Abstract
Tensile stress and tensile strain directly affect the quality of nerve regeneration after bridging nerve defects by poly(lactic-co-glycolic acid) conduit transplantation and autogenous nerve grafting for sciatic nerve injury. This study collected the sciatic nerve from the gluteus maximus muscle from fresh human cadaver, and established 10-mm-long sciatic nerve injury models by removing the ischium, following which poly(lactic-co-glycolic acid) conduits or autogenous nerve grafts were transplanted. Scanning electron microscopy revealed that the axon and myelin sheath were torn, and the vessels of basilar membrane were obstructed in the poly(lactic-co-glycolic acid) conduit-repaired sciatic nerve following tensile testing. There were no significant differences in tensile tests with autogenous nerve graft-repaired sciatic nerve. Following poly(lactic-co-glycolic acid) conduit transplantation for sciatic nerve repair, tensile test results suggest that maximum tensile load, maximum stress, elastic limit load and elastic limit stress increased compared with autogenous nerve grafts, but elastic limit strain and maximum strain decreased. Moreover, the tendencies of stress-strain curves of sciatic nerves were similar after transplantation of poly(lactic-co-glycolic acid) conduits or autogenous nerve grafts. Results showed that after transplantation in vitro for sciatic nerve injury, poly(lactic-co-glycolic acid) conduits exhibited good intensity, elasticity and plasticity, indicating that poly(lactic-co-glycolic acid) conduits are suitable for sciatic nerve injury repair.
Keywords: neural regeneration, peripheral nerve injury, sciatic nerve, injury model, poly(lactic-co-glycolic acid), transplantation, stress, strain, mechanical property, grants-supported paper, neuroregeneration
Research Highlights
-
(1)
After poly(lactic-co-glycolic acid) conduit transplantation and autogenous nerve grafting of in vitro models of sciatic nerve injury using human cadaver sciatic nerves, longitudinal tensile tests were conducted, and stress and strain data of sciatic nerves were obtained. This provided biomechanical basis for poly(lactic-co-glycolic acid) conduit transplantation in the repair of injured sciatic nerve.
-
(2)
Poly(lactic-co-glycolic acid) conduits (ratio of lactic acid to glycolic acid = 70:30) were fabricated using NaCl as a pore-foaming agent. The mass ratio of poly(lactic-co-glycolic acid) to NaCl was 1:9. After transplantation, the poly(lactic-co-glycolic acid) conduit exhibited a definite intensity, elasticity and plasticity and can be used for sciatic nerve injury transplantation.
INTRODUCTION
The conventional method for repair of peripheral nerve injury is autogenous nerve grafting, but sources of autogenous nerve are limited. Furthermore, neurological deficits in the donor site and painful neuroma can occur following surgery. The use of allogeneic nerve grafts is limited because of host immune rejection. One study investigating preconditioning for allogeneic nerve grafting to remove or reduce its antigenicity is ongoing[1].
In recent years, various absorbable biomaterials have been used in the repair of nerve defects, and achieved a certain degree of repair of a 10-mm-long nerve injury[2]. Poly(lactic-co-glycolic acid), a copolymer of two monomers, lactic acid and glycolic acid, is an extensively used material in the research and application of tissue engineering[3,4,5]. A number of studies have addressed the biological properties, biocompatibility and biomechanics of peripheral nerve injury models after transplantation of poly(lactic-co-glycolic acid) material, poly(lactic-co-glycolic acid) conduits and autogenous nerve grafting[6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33]. Peng et al [34] performed a one-dimensional tensile test in 10-mm-long sciatic nerve injury models of human cadaver after autogenous nerve and human amnion