Abstract
Scutellaria baicalensis stem-leaf total flavonoid might attenuate learning/memory impairment and neuronal loss in rats induced by amyloid beta-peptide. This study aimed to explore the effects of Scutellaria baicalensis stem-leaf total flavonoid on amyloid beta-peptide-induced neuronal apoptosis and the expression of apoptosis-related proteins in the rat hippocampus. Male Wistar rats were given intragastric administration of Scutellaria baicalensis stem-leaf total flavonoid, 50 or 100 mg/kg, once per day. On day 8 after administration, 10 μg amyloid beta-peptide (25–35) was injected into the bilateral hippocampus of rats to induce neuronal apoptosis. On day 20, hippocampal tissue was harvested and probed with the terminal deoxyribonucleotidyl transferase-mediated biotin-16-dUTP nick-end labeling assay. Scutellaria baicalensis stem-leaf total flavonoid at 50 and 100 mg/kg reduced neuronal apoptosis induced by amyloid beta-peptide (25–35) in the rat hippocampus. Immunohistochemistry and western blot assay revealed that expression of the pro-apoptotic protein Bax, cytochrome c and caspase-3 was significantly diminished by 50 and 100 mg/kg Scutellaria baicalensis stem-leaf total flavonoid, while expression of the anti-apoptotic protein Bcl-2 was increased. Moreover, 100 mg/kg Scutellaria baicalensis stem-leaf total flavonoid had a more dramatic effect than the lower dosage. These experimental findings indicate that Scutellaria baicalensis stem-leaf total flavonoid dose-dependently attenuates neuronal apoptosis induced by amyloid beta-peptide in the hippocampus, and it might mediate this by regulating the expression of Bax, cytochrome c, caspase-3 and Bcl-2.
Keywords: neural regeneration, traditional Chinese medicine, neurodegenerative disease, Scutellaria baicalensis stem-leaf total flavonoid, amyloid beta-peptide, neurons, apoptotic protein, cytochrome c, Alzheimer's disease, grants-supported paper, neuroregeneration
Research Highlights
(1) Based on the objective criteria for drug effects described in previous in vivo studies, apoptosis was examined in this study, and the expression of apoptosis-related proteins was qualitatively and semi-quantitatively assayed using immunohistochemistry. Furthermore, western blot analysis was used for quantitating protein expression. Bax, Bcl-2, cytochrome c and caspase-3 were selected for assessment.
(2) Scutellaria baicalensis stem-leaf total flavonoid can attenuate apoptosis in the hippocampus induced by amyloid beta-peptide, reduce the expression of Bax, cytochrome c and caspase-3, and increase the expression of the anti-apoptotic protein Bcl-2.
(3) Scutellaria baicalensis stem-leaf total flavonoid has inhibitory effects against neuronal apoptosis in amyloid beta-peptide (25–35)-treated rats, and the effect is dose-dependent.
INTRODUCTION
Alzheimer's disease is a degenerative disease of the central nervous system, characterized by progressive cognitive dysfunction and memory impairment. Amyloid beta-protein is widely regarded as the main culprit in the pathogenesis of Alzheimer's disease[1]. Intracellular accumulation of amyloid beta oligomer may trigger endoplasmic reticulum stress-induced apoptosis[2]. Amyloid beta-peptide (25–35) can induce PC12 cell apoptosis[3] and caspase-dependent and -independent apoptosis of human embryonic neurons[4].
Scutellaria baicalensis stem-leaf total flavonoid is extracted from the stems and leaves of Scutellaria baicalensis Georgi[5]. Scutellaria baicalensis stem-leaf total flavonoid has anti-inflammatory and immune modulatory effects, and can alleviate myocardial ischemia-reperfusion injury[6]. In addition, the antioxidative effects of Scutellaria baicalensis stem-leaf total flavonoid help to attenuate learning and memory impairment, and prevent neuronal loss and ultrastructural damage in the hippocampal CA1 region after injection of amyloid beta[7,8]. 5,7-Dihydroxy flavanone might inhibit mitochondrial-mediated apoptosis and regulate the expression of apoptosis-related proteins after intraventricular injection of amyloid beta-peptide (25–35) into mice[9]. Curcumin was shown to mediate neuroprotection against the mitochondrial apoptosis pathway[10]. However, there is little research on the effects of Scutellaria baicalensis stem-leaf total flavonoid on apoptosis.
In this study, we sought to explore the effects of Scutellaria baicalensis stem-leaf total flavonoid on amyloid beta-induced neuronal apoptosis in the hippocampus. We also examined its effects on the expression of the apoptosis-related proteins Bax, Bcl-2, caspase-3 and cytochrome c using the terminal deoxyribonucleotidyl transferase-mediated biotin-16-dUTP nick-end labeling (TUNEL) assay, immunohistochemistry, and western blot analysis.
RESULTS
Quantitative analysis of experimental animals
Forty male Wistar rats were randomly divided into a control group (intragastric administration of saline + hippocampal injection of sterile saline), model group [intragastric administration of saline + hippocampal injection of amyloid beta-peptide (25–35)], high-dose treatment group [intragastric administration of 100 mg/kg Scutellaria baicalensis stem-leaf total flavonoid + hippocampal injection of amyloid beta-peptide (25–35)], and low-dose treatment group [intragastric administration of 50 mg/kg Scutellaria baicalensis stem-leaf total flavonoid + hippocampal injection of amyloid beta-peptide (25–35)]. All 40 rats were involved in the final analysis.
