Abstract
The traditional Chinese medicine Jiaweisinisan has antidepressant effects, and can inhibit hypothalamus-pituitary-adrenal gland axis hyperactivity in stress-induced depression. In this study, rat hippocampal neural precursor cells were cultured in serum-free medium in vitro and a stress damage model was established with 120 μM corticosterone. Cells were treated with 10% (v/v) Jiaweisinisan drug-containing serum and the corticosterone antagonist RU38486. Results of the 3-(4,5-dimethylthiazol-2-yl)-3,5-di-phenytetrazoliumromide assay showed that both Jiaweisinisan drug-containing serum and RU38486 promoted the proliferation of neural precursor cells after corticosterone exposure. Immunofluorescence detection showed that after Jiaweisinisan drug-containing serum and RU38486 treatment, the 5-bromo-2-deoxyuridine/terminal deoxynucleotidyl transferase dUTP nick end labeling ratio in hippocampal neural precursor cells significantly increased, and the apoptotic rates of glial cells reduced, and neuron-like cell differentiation from neural precursor cells significantly increased. Our experimental findings indicate that Jiaweisinisan promotes hippocampal neurogenesis after stress damage.
Keywords: neural regeneration, traditional Chinese medicine, Jiaweisinisan; corticosterone, hippocampal neural precursor cells, proliferation, differentiation, apoptosis, grants-supported paper, neuroregeneration
Research Highlights
(1) Jiaweisinisan, a traditional Chinese medicine, has been suggested to prevent stress injury.
(2) This study aimed to observe the influence of Jiaweisinisan drug-containing serum on the proliferation and differentiation of hippocampal neural precursor cells following corticosterone-induced damage.
(3) Jiaweisinisan can promote the proliferation of hippocampal neural precursor cells, and inhibit the apoptosis of glial cells and neurons in the presence of high concentrations of corticosterone.
INTRODUCTION
Neural stem cells show characteristic properties such as self-renewal, multi-directional differentiation, proliferation, division, and the ability to respond to injury and disease. Neural precursor cells, a kind of neural stem cell that exists in the nervous system, can proliferate and differentiate into neurons and glial cells. The proliferation, migration and differentiation of neural precursor cells are key stages in neurogenesis. Neural precursor cells are mostly in a resting state in the brain, exhibiting only a small amount of neurogenesis. Under the stimulation of ischemia and hypoxia, neural precursor cells can proliferate and differentiate again, resulting in the generation of new neurons and glial cells[1].
The pathogenesis of depression is poorly understood. The classical “monoamine theory” and “receptor theory” suggest that depression is mainly attributed to low expression levels of monoamine neurotransmitters such as norepinephrine and 5-hydroxytryptamine, and the hypersensitivity of relative receptors[2]. Conventional clinical treatment focuses on the regulation of monoamine neurotransmitter levels in the central nervous system through the application of antidepressants. These antidepressants include tricyclic antidepressants and selective norepinephrine reuptake inhibitors, which directly or indirectly increase the monoamine neurotransmitters in the synaptic cleft[3]. In fact, the traditional monoamine neurotransmitter and receptor theory cannot explain the clinical lag effect of antidepressants adequately, suggesting that the change of monoamine neurotransmitters may be only a signal link or cause in depression pathogenesis. Duman et al[4] first proposed the hypothesis of an obstacle in hippocampal neurogenesis in depression, suggesting that dysregulation of neurogenesis may lead to loss of nerve cells and result in depression. Evidence supporting this hypothesis comes from a lack of neurogenesis observed in hippocampal tissue biopsies from depressive patients[5]. Subsequently, numerous studies confirmed that hippocampal neurogenesis was involved in the pathogenesis of depression[6]. The hippocampus is the regulatory center of the stress response, and plays an important role in regulating emotion, learning, and memory. The hippocampal gyrus, including the CA1, CA2, CA3, and CA4 regions, mainly consist of pyramidal neurons, while the dental gyrus mainly comprises granule neurons. Because Eriksson et al[7] found that the human hippocampus has the ability to generate neurons throughout life, neurogenesis has become a research hotspot for central nervous system diseases, including depression.
Stress-induced hypercortisolism may be the most direct biochemical evidence that induces depression[8]. This biochemical change can lead to morphological changes in the hippocampus and other brain regions, ultimately affecting its function[9]. Stress-induced hippocampal neuron damage may be the key point of many central stress responses. Thus, protecting central neurons from injury, especially hippocampal neurons, is one of the most important measures in the prevention of stress-related diseases.
The traditional Chinese medicine Jiaweisinisan exerts a significant antidepressant effect by inhibiting the hyperactivity of the hypothalamus-pituitary-adrenal gland axis in stress-induced depression and regulating functional disorders of numerous neurotransmitters and receptors in the central nervous system[10,11,12,13]. This study, from the perspective of regulating hippocampal neurogenesis, aimed to observe the influence of Jiaweisinisan drug-containing serum on the proliferation and differentiation of hippocampal nerve precursor cells at a high corticosterone concentration. We further explored the protective effect of Jiaweisinisan on stress-induced hippocampal damage and its antidepressant mechanism.
RESULTS
Cultivation, passage and identification of hippocampal neural precursor cells
Hippocampal neural precursor cells became larger, began to split on day 2, and gradually formed a cell mass. In addition, neurospheres with clear boundaries were suspended in the culture medium on day 7 (Figure 1).
Figure 1.
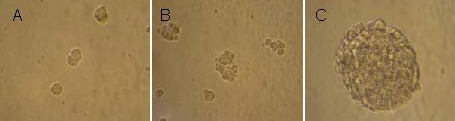
Primary cultures of rat embryonic hippocampal neural precursor cells (inverted microscope, × 250).
(A) Cells became enlarged and began to split on day 2.
(B) Cell colonies formed on day 3.
(C) Formation of neurospheres was visible on day 6.
The neurospheres exhibited suspended growth, with no obvious protrusions. After trypsin digestion and gentle mechanical disruption, cells were subcultured, each generation of 6–8 days. Suspended neurospheres seeded in various differentiation media began to adhere, and gradually formed differentiated cells (Figure 2). After differentiation for 7 days, protrusions between cells began to connect and form networks.
Figure 2.
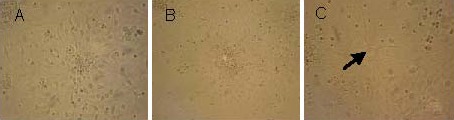
Induced differentiation of rat embryonic hippocampal neural precursor cell-derived neurospheres in differential medium (inverted microscope, × 250).
(A) Suspended neurospheres cultured in differential medium began to adhere and migrate peripherally on day 2.
(B) There were obvious processes in some differential cells, including included nerve cells and glial cells, on day 4.
(C) Processes among cells interconnected and formed a network on day 7. Arrow indicates differentiated nerve cells.
