate, organelle disappearance, and morphology similar to that of dying cells. Moderate regeneration: Severity between mild regeneration and severe degeneration.
DISCUSSION
Astrocytes were previously thought to only contribute to the protection of neurons, but increasing interest has been paid to the role that astrocytes play in the central nervous system[17]. The reactive hyperplasia of astrocytes after an insult may help the damaged central nervous system to repair itself, and could also aid in the recovery of neuronal function following cerebral ischemia[18,19].
Astrocytes extend numerous podocytes in all directions to make contact with neurons and synapses. Some astrocytes are even wrapped around various parts of the neuron, including cell bodies, dendrites, axons, and synapses. In this way, astrocytes are involved in neuronal nutrition, protection, support, electrophysiological activity and signal transduction[20,21]. Astrocytes take up and release neurotransmitters to ensure that synaptic activity is effective and precise.
Astrocytes play an important role in the regulation of water and electrolyte balance in the micro-environment, especially the buffering action of H+ and K+[22]. When ischemic brain injury occurs, glial cells release various neurotrophic factors to promote the repair and regeneration of neurons, as well as enhance neuronal plasticity, which helps to improve nervous system function.
Previous studies have found that astrocytes actively proliferate and can promote the restoration and reconstruction of damaged brain tissue by releasing basic fibroblast growth factor, neurotrophic factor, nerve growth factor and ciliary neurotrophic factor[23,24,25,26]. Furthermore, the activation of astrocytes in the ischemic penumbra has been shown to be conducive to nerve recovery[27,28]. In early brain injury, astrocytes promote neuronal development, however, neuronal growth and development is inhibited in late stage injury[29]. Electroacupuncture promotes synaptic reorganization[30,31]. This reorganization may occur following the induction of astrocyte activation, thereby enhancing the connection between astrocytes and synapses. This hypothesis may also provide a mechanism to explain the treatment of cerebral ischemia with electroacupuncture.
Lu et al[32] have shown that electroacupuncture could improve neurobehavioral scores, reduce the number of necrotic neurons in the hippocampal CA1 region, and protect the brain in mice. Electroacupuncture also promotes neuronal recovery by regulating the reactive hyperplasia of astrocytes to restrain excessive proliferation in the rat hippocampus after cerebral ischemia[33]. Luo et al[34] revealed that electroacupuncture could reduce astrocyte swelling in the ischemic zone of focal cerebral ischemia rats, and play a protective role for neurons through promoting neuronal recovery and restraining excessive proliferation after cerebral ischemia. Activated astrocytes in ischemic brain removed excitatory neurotransmitters, glutamate and H+, K+. In addition, these astrocytes increased glycogen storage and secretion of neurotrophic factor, which was conducive to improving the microenvironment of ischemic nerve cells and repairing damaged neurons. Evidence suggested that a certain degree of astrocyte activation had a protective effect on ischemic brain injury[35]. Agmatine was shown to improve prognosis of transient cerebral ischemic rats, possibly through the reduction of astrocyte edema, inhibiting proliferation and reducing apoptosis[36].
The results of this study were consistent with previous reports. We found that certain electroacupuncture frequencies not only could promote limb function recovery in cerebral ischemic rats, but also stimulate astrocyte activation, which was reflected by the increasing average absorbance of glial fibrillary acidic protein. Ischemic preconditioning may be generated through the activation of astrocytes. Ischemic preconditioning-induced ischemic tolerance significantly improved the expression of glial fibrillary acidic protein in the ischemic area[36,37]. Immunoreactive cells were significantly different in the model group (focal ischemia). This phenomenon might explain the way for astrocytes exert a neuroprotective effect[37]. Some scholars have observed that astrocyte apoptosis may promote the occurrence of secondary death in nerve cells, leading to further enlargement of the infarct area[38].