transplantation for sciatic nerve injury. They found that the tendencies of change in stress-strain curves of human amnion and sciatic nerve were similar, indicating that human amnion has good biological properties and plasticity, making it a superior graft material for peripheral nerve injury. Hou et al[35] found that myelinated nerve fiber density, average diameter of nerve fibers and myelin sheath thickness were similar in regenerating sciatic nerves treated with mesenchymal stem cell transplantation and autogenous nerve grafting at 12 weeks after 10-mm-long sciatic nerve repair with mesenchymal stem cells combined with poly(lactic-co-glycolic acid) conduits. Schakenraad et al[36] verified that thin conduits had better mechanical support for repairing 10-mm-long sciatic nerve injuries compared to glycine/DL-lactic acid copolymer conduits of various thickness. Hu et al[37] confirmed that human hair keratin can perfectly bridge a 10-mm-long injured sciatic nerve. Li et al[38] verified that after treatment with alcohol, the porosity of poly (lactic-co-glycolic acid) tubular scaffolds with a diameter of 1 660 ± 218 nm and 80.6% porosity was diminished; its glass transition temperature and thermolysis temperature were elevated; heat stability was enhanced; and breaking strength, blasting strength and suture strength were obviously increased. Zhao et al[39] found that the tensile strength of poly(lactic-co-glycolic acid) scaffolds increased with the increased proportion of polylactic acid, indicating that polylactic acid plays an important role in elevating the tensile strength of poly(lactic-co-glycolic acid) scaffolds. Poly(lactic-co-glycolic acid) is nontoxic and nonantigenic, has good biodegradability, biocompatibility and mechanical properties, and its degradation speed can be regulated by controlling the content of the ingredients[5,40,41]. Thus, the reproducibility and mechanical properties of poly(lactic-co-glycolic acid) scaffolds is high, and poly(lactic-co- glycolic acid) has been extensively used in tissue-engineered materials[5,40,41].
Previous studies mainly focused on the biological properties, biocompatibility and immunology of poly(lactic-co-glycolic acid) scaffolds. The study examining the biomechanical properties of poly(lactic-co-glycolic acid) mainly focused on the mechanical properties of poly(lactic-co-glycolic acid) materials and a poly(lactic-co-glycolic acid) tubular scaffold. However, no reports have addressed the tensile mechanical properties of injured nerve after poly(lactic-co-glycolic acid) conduit transplantation. The mechanical properties of poly(lactic-co-glycolic acid) conduits are essential for the bridging of nerve gaps and the quality of nerve regeneration. The present study sought to quantitatively illuminate the mechanical properties of sciatic nerve injury model after poly (lactic-co-glycolic acid) conduit transplantation, and to explore the feasibility of poly (lactic-co-glycolic acid) conduit transplantation in the repair of sciatic nerve injury from the angle of biomechanics.
RESULTS
Histomorphology of sciatic nerve injury models with poly(lactic-co-glycolic acid) conduit transplantation and autogenous nerve grafting
Scanning electron microscopy revealed that after tensile testing, the axon and myelin sheath were torn; myelinated nerve fibers were irregular; fiber diameter and myelin sheath thickness were unequal; a few myelinated nerve fibers were mildly swollen and the basilar membrane vessel was obstructed. There were no significant differences in the histomorphology of sciatic nerves between poly(lactic-co-glycolic acid) conduit transplantation and autogenous nerve grafting (Figure 1).
Figure 1.
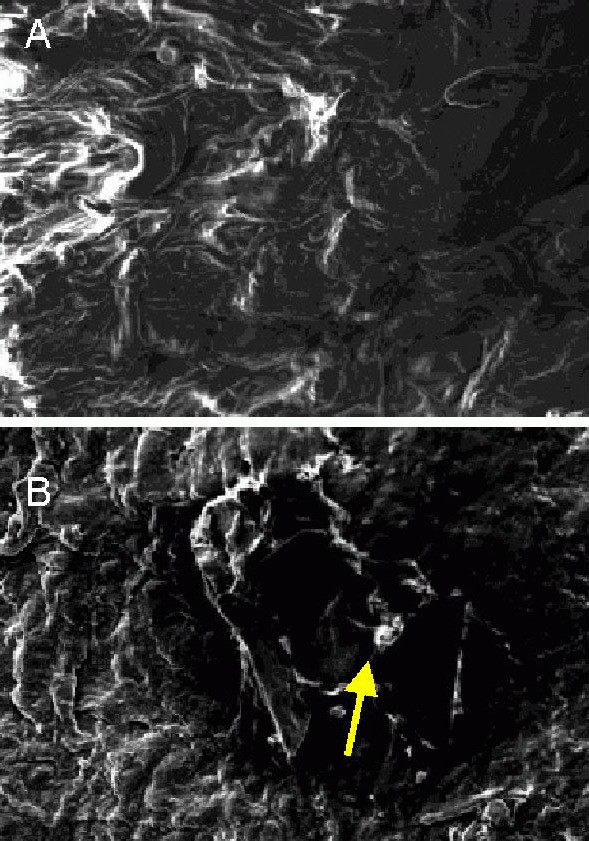
Histomorphology of sciatic nerve injury models after bridging with poly(lactic-co-glycolic acid) (PLGA) conduit and autogenous nerve (scanning electron microscope, × 400).