On day 7 after induced differentiation, the differentiated cells became more mature. Neurospheres were observed to be nestin positive by immunofluorescence staining (Figure 3). Immunofluorescence staining for 5-bromo-2-deoxyuridine (BrdU) showed that proliferating cells were visible within neurospheres, suggesting that the cultured cells had a proliferative capacity (Figure 4). Beta-tubulin-III and glial fibrillary acidic protein double immunofluorescence revealed that neurospheres formed after cells were cultured for 8 days in differentiation medium, including β-tubulin-III-positive cells (neuron-like cells) and glial fibrillary acidic protein-positive cells (astrocytes). These results indicated that the cultured cells had multiple differentiation potentials (Figure 5).
Figure 3.
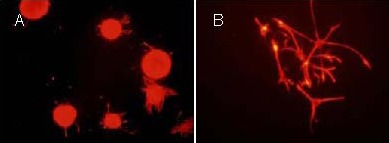
Rat embryonic hippocampal neural precursor cell-derivd neurospheres expressed nestin (immunofluorescence staining, inverted fluorescence microscope).
The fluorochrome is Cy3 and positive expression is seen as red.
(A) Expression of neural nidogen of the neurosphere (× 200).
(B) Expression of neural nidogen of the adherent single cell (× 400).
Figure 4.
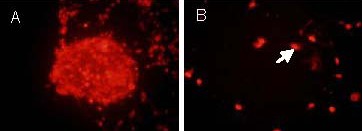
5-Bromo-2-deoxyuridine immunofluorescence staining of hippocampal neural precursor cells on day 7of induced differentiation (inverted fluorescence microscope, × 400).
The fluorochrome is Cy3 and positive expression is seen as red.
(A) The neurosphere occupying vegetative state.
(B) The single cell occupying vegetative state. Arrow indicates the Cy3 positive expression.
Figure 5.

Immunofluorescence staining of β-tubulin-III and glial fibrillary acidic protein (GFAP) of hippocampal neural precursor cells cultured in differential medium (inverted fluorescence microscope, × 400).
(A–C) Hippocampal neural precursor cells were cultured in differential medium for 8 days.
(A) Immunofluorescence staining of β-tubulin-III (red fluorescence) indicated β-tubulin-III-positive cells (arrow), and blue fluorescence represented Hoechst 33258 staining.
(B) Immunofluorescence staining of GFAP (green fluorescence) indicated GFAP-positive cells (arrow), and blue fluorescence represented Hoechst 33258 staining.
(C) Immunofluorescence double staining for β-tubulin-III and GFAP.
Jiaweisinisan drug-containing serum improved the proliferative ability of hippocampal neural precursor cells in the presence of high corticosterone concentrations
Results of MTT assay revealed that 120 μM corticosterone could significantly reduce the proliferation of neural precursor cells (P < 0.01), while the corticosterone antagonist RU38486 or 10% (v/v) Jiaweisinisan drug-containing serum could significantly improve the proliferative ability of neural precursor cells (P < 0.05 or P < 0.01; Figure 6).
Figure 6.
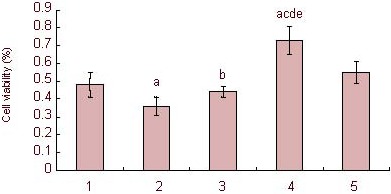
Effect of 10% (v/v) Jiaweisinisan drug-containing serum on the proliferation rate of hippocampal neural precursor cells under high corticosterone (CORT) concentrations.
Percentage of viable cells (%) = (Atreatment group – Ablank group)/(Acontrol group – Ablank group) × 100%. Data are expressed as mean ± SD and comparison between groups was performed by one-way analysis of variance. Five wells were examined in each group. aP < 0.01, vs. control group; bP < 0.05, cP < 0.01, vs. 120 μM CORT group; dP < 0.01, vs. 120 μM CORT + 2 μM RU38486 group; eP < 0.01, vs. 120 μM CORT + 10% blank serum group.
CORT-cultured cells were the stress model. RU38486 was the corticosterone antagonist. 1: Control group; 2: 120 μM CORT group; 3: 120 μM CORT + 2 μM RU38486 group; 4: 120 μM CORT + 10% Jiaweisinisan group; 5: 120 μM CORT + 10% blank serum group.
After exposure to high concentrations of corticosterone, the mean BrdU fluorescence intensity of hippocampal neural precursor cells significantly decreased, and the mean terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) fluorescence intensity significantly increased compared with the control group (P < 0.01). After RU38486 and 10% (v/v) Jiaweisinisan drug-containing serum intervention, the mean BrdU fluorescence intensity increased significantly, while mean TUNEL fluorescence intensity decreased significantly (P < 0.01). Compared with the 10% (v/v) normal serum group, the 10% (v/v) Jiaweisinisan drug-containing serum group enhanced the average BrdU fluorescence intensity (P < 0.01), while the mean TUNEL fluorescence intensity decreased (P < 0.01; Figure 7).
Figure 7.
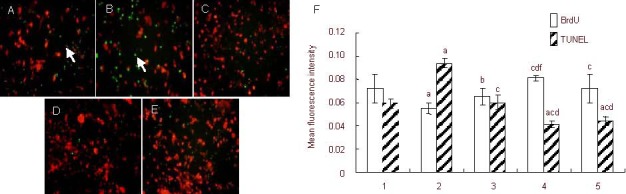
Effect of Jiaweisinisan drug-containing serum on the proliferation and apoptosis of hippocampal neural precursor cells under high corticosterone (CORT) concentrations.
(A–E) Hippocampal neural precursor cells under high CORT concentrations (inverted fluorescence microscope, × 400). Red fluorescence: Cy3-labeled BrdU; green fluorescence: TUNEL labeled (arrows). Compared with the control group (A), TUNEL-positive hippocampal neural precursor cells (green fluorescence) in the CORT group (B) increased significantly. Compared with the CORT group, TUNEL-positive cells in the 120 μM CORT + 2 μM RU38486, 120 μM CORT + 10% (v/v) Jiaweisinisan, 120 μM CORT + 10% (v/v) blank serum groups (C–E) reduced significantly, whereas the number of proliferative cells (red fluorescence) increased significantly. Corticosterone-cultured cells were used as the stress model. RU38486 was the corticosterone antagonist.
(F) BrdU- and TUNEL-positive cells represent newborn cells and apoptotic cells, respectively. 1: Control group; 2: 120 μM CORT group; 3: 120 μM CORT + 2 μM RU38486 group; 4: 120 μM CORT + 10% (v/v) Jiaweisinisan group; 5: 120 μM CORT + 10% (v/v) blank serum group. Data are expressed as mean ± SD and comparison between groups was performed by one-way analysis of variance. Experiments were performed in triplicate. aP < 0.01, vs. control group; bP < 0.05, cP < 0.01, vs. 120 μM CORT group; dP < 0.01, vs. 120 μM CORT + 2 μM RU38486 group; fP < 0.01, vs. 120 μM CORT + 10% (v/v) blank serum group.
BrdU: 5-Bromo-2-deoxyuridine; TUNEL: terminal deoxynucleotidyl transferase dUTP nick end labeling.