Therefore, blocking the astrocyte apoptosis pathway would inhibit the death of nerve cells after cerebral ischemia, which is favorable for the recovery of neurological function. Under electron microscopy, we observed that ischemic changes in nerve cells and astrocytes were significantly reduced in the hippocampus following stimulation at 15 and 30 Hz. This indicated that pretreatment was more effective in maintaining cell shape and created a neuroprotective effect[29]. At the same time, glial fibrillary acidic protein expression was significantly up-regulated and accompanied by changes in cell morphology, including cellular hypertrophy, neurite thickening, and more intense staining. It has been suggested that transient ischemic preconditioning could promote the proliferation and activation of astrocytes when ischemia occurs again. Moreover, the activated astrocytes could lessen the damage caused by the second ischemic event through inducing endogenous neuroprotective effects, which play an important role in brain ischemic tolerance. Astrocyte mediated endogenous protective effects may be achieved through a variety of mechanisms. Reuptake of excitatory amino acids and ATP/adenosine is a key link in signal transduction of astrocytes and neurons[39,40,41,42,43,44]. In addition, Ca2+ fluctuation, homeostasis, and vasomotor function adjustment mechanism may also be associated with endogenous neuroprotective effects[45,46]. These variables contribute to adjusting astrocyte proliferation, reducing astrocyte swelling and neuronal degeneration at the ischemic area, and preserve synaptic structural integrity. A large amount of glial fibrillary acidic protein expression could be a double-edged sword, however, for hypoxic-ischemic neurons. Increasing glial fibrillary acidic protein expression around the hypoxic-ischemic neurons might provide protection[9], but by contrast, the downside is that excessive gliosis would produce a mechanical barrier that impedes nerve injury repair, and could even grow into a glioma. Glial fibrillary acidic protein positive astrocytes have been shown to influence primitive progenitor cells to differentiate into mature astrocytes[47]. These cells are then involved in clearing parts of damaged neurons and sealing off the damaged area. Also, mature astrocytes participate in the surviving environment of the ischemic area and secrete a variety of neurotrophic factors in the injured area to promote central and peripheral axonal growth and survival[48,49].
Astrocytes regulate synaptic synthesis and growth, and interact with blood vessels to form the colloid vascular network. Astrocytes not only participated in the composition of the brain, but also excited and transduced signals in the exchange area, which guide the colloid vascular unit to regulate the activity of neurons by cell coordination signaling[50,51,52,53]. Moreover, astrocytes induce neurogenesis in adult neural stem cells[54]. From this study, we learned that one of the possible mechanisms underlying endogenous protection after cerebral ischemia, which was induced by electroacupuncture treatment, was to influence glial fibrillary acidic protein expression. In addition, another mechanism might be adjusting astrocyte proliferation and preserving structural integrity.
Different electroacupuncture parameters produce varying effects and different frequencies of electroacupuncture cause various analgesic effects. For example, 2/100 Hz of alternating dilatational wave could increase the release of four kinds of opioid peptides, which created an analgesic effect[55,56]. The 2 Hz electroacupuncture inhibited pain sensitivity and hypersensitivity, and multiple stimuli would take on a cumulative effect. Whereas in 100 Hz electroacupuncture, the analgesic effect was poor, and multiple stimuli did not produce a cumulative effect[56]. In this study, 15 and 30 Hz could create an optimal response, which might be associated with nervous system protection. This was not the case with the 100 Hz frequency stimulation.
Compared with previous studies, this experiment had the following differences: Former researches focused on the functional mechanism of electroacupuncture. Few studies have been published addressing the relationship between electroacupuncture frequency and functional mechanisms. Since the frequencies of electroacupuncture play an important role in clinical curative effect, it is necessary to explore how different frequencies of electroacupuncture work on cerebral ischemic rats.
To make the experimental data more accurate, we chose to make further observations from different areas within the same brain specimen, which achieved a form of internal control. In addition, we detected astrocytes, neurons and synaptic ultrastructures using electron microscopy. All experimental findings contribute further to the study of the function of electroacupuncture and the frequency required for use after cerebral ischemia.
MATERIALS AND METHODS
Design
A randomized, controlled animal experiment.
Time and setting
Experiments were conducted in the Central Laboratory and Animal Experimental Center of Guangxi Medical University, China between August 2009 and December 2010.
Materials
Healthy male Sprague-Dawley rats, weighing 250–300 g, were purchased from the Experimental Animal Center of Guangxi Medical University in China (license No. SCXK (Gui) 2003-0003). Rats were kept in a quiet and dim environment at 20 ± 2°C for 24 hours. All rats were fasted 12 hours before experiments.