(A) Torn axon and myelin sheath and irregular myelinated nerve fibers after autogenous nerve grafting.
(B) A few mildly swollen myelinated nerve fibers obstructed the basilar membrane vessels after PLGA conduit transplantation (arrow).
Tensile mechanical property of injured sciatic nerve after poly(lactic-co-glycolic acid) conduit and autogenous nerve grafting
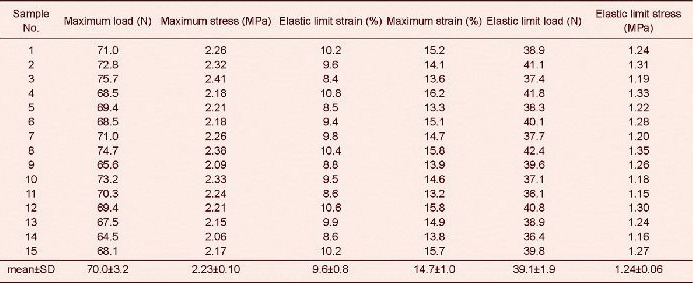
Tensile test results revealed that tensile maximum load, maximum stress, maximum strain, elastic limit load and elastic limit stress of sciatic nerve injury models were significantly lower after poly(lactic-co-glycolic acid) conduit transplantation and autogenous nerve grafting compared with the normal controls (P < 0.05)[34]. Tensile maximum load, maximum stress, elastic limit load and elastic limit stress were significantly higher (P < 0.05), but elastic limit strain and tensile maximum strain were significantly lower (P < 0.05) in sciatic nerve injury models after poly(lactic-co-glycolic acid) conduit transplantation compared with autogenous nerve grafting (Tables 1–3).
Table 1.
Tensile test results of injured sciatic nerve after autogenous nerve grafting
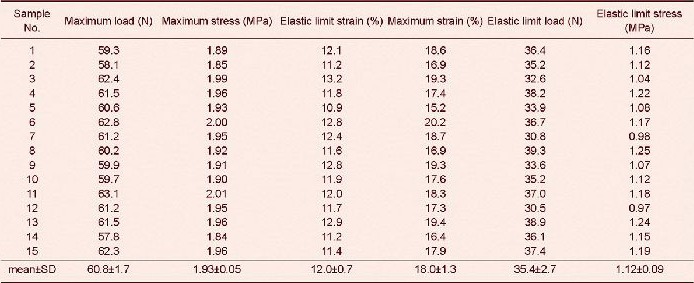
Table 3.
Difference between normal sciatic nerve and injured sciatic nerves after poly(lactic-co-glycolic acid) (PLGA) conduit transplantation and autogenous nerve grafting in tensile tests
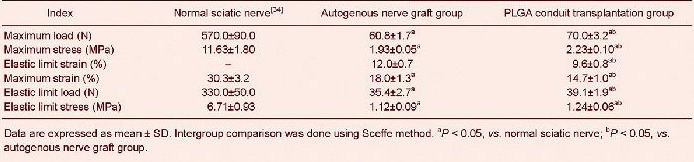
Table 2.