After high concentrations of corticosterone treatment, the ratio of BrdU/TUNEL-positive cells significantly decreased in hippocampal neural precursor cells compared with the control group (P < 0.05). After intervention with RU38486 and 10% (v/v) Jiaweisinisan drug-containing serum, the ratio of BrdU/TUNEL-positive cells in hippocampal neural precursor cells increased significantly (P < 0.05 or 0.01; Figure 8).
Figure 8.
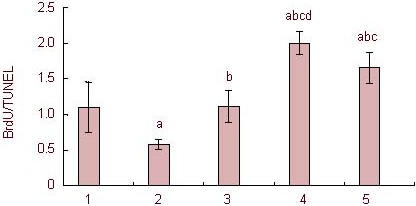
Effect of 10% (v/v) Jiaweisinisan drug-containing serum on the BrdU/TUNEL ratio of hippocampal neural precursor cells under high concentrations of corticosterone (CORT).
Data are expressed as mean ± SD and comparison between groups was performed by one-way analysis of variance. Experiments were performed in triplicate. aP < 0.01, vs. control group; bP < 0.01, vs. 120 μM CORT group; cP < 0.01, vs. 120 μM CORT + 2 μM RU38486 group; dP < 0.05, vs. 120 μM CORT + 10% (v/v) blank serum group.
1: Control group; 2: 120 μM CORT group; 3: 120 μM CORT + 2 μM RU38486 group; 4: 120 μM CORT + 10% (v/v) Jiaweisinisan group; 5: 120 μM CORT + 10% (v/v) blank serum group.
BrdU: 5-Bromo-2-deoxyuridine; TUNEL: terminal deoxynucleotidyl transferase dUTP nick end labeling.
Jiaweisinisan drug-containing serum inhibited apoptosis of glial cells and neuron-like cell differentiation from hippocampal neural precursor cells in the presence of high-concentration corticosterone
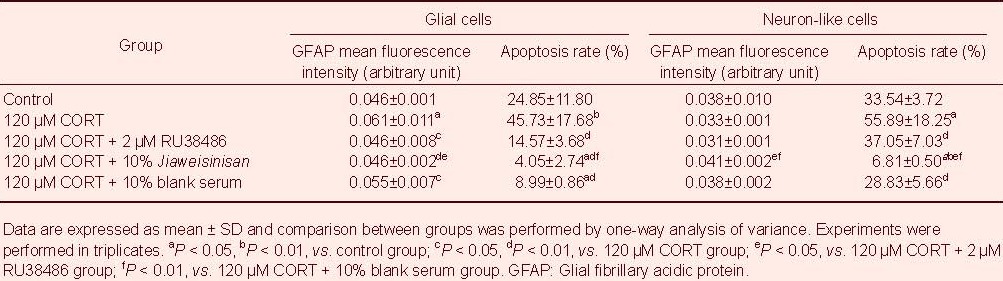
In the process of hippocampal neural precursor cell differentiation, the average glial fibrillary acidic protein fluorescence intensity of glial cells differentiating from neural precursor cells in the presence of high concentrations of corticosterone was significantly enhanced compared with the control group (P < 0.05).
The average fluorescence intensity of differentiated neuron-like cells decreased, but the difference was not significant. After intervention with RU38486 and 10% (v/v) Jiaweisinisan drug-containing serum, mean fluorescence intensity of glial cells significantly decreased (P < 0.05 or 0.01). Average fluorescence intensity of differentiated neuron-like cells from hippocampal neural precursor cells after RU38486 intervention was unaltered compared with the corticosterone group.
Under high concentrations of corticosterone, the rate of apoptosis in differentiated glial cells was significantly increased (P < 0.01). RU38486 and 10% (v/v) Jiaweisinisan drug-containing serum inhibited the apoptosis of differentiated glial cells (P < 0.01). In the presence of high concentrations of corticosterone, the rate of apoptosis in differentiated neuron-like cells was significantly increased (P < 0.01). RU38486 and 10% (v/v) Jiaweisinisan drug-containing serum inhibited apoptosis of differentiated neuron-like cells (P < 0.05 or 0.01; Figure 9, Table 1).
Figure 9.
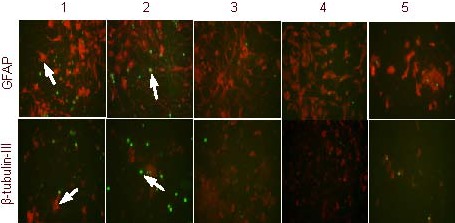
Effects of Jiaweisinisan drug-containing serum on differentiated glial cells and neuron-like cells in the presence of high concentrations of corticosterone (CORT) (× 400).
Compared with the control group, GFAP- and β-tubulin-III-positive cells (red fluorescence) in the CORT group were reduced, whereas the number of TUNEL-positive cells (green fluorescence) increased. Compared with the CORT group and the blank serum group, GFAP- and β-tubulin-III-positive cells (red fluorescence) in the two treatment groups increased, whereas the number of TUNEL-positive cells (green fluorescence) reduced.
The fluorochrome is Cy3 and positive expression is represented as red. TUNEL-positive expression is indicated in green. Arrows indicate apoptotic cells.
1: Control group; 2: 120 μM CORT group; 3: 120 μM CORT + 2 μM RU38486 group; 4: 120 μM CORT + 10% (v/v) Jiaweisinisan group; 5: 120 μM CORT + 10% (v/v) blank serum group.
BrdU: 5-Bromo-2-deoxyuridine; TUNEL: terminal deoxynucleotidyl transferase dUTP nick end labeling; GFAP: glial fibrillary acidic protein.
Table 1.
Effect of 10% (v/v) Jiaweisinisan drug-containing serum on glial cells or neuron-like cells differentiated from hippocampal neural precursor cells under high concentrations of corticosterone (CORT)
DISCUSSION
Nerve precursor cells, a type of primitive nerve cells, exist not only in the embryo but also in the adult mammalian brain. Neural precursor cells have been found in the hippocampus, cortex, cerebellum and striatum during embryonic development[14,15,16,17]. Neural precursor cells are also a kind of relatively primitive undifferentiated cells, which possesses two fundamental characteristics: a self-renewal capacity and multilineage differentiation potential. Mitogen dependence is a key feature of neural precursor cells in the in vitro culture system. In particular, neural precursor cells can maintain a proliferative and divided state only in the presence of epidermal growth factor and/or basic fibroblast growth factor. Once the mitogen is removed from the culture medium, the cells begin to differentiate[18]. During in vitro cell culture, the medium was supplemented with 20 ng/mL basic fibroblast growth factor. The cells’ soma would become larger and start to split on day 2. In theory, stem cells should maintain an undifferentiated and continuous self-renewing state indefinitely. However, it is very difficult to imitate this property under experimental conditions. To date, rodents, particularly rat embryonic neural stem cells, can survive for a few months in vitro, except for human embryonic brain neural stem cells that can be expanded in vitro for 1 year or more[19,20]. Consistent with the reports of other researchers, rat embryonic precursor cells cultured in this study could only be passaged six times. After approximately 6 weeks, cells gradually entered a subsided period, no longer proliferated, and slowly died. Nestin is an intermediate filament protein that appears in the earlier stages of nervous system development. Nestin is expressed in the majority of neuroepithelial cells in the neural tube walls. Its expression gradually reduces during the cellular differentiation process and completely disappears in mature neurons or glial cells[21]. This study reveled that most cells in the suspended neurospheres were positive for nestin. Single adherent cells would grow a long branch in the growth medium, indicating differentiation potential, whereas nestin staining was still positive. The possible reason for this result is that it takes some time for the stem cell marker nestin to disappear.