Scutellaria baicalensis stem-leaf total flavonoid inhibited amyloid beta-peptide (25–35)-induced apoptosis in the rat hippocampus
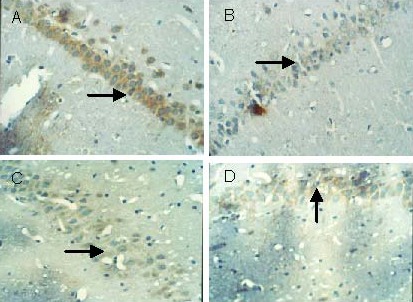
TUNEL staining revealed numerous brown nuclei in the hippocampus of rats in the model group, while only a few stained cells were found in the control group. Compared with the model group, hippocampal cells were lightly stained in the high-dose and low-dose treatment groups, and there were fewer brown cells (Figure 1).
Figure 1.

Effect of Scutellaria baicalensis stem-leaf total flavonoid on cell apoptosis (arrows) in the hippocampal CA1 region of amyloid beta-peptide (25–35)-treated rats (terminal deoxyribonucleotidyl transferase-mediated biotin-16-dUTP nick-end labeling, × 400).
(A) Control group: Cells are arranged in neat rows; a few cells exhibit light yellow nuclei.
(B) Model group: Cells are arranged in a disordered manner; a large number of cells exhibit deeply stained nuclei.
(C) Low-dose Scutellaria baicalensis stem-leaf total flavonoid group: Compared with the model group, cells are arranged tightly, the number of yellow stained cells is reduced, and the staining is light.
(D) High-dose Scutellaria baicalensis stem-leaf total flavonoid group: Compared with the model group, cells are arranged tightly, the number of yellow stained cells is significantly reduced, and the staining is light.
Statistical analysis showed that the number of TUNEL-positive cells in the model group was significantly higher than in the control group (P < 0.01). Furthermore, 50 and 100 mg/kg Scutellaria baicalensis stem-leaf total flavonoid reduced the number of TUNEL-positive hippocampal cells in animals administered amyloid beta-peptide (25–35). This effect was more evident at 100 mg/kg (P < 0.01; Figure 2).
Figure 2.
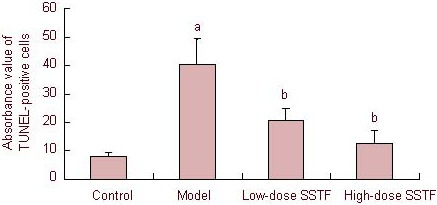
Effect of Scutellaria baicalensis stem-leaf total flavonoid (SSTF) on cell apoptosis in the hippocampal CA1 region of amyloid beta-peptide (25–35)-treated rats.
Data are expressed as mean ± SD, there were two rats in each group, and four slices (× 400) were selected from each rat. aP < 0.01, vs. control group; bP < 0.01, vs. model group (one-way analysis of variance, least significant difference between two groups).
TUNEL: Terminal deoxyribonucleotidyl transferase-mediated biotin-16-dUTP nick-end labeling.
Scutellaria baicalensis stem-leaf total flavonoid inhibited Bax expression in the hippocampus of amyloid beta-peptide (25–35)-treated rats
Immunohistochemical staining demonstrated the presence of Bax-positive cells exhibiting a brown granular cytoplasm. In the model group, most of the neurons in the hippocampal CA1 region were stained yellow. In contrast, Bax-positive cells in the low and high-dose treatment groups were lightly stained compared with the model group (Figure 3). Statistical analysis showed that Bax expression in the hippocampal CA1 region in the model group was significantly higher than in the control group (P < 0.01). Low- and high-dose Scutellaria baicalensis stem-leaf total flavonoid treatment decreased Bax expression in the hippocampal CA1 region (P < 0.01), especially the high dose (P < 0.01; Table 1).
Figure 3.

Effect of Scutellaria baicalensis stem-leaf total flavonoid on Bax expression (arrows) in the hippocampal CA1 region of amyloid beta-peptide (25–35)-treated rats (immunohistochemical staining, × 400).
(A) Control group: Cells are arranged in neat rows, and a few cells have lightly stained cytoplasm.
(B) Model group: Cells are arranged in a disorderly manner, cell count is reduced, and many cells have deeply stained cytoplasm.
(C) Low-dose Scutellaria baicalensis stem-leaf total flavonoid group: Compared with the model group, cells are arranged tightly, the number of stained cells is reduced, and the staining is light.
(D) High-dose Scutellaria baicalensis stem-leaf total flavonoid group: Compared with the model group, cells are arranged tightly, cell count is higher, the number of stained cells is significantly reduced, and the staining level is light.
Table 1.
Effect of Scutellaria baicalensis stem-leaf total flavonoid (SSTF) on Bax expression in the hippocampal CA1 region of amyloid beta-peptide (25–35)-treated rats
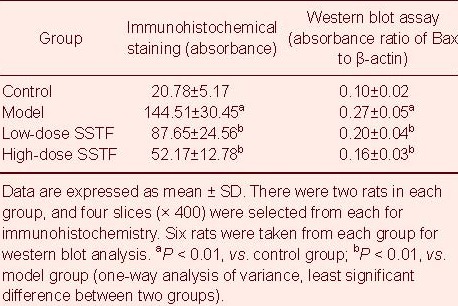
Western blot analysis results were consistent with the immunohistochemical assay findings. Amyloid beta-peptide (25-35) up-regulated Bax expression, while Scutellaria baicalensis stem-leaf total flavonoid down-regulated Bax expression in the rat hippocampus (P < 0.01). The 100 mg/kg dose had a particularly dramatic effect (P < 0.01; Figure 4, Table 1).