Methods
Establishment of cerebral ischemic model
Middle cerebral artery occlusion was established using the modified thread embolism method, as previously described[57,58]. Rats were anesthetized with 10% chloral hydrate 3.5 mL/kg given intraperitoneally. An incision was made into the neck skin following conventional disinfection, and the right common carotid artery and external carotid artery were bluntly dissected and ligated. The internal carotid artery was found using blunt dissection and a slipknot was applied using a silk suture. The far-end of the internal carotid artery was blocked with a microvascular clip. A monofilament nylon suture was introduced into the lumen of the common carotid artery through a puncture away from the carotid artery bifurcation. The silk suture around the internal carotid artery was tightened and the microvascular clip was removed. The nylon suture was then gently advanced from the carotid artery bifurcation, by 1.7–2.1 cm, to the internal carotid artery lumen. Here resistance was felt, indicating the suture had passed the middle cerebral artery origin and reached the proximal segment of the anterior cerebral artery.
Middle cerebral artery occlusion was produced in all rats. All groups, except the model group, received electroacupuncture at the specified frequency.
Successful model establishment was determined using any of the following three symptoms: (1) limb contraction at the affected side or disappearance of pain retraction; (2) the upper limb at the affected side failed to protract when tail was lifted; (3) body deflected to the affected side when crawling.
Electroacupuncture treatment
Electroacupuncture was given at the bilateral Quchi (LI11) and Zusanli (ST36) acupoints according to the commonly used acupoints in rats[59]. Stainless acupuncture needles (0.3 mm × 25 mm; Medical Supplies Factory of Suzhou, Jiangsu Province, China) were vertically inserted (about 0.5–0.8 inches) at the bilateral acupoints. The acupoints were connected to an electric acupuncture apparatus (G-6805; Shanghai Huayi Instrument Factory, Shanghai, China) stimulating at 60–80 μA current.
Electroacupuncture alternated between dense-sparse waveforms at a frequency of 15, 30 and 100 Hz for the respective groups. Acupoints were stimulated for 30 minutes a day for 5 days. The stimulus intensity was selected to match rat threshold. The model group was housed under the same conditions, but did not receive any treatment.
Neurological deficit score
Neurological score was evaluated using the “Longa” method after five days of electroacupuncture treatment[57,58]. Neurological findings were scored on a five-point score: a score of 0 indicated no neurological deficit, a score of 1 (failure to extend left forepaw fully) indicated a mild focal neurological deficit, a score of 2 (circling to the left) indicated a moderate focal neurological deficit, and a score of 3 (falling to the left) indicated a severe focal deficit. Rats with a score of 4 did not walk spontaneously and had a depressed level of consciousness.
Sample collection and processing
Immunohistochemical staining: Rats were fixed using cardiac perfusion under intraperitoneal anesthesia 5 days after middle cerebral artery occlusion and treatment (six rats were randomly selected in each group). The skull was opened immediately to remove the brain, and the brain was divided into five equal parts starting from the frontal pole to the occipital lobe. These parts were then sectioned at 3–4 μm thickness and tissue slices were fixed in 4% paraformaldehyde.
Electron microscopy: The rats were fixed by cardiac perfusion under intraperitoneal anesthesia 5 days after middle cerebral artery occlusion (three rats were randomly selected in each group). After fixing with 2.5% glutaraldehyde solution for 4 hours, brain tissue was selected from the infarct marginal zone, parietal lobe cortex, and the contralateral cortex, at a thickness of 1 mm.
Glial fibrillary acidic protein expression was detected immunohistochemically using a SABC kit (Wuhan Boster, Wuhan, Hubei Province, China). In brief, paraffin sections were dewaxed, antigen retrieval by heat repaire and added to normal goat serum. Tissue slices were then incubated with normal goat serum at room temperature for 10–15 minutes and then exposed to anti-mouse glial fibrillary acidic protein monoclonal antibody (1:200) at 4°C overnight. After washing with PBS three times for 3 minutes each, cells were incubated with biotin-labeled anti-mouse secondary antibody at 37°C for 10–15 minutes. This incubation was then also followed by three rinses with PBS times 3 minutes each, and subsequently incubated with horseradish peroxidase-labeled streptomycin avidin at 37°C for 10–15 minutes. Finally slices were rinsed (as above), and developed with 3,3’-diaminobenzidine and counterstained with hematoxylin. After rinses with dimethylbenzene, slices were mounted and observed under a light microscope. Glial fibrillary acidic protein expression was seen as brown-yellow or yellow staining.