BrdU labeling has become a common method to reflect cell proliferation and to observe differentiation of new cells. This study indicated that cultured cells possessed a proliferative capacity. Multilineage differentiation potential is another fundamental feature of neural precursor cells, which means the potential to differentiate into neuron-like cells, astrocytes and oligodendrocytes. We removed single large neurospheres to conduct induction and redifferentiation. The results demonstrated that the cells in neurospheres can differentiate into two mature nerve cell types, which were further confirmed by double-labeling immunofluorescence staining. In conclusion, the nerve cells we cultured correspond with the basic characteristics of neural precursor cells. Furthermore, there was no large amount of cell death after passage 1. All indicators were detected in passages 2–3 in this experiment.
The serum pharmacology of traditional Chinese medicine was first established by Japanese scholars in the 1980s, a research method that obtained serum from mice treated orally with traditional Chinese medicine after a certain time[22]. The impact of various factors in Chinese materia medica preparation partially overcame the difficulties in applying Chinese herbal formula in in vitro experiments directly when the serum pharmacology of traditional Chinese medicine was applied to pharmacological research, especially in molecular and cellular research. The serum pharmacology of traditional Chinese medicine has become an important method in pharmacological research in vitro; however, the method still requires further optimization.
Our previous studies have shown that 10% (v/v) Jiaweisinisan drug-containing serum significantly enhanced the decrease of PC12 cell viability caused by glutamate, down-regulated intracellular Ca2+ concentrations and up-regulated the expression of cAMP-response element binding protein and its phosphorylation levels in damaged PC12 cells. This evidence suggested that 10% (v/v) Jiaweisinisan drug-containing serum had neuroprotective and antidepressant effects[12]. This study observed the effect of 10% (v/v) Jiaweisinisan drug-containing serum on the proliferation and differentiation of hippocampal nerve precursor cells under high corticosterone concentrations to further investigate the mechanism of the anti-damage effect in the hippocampus and antidepressant effect of 10% (v/v) Jiaweisinisan drug-containing serum.
The hippocampus is rich in a variety of messenger receptors. During the stress response, the hippocampus acts as a regulating center, but is also the most sensitive area[23]. In addition, the hippocampus is vulnerable to attack by excessive corticosterone because of the high concentrations of bioactive corticosterone receptors that reside in the hippocampus. Hippocampal damage due to excessive corticosterone is associated with a reduction in hippocampus size, shrinkage of CA1 and CA3 cone cells, and inhibition of dentate gyrus granulosa cell proliferation. Chronic stress has a severe and long-lasting inhibitory effect on dentate gyrus particle cell proliferation in the hippocampus, which can eventually lead to a continuous decrease in the number of proliferating dentate gyrus cells, which is accompanied by a significant reduction in the volume of granular cells. Corticosterone antagonists, which competitively bind to the glucocorticoid receptor, have an anti-damage effect in the presence of corticosterone. The MTT assay in this study proved that high concentrations of corticosterone inhibited the proliferation of hippocampal nerve precursor cells. The proliferation rate of hippocampal nerve precursor cells increased after treatment with RU38486.
In addition, the proliferation rate of cells increased after intervention with blank serum, Jiaweisinisan drug-containing serum and RU38486. The serum and RU38486 promoted the proliferation of neural precursor cells in vitro. The role of serum may be related to its complicated composition, and may be associated with the substances that promote proliferation in serum or promote proliferation by the non-corticosterone pathway. The proliferation rate of hippocampal nerve precursor cells under high corticosterone conditions increased significantly in the 10% (v/v) Jiaweisinisan drug-containing serum group compared with the 10% (v/v) blank control serum group. Further research is needed to investigate the mechanism of this effect. TUNEL staining showed that the rate of apoptosis of cultured hippocampal neural precursor cells increased significantly under high corticosterone concentrations. Meanwhile, RU38486 decreased the rate of apoptosis. The apoptotic rate of hippocampal neural precursor cells also decreased significantly after treatment with 10% (v/v) Jiaweisinisan drug-containing serum.
Under normal circumstances, the dynamic balance between cell proliferation and cell apoptosis can help to maintain the normal function of the nervous system. BrdU/TUNEL double staining revealed that the apoptosis-to-cell proliferation ratio of hippocampal neural precursor cells in the normal group was retained at around 1:1. The ratio declined sharply under high concentrations of corticosterone, while it could be reversed by RU38486. The ratio of apoptosis-to-cell proliferation considerably increased after treatment with 10% (v/v) Jiaweisinisan drug-containing serum. The above results suggested that high concentrations of corticosterone induced nerve cell injury by inhibiting the proliferation of hippocampal neural precursor cells and promoting apoptosis of regenerative cells, whereas 10% (v/v) Jiaweisinisan drug-containing serum could protect the hippocampus by promoting the proliferation of neural precursor cells and inhibiting apoptosis.
Stress-induced hippocampal damage can be displayed in two aspects: suppressing the occurrence of dentate gyrus granular cells and decreasing the survival rate of newborn neurons. Jiaweisinisan significantly decreased the apoptosis of hippocampal neurons induced by chronic stress[24]. Based on this, we removed basic fibroblast growth factor from the medium given that cultured neural precursor cells in vitro can maintain the specific properties of stem cells only under the stimulus of mitogen. In addition, nerve growth factor and basic fibroblast growth factor have apparent proliferative effects on cultured neural precursor cells, as well as promoting nerve cell survival and synapse growth[25]. Neural precursor cells turn into a differentiated state as soon as nerve growth factor is removed from the medium. In this experiment, neural precursor cells maintained a tendency towards proliferation, rather than immediately differentiating after mitogen withdrawal and serum addition. Scattered single cells proliferated into neurospheres of unequal size, demonstrating that serum has the capacity to stimulate proliferation rather than differentiation. This observation may be associated with the proliferative effects of substances contained in serum. In addition, high concentrations of corticosterone significantly increased the rate of glial cell apoptosis. Jiaweisinisan drug-containing serum can also result in an increased number of glial cells and a decrease in apoptosis of glial cells, which was significantly different from the corticosterone group.