Figure 4.
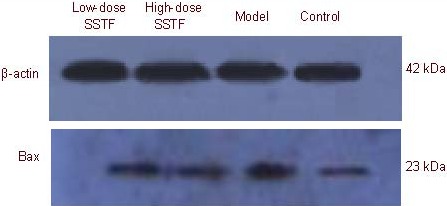
Bax expression in the hippocampus of amyloid beta-peptide (25–35)-treated rats, detected by western blot analysis.
SSTF: Scutellaria baicalensis stem-leaf total flavonoid.
Scutellaria baicalensis stem-leaf total flavonoid enhanced Bcl-2 expression in the hippocampus of amyloid beta-peptide (25–35)-treated rats
Immunohistochemistry showed that Bcl-2 was mainly expressed in the cytoplasm of rat hippocampal neurons, and was displayed as brownish yellow staining. The number of Bcl-2-positive cells in the hippocampal CA1 region in the model group was less than in the control group, with light staining. In comparison, Bcl-2-positive cells in the low- and high-dose treatment groups were more numerous than in the model group, with intense staining (Figure 5).
Figure 5.
Effect of Scutellaria baicalensis stem-leaf total flavonoid on Bcl-2 expression (arrows) in the hippocampal CA1 region of amyloid beta-peptide (25–35)-treated rats (immunohistochemical staining, × 400).
(A) Control group: Cells are arranged in neat rows and display a normal morphology, and many cells are deeply stained.
(B) Model group: Cells are arranged in a disorderly manner and exhibit abnormal morphology, and the number of stained cells is reduced.
(C) Low-dose Scutellaria baicalensis stem-leaf total flavonoid group: Compared with the model group, the number of deeply stained cells is increased.
(D) High-dose Scutellaria baicalensis stem-leaf total flavonoid group: Compared with the model group, the number of deeply stained cells is significantly increased.
Statistical analysis showed that, compared with the control group, Bcl-2 expression in the hippocampal CA1 region in the model group was significantly lower (P < 0.01). Furthermore, compared with the model group, Bcl-2 expression in the hippocampal CA1 region was increased significantly after low- and high-dose Scutellaria baicalensis stem-leaf total flavonoid treatment (P < 0.01). High-dose treatment had a more dramatic effect (P < 0.01; Table 2).
Table 2.
Effect of Scutellaria baicalensis stem-leaf total flavonoid (SSTF) on Bcl-2 expression in the hippocampal CA1 region of amyloid beta-peptide (25–35)-treated rats
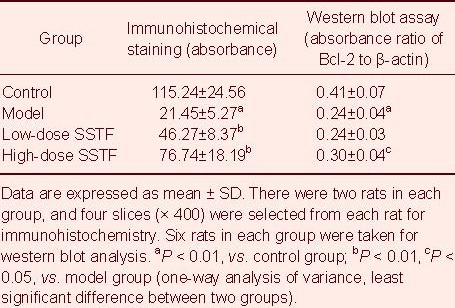
Western blot analysis results were consistent with the immunohistochemical results. Amyloid beta-peptide (25–35) down-regulated hippocampal Bcl-2 expression (P < 0.01), and 100 mg/kg Scutellaria baicalensis stem-leaf total flavonoid up-regulated Bcl-2 expression in the rat hippocampus (P < 0.05; Figure 6, Table 2).
Figure 6.

Bcl-2 expression in the hippocampus of amyloid beta-peptide (25–35)-treated rats, detected by western blot analysis.
SSTF: Scutellaria baicalensis stem-leaf total flavonoid.
Scutellaria baicalensis stem-leaf total flavonoid inhibited caspase-3 expression in the hippocampus of amyloid beta-peptide (25–35)-treated rats
Immunohistochemical staining showed that caspase-3 was expressed in the cytoplasm and nuclei of rat hippocampal neurons. The caspase-3-positive cells in the hippocampal CA1 region of rats in the model group were more numerous than in the control group and were deeply stained. Compared with the model group, the number of caspase-3-positive cells was decreased after low- and high-dose Scutellaria baicalensis stem-leaf total flavonoid treatment, and these cells were slightly stained (Figure 7). Statistical analysis revealed that caspase-3 expression in the hippocampal CA1 region in the model group was increased significantly compared with the control group (P < 0.01). Compared with the model group, caspase-3 expression was significantly reduced after low- and high-dose Scutellaria baicalensis stem-leaf total flavonoid treatment (P < 0.01), especially with high-dose treatment (P < 0.01; Table 3).
Figure 7.
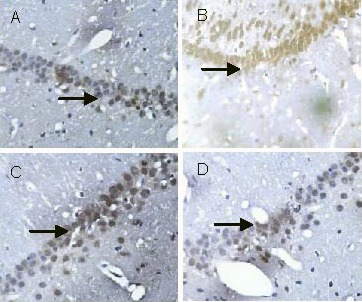
Effect of Scutellaria baicalensis stem-leaf total flavonoid on caspase-3 expression (arrows) in the hippocampal CA1 region of amyloid beta-peptide (25–35)-treated rats (immunohistochemical staining, × 400).