Tensile test results of injured sciatic nerve after poly(lactic-co-glycolic acid) conduit transplantation
Curve fitting of tensile data between autogenous nerve graft and poly(lactic-co-glycolic acid) conduit transplantation groups was performed using regression analysis, and their stress-strain curves were then obtained. First stage: when the samples in the autogenous nerve graft and poly(lactic-co-glycolic acid) conduit transplantation groups achieved 0–4.6% strain and 0–3.8% strain, respectively, the stress-strain curve showed an exponential relationship. Second stage: when the samples in the autogenous nerve graft and poly(lactic-co-glycolic acid) conduit transplantation groups achieved 4.6–12.0% strain and 3.8–9.6% strain, respectively, the stress-strain curve showed a linear relationship. Third stage: when the samples in the autogenous nerve graft and poly(lactic-co-glycolic acid) conduit transplantation groups achieved 12.0–14.3% strain and 9.6–11.2% strain, respectively, the stress-strain curve showed an exponential relationship. Fourth stage: when the samples in the autogenous nerve graft and poly(lactic-co-glycolic acid) conduit transplantation groups achieved 14.3–18.0% strain and 11.2–14.7% strain, respectively, the stress-strain curve showed plastic deformation. The deformation increased steadily, and the specimen almost lost bearing capacity and tended to be destroyed (Figure 2).
Figure 2.
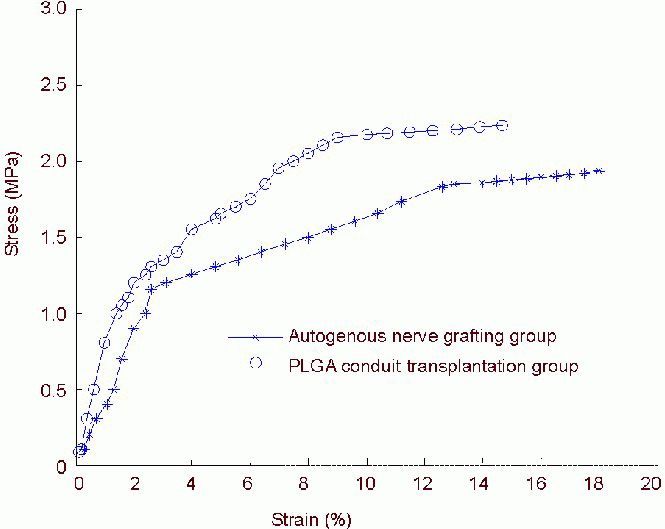
Stress-strain curve of sciatic nerve specimens in the autogenous nerve grafting and poly(lactic-co-glycolic acid) (PLGA) conduit transplantation groups.
Stress-strain curve changes result in an exponential relationship. The stress-strain curves of the two groups exhibited similarity.
DISCUSSION
Tensile testing is an important method to compare the mechanical properties of sciatic nerve after autogenous nerve grafting and poly(lactic-co-glycolic acid) conduit transplantation in a model of sciatic nerve injury. Cadaver race, gender, age, health status and vocation can introduce errors. To make the experimental data more reliable, this study adopted the following measures: sciatic nerve was obtained from fresh healthy male adult cadavers. We tried to use the specimen within 48 hours. Each specimen was preset. Sciatic nerve injury models were replicated by the same highly qualified surgeon from the Department of Microsurgery through suturing the same site as the specimen, and the number of stitches was identical. Tension speed and ambient temperature were identical. Each single sciatic nerve undergoing autogenous nerve grafting and poly(lactic-co-glycolic acid) conduit transplantation was randomly selected to receive histomorphological observation after tensile testing. Histomorphological observation results suggest that there was no significant difference in injured sciatic nerve after autogenous nerve grafting and poly(lactic-co-glycolic acid) conduit transplantation.
Using previously published results[34] of tensile testing of normal sciatic nerve as normal controls, results from our study revealed that the mechanical properties were better in the poly(lactic-co-glycolic acid) conduit transplantation group than the autogenous nerve graft group. Under loading conditions, the tendencies of change in stress-strain curves of sciatic nerve injury models were identical between autogenous nerve graft and poly(lactic-co-glycolic acid) conduit transplantation groups.
The biomaterials for sciatic nerve injury should have good biodegradation, biocompatibility, plasticity, a definite mechanical strength, and can maintain a certain shape for some time after transplantation to ensure mechanical support before tissue regeneration[42,43,44]. However, these properties can antagonize the stress produced by surrounding tissues during nerve regeneration and the stress produced by extension and flexion at the anastomotic site of nerve and nerve stump. Therefore, it is important to select suitable artificial materials and the correct method of preparation to produce a scaffold with suitable structure and mechanical properties. Experimental results suggest that a poly(lactic-co-glycolic acid) conduit (ratio of lactic acid to glycolic acid = 70:30), created using NaCl as a pore-foaming agent (mass ratio of poly(lactic-co-glycolic acid) to NaCl = 1:9), has defined intensity, elasticity and plasticity, and can be used for sciatic nerve injury transplantation.