Electron microscopy sectioning: After fixing in 2.5% glutaraldehyde for 4 hours, 1 mm3 of brain tissue was selected from different areas and rinsed 2–3 times with 0.1 M PBS, and saturated for 4 hours based on pharmaceutical infiltration speed and the thickness of the sample. The medium was then changed. After fixation, dehydration, penetration, embedding, polymerization, and dyeing, the ultrathin sections were observed under transmission electron microscopy (Hitachi High-technologies Corporation, Tokyo, Japan).
Image analysis
The anatomical structure of the brain was observed with guidance from a rat stereotaxic map[60]. As a means of quantification, each section was investigated under a 10 × 40 magnification, for positive cell numbers and positive cytoplasm absorbance using a DMR and Q550 pathological image analyzer (Hitachi High-technologies Corporation).
Statistical analysis
Data were analyzed using SPSS 13.0 software (SPSS, Chicago, IL, USA) and were expressed as mean ± SD. Comparisons among the groups were tested by one-way analysis of variance. Intergroup comparison was made using a Least Significant Difference test. A P value of less than 0.05 was considered statistically significant.
Acknowledgments
We would like to thank Guangxi Autonomous Region Large-Scale Instrument Cooperation and the Sharing Network Program for subsidizing the electron microscopy.
Footnotes
Conflicts of interest: None declared.
Funding: This study was supported by a grant from the Guangxi Administration of Traditional Chinese Medicine, No. GZZC0962.
Ethical approval: This study was approved by the Animal Ethics Committee, the First Affiliated Hospital of Guangxi Medical University, China.
(Reviewed by Apricò K, Frenchman B, Duan DM, Wang YL)
(Edited by Wang J, Yang Y, Li CH, Song LP)
REFERENCES
- [1].Takuma K, Baba A, Matsuda T. Astrocyte apoptosis: implications for neuroprotection. Prog Neurobiol. 2004;72(2):111–127. doi: 10.1016/j.pneurobio.2004.02.001. [DOI] [PubMed] [Google Scholar]
- [2].Zhang JJ. Advances in astrocytes. Zhongguo Yaolixue Tongbao. 2006;22(7):788–790. [Google Scholar]
- [3].Benarroch EE. Astrocyte-neuron interactions: Implications for epilepsy. Neurology. 2009;73(16):1323–1325. doi: 10.1212/WNL.0b013e3181bd432d. [DOI] [PubMed] [Google Scholar]
- [4].Seifert G, Schilling K, Steinhauser C. Astrocyte dysfunction in neurological disorders: a molecular perspective. Nat Rev Neurosci. 2006;7(3):194–206. doi: 10.1038/nrn1870. [DOI] [PubMed] [Google Scholar]
- [5].Panickar KS, Norenberg MD. Astrocytes in cerebral ischemic injury: morphological and general considerafions. Glia. 2005;50(4):287–298. doi: 10.1002/glia.20181. [DOI] [PubMed] [Google Scholar]
- [6].Gomes FC, Paulin D, Moura NV, et al. Glial fibrillary acidic Protein (GFAP): modulation by growth factors and its implication in estrocyte differentiation. Braz J Med Biol Res. 1999;32(5):619–631. doi: 10.1590/s0100-879x1999000500016. [DOI] [PubMed] [Google Scholar]
- [7].Oki K, Kaneko N, Kanki H, et al. Musashil as a marker of reactive astrocytes after transient focal brain ischemia. Neurosci Res. 2010;66(4):390–395. doi: 10.1016/j.neures.2009.12.013. [DOI] [PubMed] [Google Scholar]
- [8].Han X, Huang X, Wang Y, et al. A study of astrocyte activation in the periinfarct region after cerebral ischemia with electroacupuncture. Brain Inj. 2010;24(5):773–779. doi: 10.3109/02699051003610482. [DOI] [PubMed] [Google Scholar]