(A) Control group: Cells are arranged in neat rows, and a few cells are stained lightly.
(B) Model group: Cells are arranged in a disorderly manner, and the number of deeply stained cells is increased.
(C) Low-dose Scutellaria baicalensis stem-leaf total flavonoid group: Compared with the model group, cells are arranged in neat rows, the number of stained cells is decreased, and staining intensity is low.
(D) High-dose Scutellaria baicalensis stem-leaf total flavonoid group: Compared with the model group, the number of stained cells is significantly decreased, and staining intensity is low.
Table 3.
Effect of Scutellaria baicalensis stem-leaf total flavonoid (SSTF) on caspase-3 expression in the hippocampal CA1 region of amyloid beta-peptide (25–35)-treated rats
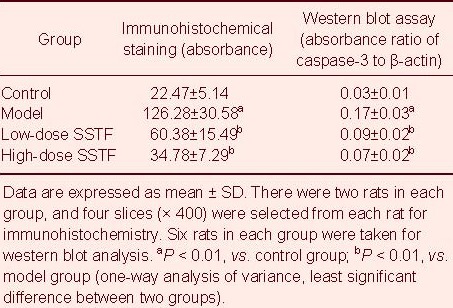
Western blot analysis results were consistent with the immunohistochemical results. Amyloid beta-peptide (25–35) increased caspase-3 expression in the hippocampus, while Scutellaria baicalensis stem-leaf total flavonoid decreased caspase-3 expression (P < 0.01), especially after 100 mg/kg treatment (P < 0.01; Figure 8, Table 3).
Figure 8.
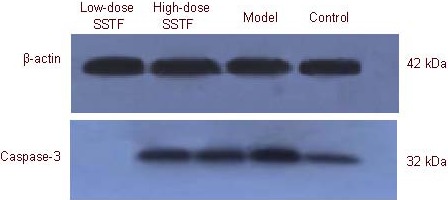
Caspase-3 expression in the hippocampus of amyloid beta-peptide (25–35)-treated rats, detected by western blot analysis.
SSTF: Scutellaria baicalensis stem-leaf total flavonoid.
Scutellaria baicalensis stem-leaf total flavonoid inhibits cytochrome c expression in the hippocampus of amyloid beta-peptide (25–35)-treated rats
Immunohistochemistry demonstrated that cytochrome c was expressed in the cytoplasm of rat hippocampal neurons and occasionally in the cell nuclei, appearing as brownish yellow staining. The number of cytochrome c-positive cells in the hippocampal CA1 region of rats in the model group was increased compared with the control group, and cells were deeply stained. Compared with the model group, cytochrome c-positive cells decreased in number and were lightly stained after low-and high-dose Scutellaria baicalensis stem-leaf total flavonoid treatment (Figure 9).
Figure 9.

Effect of Scutellaria baicalensis stem-leaf total flavonoid on cytochrome c expression (arrows) in the hippocampal CA1 region of amyloid beta-peptide (25–35)-treated rats (immunohistochemical staining, × 400).
(A) Control group: Cells are arranged in neat rows, and there are many cells, including a few deeply stained ones.
(B) Model group: Cells are arranged in a disorderly manner, cell count is reduced, and the number of deeply stained cells is increased. Even the nuclei are stained.
(C) Low-dose Scutellaria baicalensis stem-leaf total flavonoid group: Compared with the model group, the number of stained cells is reduced, and staining intensity is lower.
(D) High-dose Scutellaria baicalensis stem-leaf total flavonoid group: Compared with the model group, cells are arranged in neat rows and are more numerous. The number of stained cells is significantly reduced.
Western blot analysis results were consistent with the immunohistochemical results. Amyloid beta-peptide (25–35) treatment produced an increase in cytochrome c expression in the hippocampus, while Scutellaria baicalensis stem-leaf total flavonoid decreased cytochrome c expression (P < 0.01), especially after 100 mg/kg treatment (P < 0.01; Figure 10, Table 4).
Figure 10.
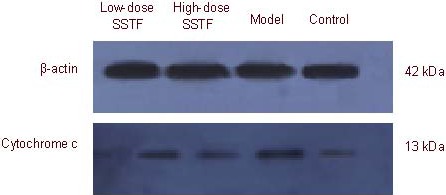
Cytochrome c expression in the hippocampus of amyloid beta-peptide (25–35)-treated rats, detected by western blot analysis.
SSTF: Scutellaria baicalensis stem-leaf total flavonoid.
Table 4.
Effect of Scutellaria baicalensis stem-leaf total flavonoid (SSTF) on cytochrome c expression in the hippocampal CA1 region of amyloid beta-peptide (25–35)-treated rats

DISCUSSION
Alzheimer's disease is a neurodegenerative disease characterized by an imbalance in pro-apoptotic and anti-apoptotic processes. Scutellaria baicalensis stem-leaf total flavonoid may attenuate learning and memory impairment and cell damage induced by amyloid beta. This function of the traditional Chinese medicine is closely related to its antioxidative effects[11]. In this study, we used the TUNEL assay, immunohistochemistry and western blot analysis to assess the hippocampal expression of the apoptosis effector molecule caspase-3, the pro-apoptotic gene Bax, cytochrome c and the anti-apoptotic gene Bcl-2. The aim of this study was to explore the effects of Scutellaria baicalensis stem-leaf total flavonoid on amyloid beta-induced damage and apoptosis.