Jiaweisinisan drug-containing serum has an apparent proliferative effect on cultured hippocampal neural precursor cells. The mean fluorescence intensity of glial cells differentiated from hippocampal neural precursor cells was higher. The newborn glial cells may have a protective effect on neuronal growth, while the specific reason needs to be further investigated. The neurons differentiated from hippocampal neural precursor cells reduced dramatically under high concentrations of corticosterone. Furthermore, the rate of apoptosis of newborn neurons was high. Jiaweisinisan drug-containing serum not only increased the number of neurons differentiating from neural precursor cells, but also decreased the rate of neuronal apoptosis. Preliminary experimental results showed that 10% Jiaweisinisan drug-containing serum promotes the proliferation of neural precursor cells, and inhibits the rate of glial cell apoptosis and neuron-like cell differentiation from neural precursor cells, which acts has an anti-damaging effect following corticosterone exposure in the hippocampus. The hippocampus and olfactory bulb of adult rodents and primates are able to generate new neurons. Neurogenesis is maintained throughout adulthood. Neural precursor cells can migrate, differentiate and ultimately integrate into existing neural networks in a variety of pathological conditions[26]. Neurogenesis plays an important role in a variety of diseases such as Parkinson's disease, stroke, depression, and schizophrenia. Furthermore, neurogenesis may be a promising new therapeutic target for these diseases[27]. Clinical and preclinical studies have shown that depressive patients suffer from hypercortisolism and hippocampal atrophy[28]. In psychopathologies such as major depression, reductions in hippocampal volume are commonly observed and have been implicated in specific symptoms of the disorders[29]. The learning and memory capacities in depressive patients are damaged. Magnetic resonance imaging indicates a reduction in hippocampal size. The extent of hippocampal atrophy is associated with the frequency of depression attacks and the duration of no treatment. The following reasons may explain the change in volume: the increasing number of apoptotic neurons within glial cells, loss of nerve fibers and neural regeneration and/or glia in the dentate gyrus[30]. The behavioral improvement in response to antidepressants is dependent on neurogenesis.
Eliminating new neurons by selective irradiation of the hippocampus results in no anxiolytic effect of antidepressants. Chronic antidepressant administration can cause neurogenesis[31]. Although the functional significance of adult neurogenesis still remains to be established, increasing evidence has implicated compromised hippocampal neurogenesis as a possible contributor in the development of major depressive disorders. Antidepressants increase hippocampal neurogenesis and there is evidence in rodent models that the therapeutic efficacy of these agents is attributable, in part, to this neurogenic effect[32]. The promotion of hippocampal neurogenesis is not only necessary for behavioral improvement after antidepressant treatment, but also acts as a new indicator to evaluate whether a compound is a potential antidepressant agent[33]. Our experimental findings have proven that under high corticosterone concentrations, 10% (v/v) Jiaweisinisan drug-containing serum promotes the proliferation of neural precursor cells, and inhibits the apoptotic rates of glial cells and neuron-like cells differentiated from neural precursor cells. We believe that Jiaweisinisan is able to promote the proliferation of hippocampal neural precursor cells, decrease apoptotic cell differentiation from neural precursor cells, stimulate hippocampal neurogenesis and protect against stress-induced hippocampal damage, all of which may be the mechanism by which Jiaweisinisan induces its antidepressant effect.
MATERIALS AND METHODS
Design
A controlled observation regarding cytology.
Time and setting
This study was performed in the Laboratory of the Department of Basic Theory of Traditional Chinese Medicine, Basic Medicine College, Guangzhou University of Chinese Medicine, China between March 2008 and May 2011.
Materials
Animals
A total of 40 healthy male Wistar rats aged 3 months, weighing 240–280 g, specific pathogen free grade, were provided by the Experimental Animal Center of Guangzhou University of Chinese Medicine in China, with certificate No. 0025178. Twenty healthy female Wistar rats at pregnant days 16–18, specific pathogen free grade, were provided by the Experimental Animal Center of Guangzhou University of Chinese Medicine, in China with certificate No. 0006962. All Wistar rats were housed in cages with water and food available ad libitum, with a 12-hour light/dark cycle at 24–26°C. Protocols were conducted in accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals, issued by the Ministry of Science and Technology of China[34].
Drugs
Jiaweisinisan consisted of bupleurum, peony root, citrus aurantium, medlar, gardenia, rehmanniae and abalone. Amounts of bupleurum, peony root, citrus aurantium, medlar, gardenia, rehmanniae and abalone were weighed according to a ratio of 1:3:1:3:1:4:6, respectively. They were purchased from Guangzhou Medicine Company and identified by the Department of Pharmacy, the First Affiliated Hospital of Guangzhou University of Chinese Medicine in China as pure herbs. All components were conventionally boiled twice, mixed, filtrated, concentrated until the crude drug concentration was 1.69 g/mL by water bath, autoclaved, and stored in a 4°C refrigerator[12,35].
Methods
Preparation of Jiaweisinisan drug-containing serum
Wistar rats aged 3 months received Jiaweisinisan intragastrically, twice per day for 3 consecutive days (8.46 g crude drug 10 mL/kg body weight). One hour after the last administration, abdominal aortic blood was taken under sterile conditions, allowed to stand for 1 hour, centrifuged at 3 000 r/min for 10 minutes, and then the separated serum was inactivated at 56°C for 30 minutes, filtrated through a 0.22 μm microporous filter and stored in a –70°C refrigerator[13].
Culture and passage of hippocampal neural precursor cells
Cells were cultured as previously reported[36]. In brief, the embryo was removed from Wistar rats at pregnant days 16–18 under sterile conditions by etherization and placed in ice-cold sterile PBS. The brain was harvested and transferred to a petri dish containing ice-cold sterile PBS, and the blood vessels and meninges were removed under a dissecting microscope (Olympus, Tokyo, Honshu, Japan). The bilateral hippocampus was separated[37,38], cut into 1 mm × 1 mm × 1 mm pieces, mixed and gently polished with a Pasteur pipette, filtered through a 200-mesh cell sieve to obtain a single cell suspension, and centrifuged at 1 000 r/min for 5 minutes. After the supernatant was discarded, cells were resuspended with stem cell Dulbecco's modified Eagle's medium/F12 media (Gibco-BRL, Gaithersburg, MD, USA) containing 2% (v/v) B27 (Gibco-BRL), 20 μg/L basic fibroblast growth factor (Peprotech, Suzhou, Jiangsu Province, China), and penicillin and streptomycin (1 × 105 U/L). Cells were counted and stained with trypan blue, then incubated at a density of 2 × 105 cells/L in T25 culture flasks at 37°C in a 5% (v/v) CO2 incubator. The culture medium was half-replenished every 2–3 days. Neurospheres were collected and digested with trypsin (Gibco-BRL) for 5–7 days and mixed gently to obtain a single cell suspension of 2 × 105 cells/L.
Induced differentiation: Neurospheres were harvested from culture flasks, centrifuged, resuspended in differentiation medium (Neurobasal medium; Gibco-BRL), and incubated on poly-L-lysine-treated glass slides in petri dishes, at 37°C in a 5% (v/v) CO2 incubator. The culture medium was half-replenished every 2–3 days.