- [9].Timmer M, Cesnulevicius K, Winkler C, et al. Fibroblast growth FGF -2 and FGF receptor 3 are required for the development of the substantia nigra and FGF-2 plays a crucial role for the rescue of dopaminergic neurons after 6-hydroxy-dopamine lesion. J Neurosci. 2007;27(3):459–471. doi: 10.1523/JNEUROSCI.4493-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Li ZR, Cui L, Gou ZL, et al. Electroacupuncture on the brain injury in rats with cerebral ischemia-reperfusion. Zhen Ci Yan Jiu. 2005;30(2):67–71. [Google Scholar]
- [11].Shen MH, Li ZR, Xiang XR, et al. Effect of electroacupuncture on cerebral cortex ultrastructure in rats with cerebral ischemia-reperfusion injury. Zhen Ci Yan Jiu. 2009;34(3):167–170. [PubMed] [Google Scholar]
- [12].Huang XQ, Chen L. Literature analyzing on the status of electric stimulation in acupuncture experiment research. Zhongguo Zhongyi Jichu Yixue Zazhi. 2001;7:73. [Google Scholar]
- [13].Jun LU, Shi YJ, Jin ZX, et al. Comparison of the effects producing by different frequency of electroacupuncture in the antidepressant model rats. Beijing Zhongyiyao Daxue Xuebao. 2003;26(6):8. [Google Scholar]
- [14].Wang XY, Shang HY, He W, et al. Effects of transcutaneous electrostimulation of auricular concha at different stimulating frequencies and duration on acute seizures in epilepsy rats. Zhen Ci Yan Jiu. 2012;37(6):447–457. [PubMed] [Google Scholar]
- [15].Wang Ke, Zhang R, Zhao GP, et al. Transcriptomics study of the transcriptional response of the spinal dorsal horn to electroacupuncture stimulation with different frequencies. Zhongguo Zhongxiyi Jiehe Zazhi. 2012;32(11):1508–1555. [PubMed] [Google Scholar]
- [16].Takano T, Oberheim N, Cotrina ML, et al. Astrocytes and ischemic injury. Stroke. 2009;40(3 Suppl):S8–12. doi: 10.1161/STROKEAHA.108.533166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Park JA, Lee HS, Ko KJ, et al. Meteorin regulates angiogenesis at the gliovaseular interface. Glia. 2008;56(3):247–258. doi: 10.1002/glia.20600. [DOI] [PubMed] [Google Scholar]
- [18].Diennl GA, Hertz L. Astrocylic contributions to bioenergetics of cerebral ischemia. Glia. 2005;50(4):362–388. doi: 10.1002/glia.20157. [DOI] [PubMed] [Google Scholar]
- [19].Takano T, Oberheim N, Cotria ML, et al. Astrocytes and ischemic injury. Stroke. 2009;40(3 Supp1):S8–12. doi: 10.1161/STROKEAHA.108.533166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Panickar KS, Norenberg MD. Astrocytes in cerebral ischemic injury: morphological and general considerafions. Glia. 2005;50(4):287–298. doi: 10.1002/glia.20181. [DOI] [PubMed] [Google Scholar]
- [21].Abbot NJ, Ronnback L, Hansson E. Astrocyte-endothelial the interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7(1):41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- [22].Haeselof RF, Blasig IE, Bauer HC, et al. In search of the astrocytic factor modulating blood-brain barrier functions in brain capilary endothelial cels in vitro. Cel Mol Neurobiol. 2005;25(1):25–39. doi: 10.1007/s10571-004-1375-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Nedergaard M, Dimagl U. Role of glial cells in cerebal ischemia. Glia. 2005;50(4):281–286. doi: 10.1002/glia.20205. [DOI] [PubMed] [Google Scholar]
- [24].Gonzalez A, Pariente JA, Salido GM. Ethanol stimulates Ros generation by mitochondria through Ca2+ mobilization and increases GFAP content in rat hippocampal astrocytes. Brain Res. 2007;1178:28–37. doi: 10.1016/j.brainres.2007.08.040. [DOI] [PubMed] [Google Scholar]
- [25].Koehler RC, Gebremedhin D, Harder DR. Role of astrocytes in cerebrovascular regulation. J Appl Physiol. 2006;100(1):307–317. doi: 10.1152/japplphysiol.00938.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Haydon PG, Carmignoto G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiol Rev. 2006;86(3):1009–1031. doi: 10.1152/physrev.00049.2005. [DOI] [PubMed] [Google Scholar]