Results of the TUNEL assay showed that amyloid beta triggered apoptosis in the hippocampus.
Immunohistochemical results revealed that caspase-3, Bax and cytochrome c expression levels in the hippocampus increased, while Bcl-2 expression decreased. Western blot analysis suggested that hippocampal expression of Bax, cytochrome c and caspase-3 in the model group was higher than in the control group, while Bcl-2 expression was lower. Collectively, the results indicate that amyloid beta can promote pro-apoptotic protein expression and inhibit anti-apoptotic protein expression.
How does amyloid beta induce neuronal apoptosis? The mitochondrial apoptotic pathway involves various proteins, especially p53, Bax, Bcl-2 and caspase[2,12]. Caspase-3 is the key factor mediating apoptosis, and its activation contributes to cytochrome c release from mitochondria, mitochondrial damage and neuronal apoptosis[13]. Bcl-2 and Bax are a pair of antagonistic proteins in the Bcl-2 gene family. Bcl-2 has significant anti-apoptotic effects, while Bax promotes apoptosis[14,15]. Bax and Bcl-2 can control the release of cytochrome c by regulating mitochondrial outer membrane permeability[16]. When Bax predominates, the cytochrome c released from the mitochondria may trigger a series of cascade reactions leading to apoptosis. The mitochondrial apoptotic pathway can be divided into caspase-dependent and independent pathways. Mitochondrial amyloid beta can increase the permeability of the mitochondrial outer membrane and promote cytochrome c release, this is called the first phase of release. The released cytochrome c stimulates inositol 1,4,5-triphosphate receptors on the endoplasmic reticulum and promotes unrestricted release of calcium. The intracellular calcium increase leads to a further increase in cytochrome c release, inducing apoptosis, which is independent of caspase[17,18]. Amyloid beta also decreases complex III (ubiquinone-cytochrome c-reductase) and IV (cytochrome c-oxidase) activity in the mitochondrial respiratory chain[19]. The reduced activity leads to a decrease in ATP, and the excess electrons directly trigger the production of superoxide anion (O2-) and other reactive oxygen species[20]. Cardiolipin is the only phospholipid in the mitochondrial inner membrane and is the target of reactive oxygen species. Reactive oxygen species may cause myocardial lipid peroxidation, and oxidized cardiolipin has a low binding affinity for cytochrome c[21], thereby leading to complete cytochrome c release, this is called the second phase of release. In the presence of ATP and dATP, cytochrome c mediates the activation of apoptosis protease-activating factor 1, inducing apoptosis complex formation and stimulating caspase-9 activation, which ultimately triggers catalytic maturation and apoptosis[22]. This apoptotic process is dependent on caspase. In this study, Bax expression increased, Bcl-2 expression decreased and cytochrome c was increased in the model group. This evidence suggests that amyloid beta may affect the expression of Bax and Bcl-2, further influencing mitochondrial outer membrane permeability, thereby regulating the release of cytochrome c and inducing neuronal apoptosis that is independent of caspase. In addition, caspase-3 expression was shown to increase. Amyloid beta causes an increase in reactive oxygen species[23]. Therefore, amyloid beta may also trigger cytochrome c release via reactive oxygen species, activating caspase and inducing caspase-dependent apoptosis.
TUNEL, immunohistochemistry and western blot assay results showed that cellular apoptosis decreased significantly, Bax expression decreased, Bcl-2 expression increased, and cytochrome c expression decreased in rats treated with Scutellaria baicalensis stem-leaf total flavonoid. This evidence suggests that Scutellaria baicalensis stem-leaf total flavonoid antagonizes amyloid beta-induced changes to apoptosis-related proteins, and alleviates caspase-independent neuronal apoptosis. Scutellaria baicalensis stem-leaf total flavonoid down-regulates caspase-3 expression and has an antioxidant effect. Thus, the anti-apoptotic effects of Scutellaria baicalensis stem-leaf total flavonoid may be mediated by its antioxidant effect, decreasing reactive oxygen species production and alleviating caspase-dependent apoptosis. Zhu et al[24] found that Hibifolin could inhibit the activation of caspase-3 and caspase-7, decrease Ca2+ flow, and reduce DNA damage in cortical neurons induced by amyloid beta. Zhang et al[11] also demonstrated that flavonoid may regulate the expression of Bcl family proteins and have an anti-apoptotic role in the mitochondrial death pathway.
In addition to antioxidation, anti-apoptosis and alleviating neurotoxicity, flavonoid also affects amyloid beta generation and fibrillation to protect neurons. Jung et al[25] found that total flavonoid extracted from Sophora plants can inhibit β-secretase activity. Thus, because amyloid precursor protein produces amyloid beta through β and γ secretase cleavage, inhibiting β-secretase activity can reduce amyloid beta production. Naiki et al[26] studied five kinds of flavonoid in vitro and found that flavonoid inhibits the formation of amyloid beta fibrils and promotes fibrillar instability. Soluble amyloid beta has no toxicity, but it will acquire toxicity after fibrillation. Lemkul et al[27] observed that morin binds to the end of fibrils and functions to block peptide adherence. It penetrates into the hydrophobic center and cleaves at aspartic acid and lysine residues, interfering with backbone hydrogen bonds, ultimately inhibiting fibrillation.