Immunofluorescence detection for nestin expression in neurospheres
Passage 1 neurospheres were collected with a Pasteur pipette and transferred to a six-well plate with poly-L-Lysine (Sigma-Aldrich, St Louis, MO, USA) treated glass slides. Fetal bovine serum (25 (v/v); Gibco-BRL) was added to the medium to promote neurosphere adhesion. Twenty-four hours later, the slides were removed and washed with PBS three times, for 5 minutes each, fixed with 4% (w/v) poly-formaldehyde at room temperature for 30 minutes, and washed with PBS containing 0.3% (v/v) Triton X-100 three times for 5 minutes each. Slices were then blocked in 5% (v/v) goat serum (Gibco-BRL) at 37°C for 30 minutes, incubated in mouse anti-rat nestin monoclonal antibody (Chemicon, San Diego, CA, USA), and diluted in PBS containing 0.3% (v/v) Triton X-100 (1:100) at 37°C for 2 hours. After washing with PBS containing 0.3% (v/v) Triton X-100, three times for 5 minutes each, cells were incubated in TRITC-labeled goat anti-mouse IgG (Chemicon), diluted in PBS containing 0.3% (v/v) Triton X-100 (1:50) at 37°C for 1 hour; washed with PBS three times for 5 minutes each; and sealed with carbonate buffer (pH 9.0–9.5) and non-fluorescent glycerol. Slices were observed and photographed under a fluorescent microscope (Olympus). The mean fluorescence intensity was analyzed by Image-Pro Plus 6.0 (Media-Cybernetics, Maryland, MD, USA) software. Experiments were performed in triplicate.
Immunofluorescence detection for BrdU expression in neurospheres
The neurospheres were dissociated and seeded into poly-L-Lysine treated glass slides within a 24-well plate. BrdU (10 μM; Sigma) was added on day 3 and cells were incubated for 2 days. Slices were removed and rinsed with PBS three times for 5 minutes each, fixed with 4% (w/v) polyformaldehyde at room temperature for 30 minutes, denatured in 2 M HCl at 37°C for 30 minutes, and neutralized in 0.1 mmol/L PBS (pH 8.5) at room temperature for 15 minutes. After washing with PBS containing 0.3% (v/v) Triton X-100 three times for 5 minutes each, cells were blocked in 5% (v/v) goat serum at 37°C for 30 minutes, then incubated with mouse anti-BrdU antibody (Chemicon), and diluted in PBS containing 0.3% (v/v) Triton X-100 (1:200) at 37°C for 2 hours. After washing with PBS containing 0.3% (v/v) Triton X-100 three times for 5 minutes each, cells were incubated with Cy3-labeled goat anti-mouse IgG (1:50; Boster, Wuhan, Hubei Province, China) at 37°C for 1 hour, rinsed with PBS three times for 5 minutes each, and sealed with carbonate buffer (pH 9.0–9.5) and non-fluorescent glycerol. Slices were observed and photographed under the fluorescent microscope. The mean fluorescence intensity was analyzed by Image-Pro Plus 6.0 software. Experiments were performed in triplicate.
Immunocytochemistry detection for the co-expression of β-tubulin-III and glial fibrillary acidic protein in neurospheres
Single large neurospheres were removed from the flask, dissociated in 1 mL differentiation medium, gently shaken, and incubated in 24-well plates coated with poly-L-Lysine treated glass slides. After 8 days of differentiation, the slide was removed and washed with PBS three times for 5 minutes each, fixed with 4% (w/v) poly-formaldehyde at room temperature for 30 minutes; washed with PBS containing 0.3% (v/v) Triton X-100 three times for 5 minutes each; stained with Hoechst 33258 (Biotium, Hayward, CA, USA) in the dark for 15 minutes; washed with PBS three times for 5 minutes each; fixed with 4% (w/v) paraformaldehyde at room temperature for 15 minutes, and washed with PBS containing 0.3% (v/v) Triton X-100 three times for 5 minutes each. Subsequently, cells were blocked with 5% (v/v) goat serum at 37°C for 30 minutes and incubated in mouse anti-β-rat tubulin-III monoclonal antibody (Neomarkers, Fremont, CA, USA; 1:400) and rabbit anti-glial fibrillary acidic protein antibody (Chemicon; 1:500), diluted in PBS containing 0.3% (v/v) Triton X-100 at 37°C for 2 hours. After washing with PBS containing 0.3% (v/v) Triton X-100 three times for 5 minutes each, cells were incubated with Cy3-labeled goat anti-mouse of IgG (1:100) and FITC-labeled goat anti-rabbit IgG (Boster, Wuhan, China; 1:50), diluted in PBS containing 0.3% (v/v) Triton X-100. Finally, slices were sealed with carbonate buffer (pH 9.0–9.5) and non-fluorescent glycerol, and observed under the fluorescent microscope. The mean fluorescence intensity was analyzed using Image-Pro Plus 6.0 (Media-Cybernetics) software. Experiments were performed in triplicate.
Grouping and treatment of neural precursor cells
Neural precursor cells cultured in 96-well plates were randomly divided into five groups. Control group: cells were cultured with stem cell growth medium for 3 days; 120 μM corticosterone group: cells cultured with stem cell growth medium containing 120 μM corticosterone (Sigma-Aldrich) for 3 days; 120 μM corticosterone + RU38486 group: cells cultured with stem cell growth medium containing 120 μM corticosterone and 2 μM RU38486 (Sigma-Aldrich) for 3 days; 120 μM corticosterone + 10% (v/v) Jiaweisinisan drug-containing serum group: cells cultured with stem cell growth medium containing 120 μM corticosterone + 10% (v/v) Jiaweisinisan drug-containing serum for 3 days; 120 μM corticosterone + 10% (v/v) blank serum group: cells cultured with 10% (v/v) blank serum containing 120 μM corticosterone for 3 days. Each group contained 16 wells.
Cell viability measured by MTT assay
Neural precursor cells dissociated from rat embryos were subjected to a suspending culture. Passage 1 neurospheres were dispersed into single cells, resuspended with stem cell growth medium, and seeded in 96-well plates at a density of 4 × 104 cells/well for 3 days. MTT (25 μL of a 5 g/L stock; Sigma-Aldrich) was added to each well, and cells were cultured for 4 hours and centrifuged at 3 000 r/min. The supernatant (80 μL) was carefully removed, and 180 μL of dimethyl sulfoxide (Sigma-Aldrich) was added with gentle shaking for 15 minutes. Absorbance was measured at 490 nm with a microplate reader (Bio-rad, Hercules, CA, USA). The mean absorbance of five wells in the indicated groups was used to calculate the percentage of cell viability as follows: percentage of cell viability = (Atreatment group – Ablank group)/(Acontrol group – Ablank group) × 100%[38]. The experiment was performed in triplicate.