A previous study reported the tensile mechanical properties of normal sciatic nerve specimen of gluteus maximus muscle of normal Chinese adult male cadavers[34]. Thus, in our study we did not set a normal control group, but used the normal control group from the previous study[34]. Abundant previous studies mainly examined the biocompatibility, biodegradation and mechanical strength of poly(lactic-co-glycolic acid) materials and conduits, and the mechanical properties of the injured sciatic nerve after poly(lactic-co-glycolic acid) conduit transplantation in animal in vitro studies, such as in rats[45,46,47,48]. The difference of our study to these other studies is that we selected sciatic nerve specimens from fresh human cadavers to replicate sciatic nerve injury models. The injured sciatic nerve received autogenous nerve grafting and poly(lactic-co-glycolic acid) conduit transplantation, followed by tensile testing. Stress-strain curve fitting between autogenous nerve grafting and poly(lactic-co-glycolic acid) conduit transplantation groups was conducted using regression analysis to compare the difference in mechanical properties of sciatic nerves. This study provided a biomechanical basis for poly(lactic-co-glycolic acid) conduit transplantation for sciatic nerve injury. Although it is an in vitro experiment, it is similar to clinical sciatic nerve injury repaired with poly(lactic-co-glycolic acid) conduit transplantation. Human cadaver sciatic nerve specimens are limited, so this study only simulated 10-mm long sciatic nerve injury models. Further studies addressing various injury models and various types of stresses deserve further investigations. Because of individual differences of various biomaterials, in vitro experimental data have some definite limitations, but is nonetheless still valuable for the clinic. After transplantation, poly(lactic-co-glycolic acid) conduits exhibited good intensity, elasticity and plasticity, indicating that poly(lactic-co-glycolic acid) conduit is fit for the repair of sciatic nerve injury.
MATERIALS AND METHODS
Design
Contrast observation, biomechanics experiment.
Time and setting
Experiments were performed at the Mechanics Experiment Center, Jilin University, China from December 2009 to August 2012.
Materials
Bilateral sciatic nerve specimens of gluteus maximus muscle were obtained from eight fresh normal Chinese adult male cadavers who died from acute head trauma, aged 25–30 years old. The cadavers were provided by the Norman Bethune Health Science Centre of Jilin University, China. A total of 16 bilateral sciatic nerves of the gluteus maximus muscle were collected within 24 hours after death, and stored in a container containing saline.
Poly(lactic-co-glycolic acid) (ratio of lactic acid and glycolic acid = 70:30) was purchased from Changchun Sinobiomaterials Co., Ltd. (Changchun, Jilin Province, China).
Methods
Preparation of poly(lactic-co-glycolic acid) conduit
In accordance with previously published studies[32,39], poly(lactic-co-glycolic acid) was dissolved in dichloromethane. NaCl particles of 200–300 μm served as pore-foaming agent. Poly(lactic-co-glycolic acid) was mixed with NaCl at a mass ratio of 1:9. The mixture was poured into a prefabricated mold, and made into 30 conduits of 10 mm length, 11 mm outer diameter and 9 mm inner diameter. The mixture in the mold was placed at room temperature and volatilized in a fume hood for 96 hours. After stripping, the scaffolds were obtained and placed in a vacuum oven for drying at 37°C for 48 hours, and then immersed in an 800-mL beaker containing deionized water for 96 hours. The deionized water was replaced once every 4 hours. The scaffolds were dried in a drying box at 37°C for 48 hours, in a vacuum oven for drying at 37°C for 48 hours, and then placed in a dryer.
Preparation of sciatic nerve specimens
After 1 day of storage, each of 15 sciatic nerves of gluteus maximus muscle was cut into two samples with a scalpel by the same highly qualified surgeon from the Department of Microsurgery. Thirty 40-mm-long samples were equally and randomly assigned to autogenous nerve graft and poly(lactic-co-glycolic acid) conduit transplantation groups.