In summary, Scutellaria baicalensis stem-leaf total flavonoid can attenuate neuronal apoptosis induced by amyloid beta. It accomplishes this by modulating the expression of apoptosis-related genes and by having an antioxidant effect. Our findings provide an experimental basis for the application of Scutellaria baicalensis stem-leaf total flavonoid in the prevention and treatment of neurodegenerative diseases.
MATERIALS AND METHODS
Design
A randomized, controlled, animal experiment.
Time and setting
The experiment was performed in the Animal Laboratory of Chengde Medical College, China from June 2009 to June 2010.
Materials
Animals
Forty healthy, clean, male, Wistar rats, aged 10–12 weeks, weighing 280 ± 20 g, were provided by the Experimental Animal Center of Hebei Province (license No. SYXK (Ji) 2009-0022). All experiments were performed in the specific pathogen free level at 22 ± 2°C and 50 ± 5% humidity, allowing free access to water and food. All procedures were in accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals, formulated by the Ministry of Science and Technology of China[28].
Drugs
Scutellaria baicalensis stem-leaf total flavonoid was provided by the Institute of Traditional Chinese Medicine, Chengde Medical College, China. In brief, stems and leaves were crushed into pieces and extracted two or three times in aqueous solution. The supernatant was acidized, isolated, adsorbed, desorbed, condensed and dried to prepare a brown powder of 61.88% purity[5]. Scutellaria baicalensis stem-leaf total flavonoid was dissolved in distilled water and adjusted for pH 7.0.
Amyloid beta-peptide (25–35) (purity 97%; Sigma, St. Louis, MO, USA) was tested with high performance liquid chromatography. Amyloid beta-peptide (25–35), 1 mg, was dissolved in 500 μL sterile saline to prepare a 2 g/L solution, which was stored at –20°C. Prior to the experiment, the solution was incubated at 37°C for 5–7 days.
Methods
Drug intervention
Rats in the control and model groups were intragastrically given distilled water, and those in the low-dose and high-dose treatment groups were treated with Scutellaria baicalensis stem-leaf total flavonoid, 50 or 100 mg/kg per day, respectively, for 20 consecutive days. On day 8 after administration, 5 μL of sterile saline was injected into the bilateral hippocampus of rats in the control group, while 5 μL amyloid beta-peptide (25–35), 10 μg, was injected into the bilateral hippocampal CA1 region of rats (anteroposterior: –3.5 mm; mediolateral ± 2 mm; dorsoventral: 2.7 mm[29]) in the three remaining groups.
Tissue preparation
All animals were killed after 20 days of intragastric administration, and four rats from each group were selected for preparation of brain tissue paraffin specimens. Blood samples were collected under anesthesia, and 250 mL saline was perfused via the left ventricle. Brain tissue was fixed in PB solution, 250 mL, containing paraformaldehyde, for 1 hour. Then, the bilateral hippocampi were cut along the coronal plane, and the tissue block was trimmed and fixed in 4% paraformaldehyde overnight. Brain tissue was rinsed with PBS, dehydrated in graded ethanol, cleared with xylene, and embedded in paraffin. Two pieces of tissue were embedded in a piece of wax and cut into serial coronal slices of 5 μm thickness, containing the hippocampus, for TUNEL and immunohistochemical staining.
TUNEL staining for hippocampal cell apoptosis
The paraffin slices were dewaxed, hydrated and assayed according to instructions in the apoptosis detection kit (Roche, Basel, Switzerland). Slices were incubated with reaction liquid and converter-POD, developed with 3,3’-diaminobenzidine, counterstained with hematoxylin, dehydrated in graded ethanol, cleared with xylene, and mounted with neutral gum. Brain tissue was observed under an optical microscope (Olympus, Tokyo, Japan). A cell with its cytoplasm or nucleus stained brown-yellow indicated an apoptotic cell.
Immunohistochemical staining for Bax, Bcl-2, caspase-3 and cytochrome c expression in the rat hippocampus
Brain slices were dewaxed with xylene, hydrated with gradient ethanol, and placed in a 95°C water bath for antigen retrieval. Slices were incubated with rabbit anti-Bax polyclonal antibody (1:100), rabbit anti-caspase-3 polyclonal antibody (1:200), rabbit anti-Bcl-2 polyclonal antibody (1:75) or mouse anti-rat cytochrome c monoclonal antibody (1:75) at 37°C for 2.5 hours. All antibodies were purchased from Santa Cruz Biotechnology, Santa Cruz, CA, USA. Then, slices were incubated with horseradish peroxidase-conjugated goat anti-rabbit/mouse IgG (1:3 000; Beijing Zhong Shan-Golden Bridge Biological Technology Co., Ltd., Beijing, China) at 37°C for 15 minutes, developed with 3,3’-diaminobenzidine, counterstained with hematoxylin, and mounted with neutral gum. Brain tissue was observed under the light microscope. Negative control was incubated with PBS, rather than antibodies.
Image analysis
Two rats in each group were selected for preparation of brain tissue paraffin sections for TUNEL and immunohistochemical staining. Four consecutive sections from the hippocampal CA1 region were observed by light microscopy, and the absorbance was measured using Image-Pro Plus software (Media Cybernetics, Inc., Bethesda, MD, USA) under 400 × magnification and averaged.