Immunohistochemical detection of cell proliferation and apoptosis
Passage 1 neurospheres were digested with trypsin, mixed gently to form a single suspension, then seeded into a 24-well plate with poly-L-Lysine treated glass slides for 2 days. BrdU (10 μM) was added in each well, and cells were incubated for 48 hours. Subsequently, slides were removed for immunocytochemical staining, washed with PBS three times for 5 minutes, fixed with 4% (w/v) paraformaldehyde at room temperature for 30 minutes, washed with PBS containing 0.3% (v/v) Triton X-100 three times for 5 minutes, incubated in 0.1% (w/v) sodium citrate solution containing 0.3% (v/v) Triton X-100 on ice for 2 minutes, and washed with PBS three times for 5 minutes each. TUNEL reaction mixture (30 μL; Roche, Basel, Switzerland; two negative controls and a positive control were included) was added, and cells were incubated at 37°C for 60 minutes. The following procedures were performed in the dark. Slides were washed with PBS three times for 5 minutes each, fixed with 4% (w/v) poly-formaldehyde at room temperature for 30 minutes, denatured at 37°C for 30 minutes, and neutralized in 0.1 M borate buffer (pH 8.5) at room temperature for 15 minutes. After washing with PBS containing 0.3% (v/v) Triton X-100 three times for 5 minutes each, cells were blocked in 5% (v/v) goat serum at 37°C for 30 minutes and incubated with mouse anti-BrdU antibody (diluted in PBS containing 0.3% (v/v) Triton X-100 in 1:100) at 37°C for 2 hours. After washing with PBS containing 0.3% (v/v) Triton X-100 three times for 5 minutes, cells were incubated with Cy3-labeled goat anti-mouse IgG (diluted in PBS containing 0.3% (v/v) Triton X-100 in 1:100) at 37°C for 1 hour. After washing with PBS three times for 5 minutes each, the slides were observed and photographed under the fluorescent microscope. The nuclei of positive Cy3-labled BrdU cells were stimulated to emit red fluorescence under green exciting light, while the nuclei of TUNEL-positive cells were stimulated to emit green fluorescence under blue exciting light. BrdU- and TUNEL-positive cells indicated newborn cells and apoptotic cells, respectively. The mean fluorescence intensity was analyzed by Image-Pro Plus 6.0 software. Experiments were performed in triplicate.
Double-labeled immunofluorescence detection for β-tubulin-III, glial fibrillary acidic-protein and TUNEL-positive cells
The detected groups were treated as previously described. In brief, slides were washed with PBS three times for 5 minutes each, fixed with 4% (w/v) polyformaldehyde at room temperature for 30 minutes, washed with PBS containing 0.3% (v/v) Triton X-100 three times for 5 minutes, and blocked in 5% (v/v) goat serum at 37°C for 30 minutes. Subsequently, cells were incubated with mouse anti-β-tubulin-III antibody (diluted in PBS containing 0.3% (v/v) Triton X-100; 1:500) or goat anti-glial fibrillary acidic protein polyclonal antibody (Chemicon; diluted in PBS containing 0.3% (v/v) Triton X-100; 1:400) at 37°C for 2 hours. After washing with PBS containing 0.3% (v/v) Triton X-100 three times for 5 minutes, cells were incubated with Cy3-labeled goat anti-mouse IgG (Chemicon; diluted in PBS containing 0.3% (v/v) Triton X-100; 1:50) at 37°C for 1 hour. Cells were again washed with PBS three times for 5 minutes, fixed with 4% (w/v) polyformaldehyde at room temperature for 30 minutes, washed with PBS containing 0.3% (v/v) Triton X-100 three times, and incubated with penetrated solution on ice for 2 minutes. After washing with PBS three times for 5 minutes each, cells were incubated with 30 μL TUNEL reaction mixture at 37°C for 60 minutes. Two negative and positive controls were also included. After washing with PBS three times for 5 minutes each, the slides were observed and photographed under a fluorescent microscope. Positive Cy3-labled β-tubulin-III and glial fibrillary acidic protein cells were stimulated to emit red fluorescence under green exciting light, while the nuclei of TUNEL-positive cells were stimulated to emit green fluorescence under blue exciting light. The mean fluorescence intensity was analyzed using Image-Pro Plus 6.0 software. Experiments were performed in triplicate.
Statistical analysis
Data were expressed as mean ± SD. Statistical analysis was performed between groups by one-way analysis of variance using SPSS 16.0 software (SPSS, Chicago, IL, USA). A probability value of P < 0.05 was considered a significant difference.
Footnotes
Conflicts of interest: None declared.
Funding: This work was supported by the National Natural Science Foundation of China, No. 30500660.
Ethical approval: This work has been approved by the Ethics Committee of Experimental Animals, Guangzhou University of Chinese Medicine in China.
(Reviewed by Diwakarla S, Stow A, Huang SJ, Yang XF)
(Edited by Yu, J, Yang Y, Li CH, Song LP)
REFERENCES
- [1].O’Connor SM, Stenger DA, Shaffer KM, et al. Primary neural precursor cell expansion, differentiation and cytosolic Ca(2+) response in three-dimensional collagen gel. J Neurosci Methods. 2000;102(2):187–195. doi: 10.1016/s0165-0270(00)00303-4. [DOI] [PubMed] [Google Scholar]
- [2].Zemlan FP, Garver DL. Depression and antidepressant therapy: receptor dynamics. Prog Neuropsychopharmacol Biol Psychiatry. 1990;14(4):503–523. doi: 10.1016/0278-5846(90)90004-z. [DOI] [PubMed] [Google Scholar]
- [3].Castrén E, Rantamäki T. Neurotrophins in depression and antidepressant effects. (87-93).Novartis Found Symp. 2008;289:43–50. doi: 10.1002/9780470751251.ch4. [DOI] [PubMed] [Google Scholar]
- [4].Duman RS, Malberg J, Nakagawa S, et al. Neuronal plasticity and survival in mood disorders. Biol Psychiatry. 2000;48(8):732–739. doi: 10.1016/s0006-3223(00)00935-5. [DOI] [PubMed] [Google Scholar]
- [5].Dwivedi Y. Evidence demonstrating role of microRNAs in the etiopathology of major depression. J Chem Neuroanat. 2011;42(2):142–156. doi: 10.1016/j.jchemneu.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Becker S, Macqueen G, Wojtowicz JM. Computational modeling and empirical studies of hippocampal neurogenesis-dependent memory: Effects of interference, stress and depression. Brain Res. 2009;1299:45–54. doi: 10.1016/j.brainres.2009.07.095. [DOI] [PubMed] [Google Scholar]
- [7].Eriksson PS, Perfilieva E, Björk-Eriksson T, et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4(11):1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- [8].Duman RS, Malberg J, Nakagawa S. Regulation of adult neurogenesis by psychotropic drugs and stress. J Pharmacol Exp Ther. 2001;299(2):401–407. [PubMed] [Google Scholar]