- [27].Hertz L, Zielke HR. Astrocytic control of glutamatergic activity:astrocytes as star of the show. Trends Neurosci. 2004;27(12):735–743. doi: 10.1016/j.tins.2004.10.008. [DOI] [PubMed] [Google Scholar]
- [28].Wang YD, Qiu Y, Pan JR, et al. Relationship between long-tern presence of hypoxic tissue and astrocyte activation after cerebral infarction in rats. Zhonghua Shenjing Yixue Zazhi. 2009;8(8):781–784. [Google Scholar]
- [29].Van Beek J, Chan P, Bernaudin M, et al. Glial responses,clusterin, and complement in permanent focal cerebral ischemia in the mouse. Glia. 2000;31(1):39–50. doi: 10.1002/(sici)1098-1136(200007)31:1<39::aid-glia40>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- [30].Wang GB, Xu NG, She SF, et al. Effect of electroacupuncture on the synaptic structure in the ischemic areas of rats with focal cerebral ischemia. Zhongguo Linchuang Kangfu. 2005;9(5):115–117. [Google Scholar]
- [31].Luo Y, Xu NG, Yi W, et al. Study on the correlation between synaptic reconstruction and astrocyte after ischemia and the influence of electroacupuncture on rats. Chin J Integr Med. 2011;17(10):750–757. doi: 10.1007/s11655-011-0754-7. [DOI] [PubMed] [Google Scholar]
- [32].Lu ZH, Rui M, Yi H, et al. Electroacupuncture precondtioning induces neuroprotection against forebrain ischemia/reperfusion injury in C57BL6mice. Zhonghua Shenjing Jibing Waike Yanjiu Zazhi. 2009;8(5):434. [Google Scholar]
- [33].Yu HB, Yang ZX, Wang L, et al. Effect of electroacupuncture at Ren channel and Shu acupoint on astrocytes in hippocampus of rats with cerebral ischemia. Zhongguo Linchuang Kangfu. 2006;10(31):93–95. [Google Scholar]
- [34].Luo Y, Xu NG, Yi W, et al. Effect of eletroacupuncture on EAAT1 and EAAT2 of astrocyte in the marginal zone of cerebral ischemia in rats. Anhui Zhongyi Xueyua Xuebao. 2009;28(1):30–33. [Google Scholar]
- [35].Pandapani KM, Brann DW. Estrogen astrocyte interactions: implications for neuroprotection. BMC Neurosci. 2002;3(1):6. doi: 10.1186/1471-2202-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Wang CC, Chio CC, Chang CH, et al. Beneficial effect of agmatine on brain apoptosis, astrogliosis, and edema after rat transient cerebral ischemia. BMC Pharmacol. 2010;10:11. doi: 10.1186/1471-2210-10-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Chen J, Yan SJ, Chen Y. Focal cerebral ischemic preconditioning in rat astrocytes GFAP expression. Zhongguo Laonianxue Zazhi. 2012;32(6):1189–1190. [Google Scholar]
- [38].Gibson CI, Coughlan TC, Murphy SP. Glial nitric oxide an isehemia. Glia. 2005;50(4):417–426. doi: 10.1002/glia.20143. [DOI] [PubMed] [Google Scholar]
- [39].Koizumi S, Fujishita K, Tsuda M, et al. Dynamic inhibition of excitatory synaptic transmission by astrocyte-derived ATP in hippocampal cultures. Proc Natl Acad Sci U S A. 2003;100(19):11023–11028. doi: 10.1073/pnas.1834448100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Pascual O, Casper KB, Kubera C, et al. Astrocytic purinergic signaling coordinates synaptic networks. Science. 2005;310(5745):113–116. doi: 10.1126/science.1116916. [DOI] [PubMed] [Google Scholar]
- [41].Zhang JM, Wang HK, Ye CQ, et al. ATP released by astrocytes mediates glutamatergic activity-dependent heterosynaptic suppression. Neuron. 2003;40(5):971–982. doi: 10.1016/s0896-6273(03)00717-7. [DOI] [PubMed] [Google Scholar]
- [42].Kang J, Jiang L, Goldman SA, et al. Astrocyte-rneAiated potentiation of inhibitory synaptic transmission. Nat Neurosci. 1998;1(8):683–692. doi: 10.1038/3684. [DOI] [PubMed] [Google Scholar]