Western blot analysis for Bax, Bcl-2, caspase-3 and cytochrome c expression in the rat hippocampus
Hippocampal tissue, 0.15–0.20 g, from six rats in each group was incubated with 1 mL PBS-NaF, ground on ice, centrifuged, and homogenized with RIPA lysate (Beyotime Institute of Biotechnology, Shanghai, China). Protein content was measured according to instructions in the bicinchoninic acid kit (BestBio, Shanghai, China). Samples were loaded onto sodium dodecyl sulfate polyacrylamide gel, electrophoresed, transferred onto a polyvinylidene difluoride membrane (IPVH00010; thickness: 0.45 μm, 0.22 μm; Millipore, Billerica, MA, USA), and blocked overnight. Then, slices were incubated with anti-Bax polyclonal antibody (1:200), rabbit anti-caspase-3 polyclonal antibody (1:200), rabbit anti-Bcl-2 polyclonal antibody (1:100), mouse anti-rat cytochrome c monoclonal antibody (1:200) and mouse-rat β-actin monoclonal antibody (1:1 000; Santa Cruz Biotechnology) at room temperature for 2 hours. Afterwards, slices were incubated with horseradish peroxidase-conjugated goat anti-rabbit IgG (1:3 000) and goat anti-mouse IgG (1:5 000) for 1 hour. Images were developed and fixed using enhanced chemiluminescence (Applygen Technologies Inc., Beijing, China). Absorbance was measured with Gelpro4 software, and β-actin was taken as the internal reference.
Statistical analysis
SPSS 11.5 software (SPSS, Chicago, IL, USA) was used for one-way analysis of variance, and two groups were compared using the least significant difference test method. Measurement data were expressed as mean ± SD. A P value < 0.05 was regarded to indicate a significant difference.
Acknowledgments:
We are grateful to Professor Jianxin Zhang from the Hebei Academy of Medical Sciences; Professor Jiming Tong from the Institute of Traditional Chinese Medicine of Chengde Medical College; Xin Li, Yong Yan, Xiaoguang Wu, Long Chen, Qian Xu and Xiaochun Zhou from the Institute of Basic Sciences of Chengde Medical College; Professor Shumin Zhao and all staff from the Department of Electron Microscopy of Chengde Medical College, China; Liqun Ren, Lixin Mei and Yingchun Zhang from the Department of Pathology of Chengde Medical College, China; Professor Lixin Sun from the Central Laboratory of Affiliated Hospital of Chengde Medical College, China; and Xiaoping Jin from the Department of Pathology in the Affiliated Hospital of Chengde Medical College, China for help with experimental guidance.
Footnotes
Conflicts of interest: None declared.
Funding: This study was supported by grants from Hebei Provincial Science and Technology Bureau, No. 08276101D-21.
Ethical approval: This study was approved by the Animal Ethics Committee of Chengde Medical College in China.
(Reviewed by Patel B, Stow A, Liu P, Ma XL)
(Edited by Wang LM, Yang Y, Li CH, Song LP)
REFERENCES
- [1].Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297(5580):353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- [2].Nishitsuji K, Tomiyama T, Ishibashi K, et al. The E693Delta mutation in amyloid precursor protein increases intracellular accumulation of Aβ oligomers and causes endoplasmic reticulum stress-induced apoptosis in cultured cells. Am J Pathol. 2009;174(3):957–969. doi: 10.2353/ajpath.2009.080480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Zhi-Kun S, Hong-Qi Y, Zhi-Quan W, et al. Erythropoietin prevents PC12 cells from beta-amyloid-induced apoptosis via PI3K/Akt pathway. Transl Neurodegener. 2012;1(1):7. doi: 10.1186/2047-9158-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Jhamandas JH, Li Z, Westaway D, et al. Actions of β-amyloid protein on human neurons are expressed through the amylin receptor. Am J Pathol. 2011;178(1):140–149. doi: 10.1016/j.ajpath.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Zhao TH, Kang SW, Tong JM, et al. China: 2005. Oct 19, Extraction of total flavonoid from Scutellaria Baicalensis Stem-leaf. CN200510008584.8. [Google Scholar]
- [6].Zhao SM, Liu S, Yang HG, et al. Protective effect of Scutellaria baicalensis stem-leaf total flavonoid on lipid peroxidation induced by myocardial ischemia reperfusion in rats. Jiepou Xuebao. 2006;29(4):450–452. [Google Scholar]
- [7].Wang RT, Zhang JX, Dong YJ. Protective effect of Scutellaria baicalensis stem-leaf total flavonoid against hippocampus neurodamage induced by injection Aβ25-35. Zhongguo Laonian Xue Zazhi. 2010;30(7):926–928. [Google Scholar]
- [8].Wang RT, Shen XB, Zhou J, et al. Effect of total flavonoid from Scutellaria baicalensis stem-leaf on hippocampal injury induced by injection Aβ25-35. Zhongguo Xinyao Zazhi. 2010;19(11):982–985. [Google Scholar]