- [9].Barha CK, Barker JM, Brummelte S, et al. Hormone Regulation of Adult Hippocampal Neurogenesis in the Mammalian Brain. In: Arnold AP, Etgen AM, Fahrbach SE, et al., editors. Hormones, Brain and Behavior. 2nd ed. Waltham: Academic Press; 2009. [Google Scholar]
- [10].Yan C, Xu ZW, Li Y, et al. Comparative study on effects of liver-regulating, kidney-tonifying and spleen-invigorating recipes on monoamine neurotransmitter in plasma and hypothalamus of rats with chronic psychological stress. Zhongguo Zhongxiyi Jiehe Zazhi. 2002;22(12):925–928. [Google Scholar]
- [11].Yan C, Xu ZW, Li Y, et al. Regulatory effect of liver-regulating, spleen-strengthening and kidney-tonifying herbs on nerve center of rats with repeated psychological stress. Guangzhou Zhongyiyao Daxue Xuebao. 2003;20(2):143–146. [Google Scholar]
- [12].Yan C, Wu LL, Xu ZW, et al. The effects of different therapies and formulas on CRH mRNA expression in Hypothalamus of rats with chronic psychological stress. Zhongguo Yaoli Xue Tongbao. 2004;20(10):1164–1166. [Google Scholar]
- [13].Wu LL, Yan C, Ding SY, et al. The initial research on the effect of anti-stressed depression of Jiaweisinisan and it's mechanism of N-methyl-D-aspartate receptor channel in hippocampus. Zhongguo Yaoli Xue Tongbao. 2007;23(11):1425–1430. [Google Scholar]
- [14].Vicario-Abejón C, Collin C, Tsoulfas P, et al. Hippocampal stem cells differentiate into excitatory and inhibitory neurons. Eur J Neurosci. 2000;12(2):677–688. doi: 10.1046/j.1460-9568.2000.00953.x. [DOI] [PubMed] [Google Scholar]
- [15].Williams BP, Price J. Evidence for multiple precursor cell types in the embryonic rat cerebral cortex. Neuron. 1995;14(6):1181–1188. doi: 10.1016/0896-6273(95)90265-1. [DOI] [PubMed] [Google Scholar]
- [16].Kilpatrick TJ, Bartlett PF. Cloned multipotential precursors from the mouse cerebrum require FGF-2, whereas glial restricted precursors are stimulated with either FGF-2 or EGF. J Neurosci. 1995;15(5 Pt 1):3653–3661. doi: 10.1523/JNEUROSCI.15-05-03653.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Reynolds BA, Tetzlaff W, Weiss S. A multipotent EGF-responsive striatal embryonic progenitor cell produces neurons and astrocytes. J Neurosci. 1992;12(11):4565–4574. doi: 10.1523/JNEUROSCI.12-11-04565.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gage FH. Mammalian neural stem cells. Science. 2000;287(5457):1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- [19].Svendsen CN, Skepper J, Rosser AE, et al. Restricted growth potential of rat neural precursors as compared to mouse. Brain Res Dev Brain Res. 1997;99(2):253–258. doi: 10.1016/s0165-3806(97)00002-3. [DOI] [PubMed] [Google Scholar]
- [20].Ostenfeld T, Joly E, Tai YT, et al. Regional specification of rodent and human neurospheres. Brain Res Dev Brain Res. 2002;134(1-2):43–55. doi: 10.1016/s0165-3806(01)00291-7. [DOI] [PubMed] [Google Scholar]
- [21].Lendahl U, Zimmerman LB, McKay RD. CNS stem cells express a new class of intermediate filament protein. Cell. 1990;60(4):585–595. doi: 10.1016/0092-8674(90)90662-x. [DOI] [PubMed] [Google Scholar]
- [22].Iwama H, Amagaya S, Ogihara Y. Effect of shosaikoto, a Japanese and Chinese traditional herbal medicinal mixture, on the mitogenic activity of lipopolysaccharide: a new pharmacological testing method. J Ethnopharmacol. 1987;21(1):45–53. doi: 10.1016/0378-8741(87)90093-6. [DOI] [PubMed] [Google Scholar]
- [23].Hegde P, Singh K, Chaplot S, et al. Stress-induced changes in sleep and associated neuronal activity in rat hippocampus and amygdala. Neuroscience. 2008;153(1):20–30. doi: 10.1016/j.neuroscience.2008.01.042. [DOI] [PubMed] [Google Scholar]
- [24].Wu LL, Wang WZ, Yan C, et al. The protective effect of modified Sini Powder on hippocampus injury in chronic stressed depression rats. Zhongyi Zazhi. 2008;49(4):353–355. [Google Scholar]
- [25].Carpenter MK, Cui X, Hu ZY, et al. In vitro expansion of a multipotent population of human neural progenitor cells. Exp Neurol. 1999;158(2):265–278. doi: 10.1006/exnr.1999.7098. [DOI] [PubMed] [Google Scholar]
- [26].Balu DT, Lucki I. Adult hippocampal neurogenesis: regulation, functional implications, and contribution to disease pathology. Neurosci Biobehav Rev. 2009;33(3):232–252. doi: 10.1016/j.neubiorev.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Elder GA, De Gasperi R, Gama Sosa MA. Research update: neurogenesis in adult brain and neuropsychiatric disorders. Mt Sinai J Med. 2006;73(7):931–940. [PubMed] [Google Scholar]
- [28].Mao QQ, Ip SP, Ko KM, et al. Peony glycosides protect against corticosterone-induced neurotoxicity in PC12 cells. Cell Mol Neurobiol. 2009;29(5):643–647. doi: 10.1007/s10571-009-9357-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Novati A, Hulshof HJ, Koolhaas JM, et al. Chronic sleep restriction causes a decrease in hippocampal volume in adolescent rats, which is not explained by changes in glucocorticoid levels or neurogenesis. Neuroscience. 2011;190:145–155. doi: 10.1016/j.neuroscience.2011.06.027. [DOI] [PubMed] [Google Scholar]
- [30].Murray F, Smith DW, Hutson PH. Chronic low dose corticosterone exposure decreased hippocampal cell proliferation, volume and induced anxiety and depression like behaviours in mice. Eur J Pharmacol. 2008;583(1):115–127. doi: 10.1016/j.ejphar.2008.01.014. [DOI] [PubMed] [Google Scholar]
- [31].Wang JW, David DJ, Monckton JE, et al. Chronic fluoxetine stimulates maturation and synaptic plasticity of adult-born hippocampal granule cells. J Neurosci. 2008;28(6):1374–1384. doi: 10.1523/JNEUROSCI.3632-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Fournier NM, Duman RS. Role of vascular endothelial growth factor in adult hippocampal neurogenesis: implications for the pathophysiology and treatment of depression. Behav Brain Res. 2012;227(2):440–449. doi: 10.1016/j.bbr.2011.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Santarelli L, Saxe M, Gross C, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301(5634):805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- [34].The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006 Sep 30; [Google Scholar]
- [35].Xu SY. Beijing: People's Medical Publishing House; 2003. Methodology of Pharmacological Experiment. [Google Scholar]
- [36].Zheng ZX, Lin L. Beijing: Science Press; 2002. Theory and Practice of Nerve Cell. [Google Scholar]
- [37].Bao XM, Shu SY. Beijing: People's Medical Publishing House; 1991. The Stereotaxic Atlas of the Rat Brain. [Google Scholar]
- [38].Li CL, Cui ZY, Li SZ. Improvement and preliminary application of MTT colorimetric analysis. Shanghai Mianyi Xue Zazhi. 1996;16(5):306–307. [Google Scholar]