- [43].Nedergaard M, Takano L, Hansen AJ. Beyond the role of glutamute as a neurotransmitter. Nat Rev Neurosci. 2002;3(9):748–755. doi: 10.1038/nrn916. [DOI] [PubMed] [Google Scholar]
- [44].Pascual O, Casp Mac Vicar BA. Calcium transients in astrocyte endfeet cause cerebrovascular constrictions. Nature. 2004;431(7005):195–199. doi: 10.1038/nature02827. [DOI] [PubMed] [Google Scholar]
- [45].Takano T, Tian GF, Peng W, et al. Astrocyte-mediared control of cerebral blood flow. Nat Neurosci. 2006;9(2):260–267. doi: 10.1038/nn1623. [DOI] [PubMed] [Google Scholar]
- [46].Zonta M, Angulo MC, Gobbo S, et al. Neuron-to-astrocyte signaling is central to the dynalmic control ofbrain microcirculation. Nat Neurosci. 2003;6(1):43–50. doi: 10.1038/nn980. [DOI] [PubMed] [Google Scholar]
- [47].Karwacki Z, Kowianski P, Dziewiatkowski J, et al. The influence of sevoflurane on the reactivity of astrocytes in the course of the experimental intracerebral haemorrhage in rat. J Physiol Pharmacol. 2005;56(3):455–469. [PubMed] [Google Scholar]
- [48].Wan SY, Tan F, Wu HK, et al. Effects of electric acupuncture on glial fibrillary acidic protein and vascular endothelial growth factor expressions and ultrastructure of gliavascular net of cerebral ischemic tissue in hypertensive rats. Zhongguo Zhongxiyi Jiehe Jijiu Zazhi. 2001;7(4):226–229. [Google Scholar]
- [49].Koehler RC, Gebremedhin D, Harder DR. Role of astrocytes in cerebrovascular regulation. J Appl Physiol. 2006;100(1):307–317. doi: 10.1152/japplphysiol.00938.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Kim JH, Park JA, Lee SW, et al. Blood-neural barrier: Intercel-lular communication at glio-vascular interface. J Biochem Mol Biol. 2006;39(4):339–345. doi: 10.5483/bmbrep.2006.39.4.339. [DOI] [PubMed] [Google Scholar]
- [51].Fellin T, Sul JY, D’Ascenzo M, et al. Bidirectional astrocyte-neuron communication: the many roles of glutamate and ATP. Novartis Found Symp. 2006;276:208–217. doi: 10.1002/9780470032244.ch16. [DOI] [PubMed] [Google Scholar]
- [52].Slezak M, Pfrieger FW, Soltys A. Synaptic plasticity, astrocytes and morphological homeostasis. J Physiol (Paris) 2006;99(2-3):84–91. doi: 10.1016/j.jphysparis.2005.12.082. [DOI] [PubMed] [Google Scholar]
- [53].Liu QS, Xu Q, Arcuino G, et al. Astrocyte-mediated activation of neuronal kainite receptors. Proc Natl Acad Sci U S A. 2004;101(9):3172–3177. doi: 10.1073/pnas.0306731101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Song H, Stevens CF, Gage FH. Astroglia induce neurogenesis from adult neural stem cells. Nature. 2002;417(6884):39–44. doi: 10.1038/417039a. [DOI] [PubMed] [Google Scholar]
- [55].Han JS, Chen XH, Sun SL, et al. Effect of low and high frequency TENS on Met-enkephalin-arg-phe and dynorph in A immunoreactivity in human lumbar CSF. Pain. 1991;47(3):295–298. doi: 10.1016/0304-3959(91)90218-M. [DOI] [PubMed] [Google Scholar]
- [56].Han JS. New evidence to substantiate the frequency specificity of acupuncture-induced analgesia. Zhen Ci Yan Jiu. 2001;26(3):224–227. [Google Scholar]
- [57].Bederson JB, Pitts LH, Tsuji M, et al. Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke. 1986;17(3):472–476. doi: 10.1161/01.str.17.3.472. [DOI] [PubMed] [Google Scholar]
- [58].Long EA, Weinstein PR, Carlson S, et al. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20(1):84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- [59].Li ZR. 7th ed. Beijing: China Press of Traditional Chinese Medicine; 2003. Experimental Acupuncture. [Google Scholar]
- [60].Zhuge QC. Beijing: People's Medical Publishing House, China; 2005. Rats Stereotaxic Map. [Google Scholar]