- [9].Liu R, Wu CX, Zhou D, et al. Pinocembrin protects against β-amyloid-induced toxicity in neurons through inhibiting receptor for advanced glycation end products (RAGE)-independent signaling pathways and regulating mitochondrion-mediated apoptosis. BMC Med. 2012;10:105. doi: 10.1186/1741-7015-10-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Huang HC, Xu K, Jiang ZF. Curcumin-mediated neuroprotection against amyloid-β-induced mitochondrial dysfunction involves the inhibition of GSK-3β. J Alzheimers Dis. 2012;32(4):981–996. doi: 10.3233/JAD-2012-120688. [DOI] [PubMed] [Google Scholar]
- [11].Zhang J, Zhao CC, Han WN, et al. Effects of flavonoid on myocardial apoptosis. Zhongguo Yaoli Xue Tongbao. 2008;24(5):635–639. [Google Scholar]
- [12].Silva DF, Selfridge JE, Lu J, et al. Mitochondrial abnormalities in Alzheimer's disease: possible targets for therapeutic intervention. Adv Pharmacol. 2012;64:83–126. doi: 10.1016/B978-0-12-394816-8.00003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Musatov A, Robinson NC. Susceptibility of mitochondrial electron-transport complexes to oxidative damage. Focus on cytochrome c oxidase. Free Radic Res. 2012;46(11):1313–1326. doi: 10.3109/10715762.2012.717273. [DOI] [PubMed] [Google Scholar]
- [14].Cheng EH, Wei MC, Weiler S, et al. BCL-2, BCL-X(L) sequester BH3 domain-only molecules preventing BAX-and BAK-mediated mitochondrial apoptosis. Mol Cell. 2001;8(3):705–711. doi: 10.1016/s1097-2765(01)00320-3. [DOI] [PubMed] [Google Scholar]
- [15].Marani M, Tenev T, Hancock D, et al. Identification of novel isoforms of the BH3 domain protein Bim which directly activate Bax to trigger apoptosis. Mol Cell Biol. 2002;22(11):3577–3589. doi: 10.1128/MCB.22.11.3577-3589.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Breckenridge DG, Xue D. Regulation of mitochondrial membrane permeabilization by BCL-2 family proteins and caspases. Curr Opin Cell Biol. 2004;16(6):647–652. doi: 10.1016/j.ceb.2004.09.009. [DOI] [PubMed] [Google Scholar]
- [17].Goldstein JC, Waterhouse NJ, Juin P, et al. The coordinate release of cytochrome c during apoptosis is rapid, complete and kinetically invariant. Nat Cell Biol. 2000;2(3):156–162. doi: 10.1038/35004029. [DOI] [PubMed] [Google Scholar]
- [18].Boehning D, Patterson RL, Sedaghat L, et al. Cytochrome c binds to inositol (1,4,5) trisphosphate receptors, amplifying calcium-dependent apoptosis. Nat Cell Biol. 2003;5(12):1051–1061. doi: 10.1038/ncb1063. [DOI] [PubMed] [Google Scholar]
- [19].Casley CS, Canevari L, Land JM, et al. Beta-amyloid inhibits integrated mitochondrial respiration and key enzyme activities. J Neurochem. 2002;80(1):91–100. doi: 10.1046/j.0022-3042.2001.00681.x. [DOI] [PubMed] [Google Scholar]
- [20].Manczak M, Anekonda TS, Henson E, et al. Mitochondria are a direct site of A beta accumulation in Alzheimer's disease neurons: implications for free radical generation and oxidative damage in disease progression. Hum Mol Genet. 2006;15(9):1437–1449. doi: 10.1093/hmg/ddl066. [DOI] [PubMed] [Google Scholar]
- [21].Kagan VE, Borisenko GG, Tyurina YY, et al. Oxidative lipidomics of apoptosis: redox catalytic interactions of cytochrome c with cardiolipin and phosphatidylserine. Free Radic Biol Med. 2004;37(12):1963–1985. doi: 10.1016/j.freeradbiomed.2004.08.016. [DOI] [PubMed] [Google Scholar]
- [22].Otera H, Ohsakaya S, Nagaura Z, et al. Export of mitochondrial AIF in response to proapoptotic stimuli depends on processing at the intermembrane space. EMBO J. 2005;24(7):1375–1386. doi: 10.1038/sj.emboj.7600614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Wang RT, Guan LH, Zhou J, et al. Effect of Scutellaria baicalensis stem-leaf total flavonoid on learning and memory impairment induced by Aβ25-35 and activity of anti-oxidative enzyme of hippocampus in rats. Shenjing Yaoli Xuebao. 2011;1(2):14–18. [Google Scholar]
- [24].Zhu JT, Choi RC, Xie HQ, et al. Hibifolin, a flavonol glycoside, prevents beta-amyloid-induced neurotoxicity in cultured cortical neurons. Neurosci Lett. 2009;461(2):172–176. doi: 10.1016/j.neulet.2009.06.010. [DOI] [PubMed] [Google Scholar]
- [25].Jung HA, Yokozawa T, Kim BW, et al. Selective inhibition of prenylated flavonoid from Sophora flavescens against BACE1 and cholinesterases. Am J Chin Med. 2010;38(2):415–29. doi: 10.1142/S0192415X10007944. [DOI] [PubMed] [Google Scholar]
- [26].Naiki H, Hasegawa K, Ono K, et al. Search for antiamyloidogenic compounds based on a nucleation-dependent polymerization model. Yakugaku Zasshi. 2010;130(4):503–509. doi: 10.1248/yakushi.130.503. [DOI] [PubMed] [Google Scholar]
- [27].Lemkul JA, Bevan DR. Destabilizing Alzheimer's Abeta(42) protofibrils with morin: mechanistic insights from molecular dynamics simulations. Biochemistry. 2010;49(18):3935–3946. doi: 10.1021/bi1000855. [DOI] [PubMed] [Google Scholar]
- [28].The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006 Sep 30; [Google Scholar]
- [29].Paxions G, Watson C. 6th ed. San Diego: Academic Press; 2005. The Rat Brain in Stereotaxic Coordinates. [Google Scholar]