Replication of sciatic nerve injury models
In accordance with a previous study[34], models of sciatic nerve injury were replicated by cutting the sample to make a 10-mm-long defect by a highly qualified physician with a S-5 aseptic plastic-handled scalpel (Huaian Lianhe Yikang Medical Supplies Co., Ltd., Xuyi, Jiangsu Province, China).
Repair of sciatic nerve injury
The samples were separately bridged with autogenous nerve graft and poly(lactic-co-glycolic acid) conduit using 7-0 nylon thread (Qingdao Nesco Medical Co., Ltd., Qingdao, Shandong Province, China). Eight stitches were sutured in each sample at the same site.
Tensile test
Tensile testing was conducted on an Autocontrol Electronic Universal Testing Machine (Changchun Research Institute for Testing, Changchun, Jilin Province, China). The length and diameter of each sample were measured under a reading microscope (Third Optical Instrument Factory, Changchun, Jilin Province, China). In the autogenous nerve graft group, the sample was 40 mm long, 11.1–11.3 mm outer diameter and 9.1–9.2 mm inner diameter. In the poly(lactic-co-glycolic acid) conduit graft group, the sample was 40 mm long, 11.0–11.2 mm outer diameter and 9.0–9.3 mm inner diameter. In accordance with a previously published method[49], each sample was subjected to loading and unloading 10 times for presetting. This experiment simulated normal human body temperature of 36.5 ± 1.0°C. The samples in the two groups were placed in the chuck of the testing machine, and underwent tension at 5 mm/min. After testing, the Autocontrol Electronic Universal Testing Machine automatically output maximum load, maximum stress, elastic limit strain, maximum strain, elastic limit load and elastic limit stress and stress-strain curve.
Histological observation
Cross sections of one sciatic nerve at the same site from the autogenous nerve graft and poly(lactic-co-glycolic acid) conduit transplantation groups were separately and randomly selected, prefixed in 4% glutaral, postfixed in 1% osmic acid, dehydrated in acetone, and clinically point dried, followed by vacuum coating with gold palladium by direct-current sputtering. Nerve cross sections were observed under a field emission scanning electron microscope (Carl Zeiss, Jena, Germany) to compare the changes in nerve cells, myelin sheaths, axons and basilar membranes.
Statistical analysis
The data were analyzed using SPSS 16.0 software (SPSS, Chicago, IL, USA) and expressed as mean ± SD. One-way analysis of variance was used. Intergroup comparison was performed using Sceffe method. A value of P < 0.05 was considered statistically significant.
Footnotes
Funding: This project was funded by the Technology Development Project of Jilin Province, No. 20110492.
Conflict of Interest: None declared.
Ethical approval: This study was approved by the Ethics Committee, College of Medicine, Jilin University, China.
(Reviewed by Wallace M, Frenchman B, Zheng XY, Chen ZG)
(Edited by Yu J, Qiu Y, Li CH, Song LP, Liu WJ, Zhao M)
REFERENCES
- [1].Wang JY, Liu XL, Zhu JK, et al. Study of planting isogenous Schwann cells into chemical extracted allogenous nerve in vitro. Zhonghua Xianwei Waike Zazhi. 2002;25(3):189–191. [Google Scholar]
- [2].Zhang KF, Ou XC, Yang ZY, et al. Repair of sciatic nerve gap of rats with chitosan tube combined with basic fibroblast growth factor. Zhongguo Kangfu Lilun yu Shijian. 2008;14(12):1133–1135. [Google Scholar]
- [3].Lu L, Peter SJ, Lyman MD, et al. In vitro and in vivo degradation of porous poly(DL-lactic-co-glycolic acid) foams. Biomaterials. 2000;21(18):1837–1845. doi: 10.1016/s0142-9612(00)00047-8. [DOI] [PubMed] [Google Scholar]
- [4].Liu Y, Ruan JM, Zhang HB, et al. Thermal stability for Poly L-lactide(PLLA) Fenmo Yejin Cailiao Kexue yu Gongcheng. 2006;11(6):367–371. [Google Scholar]
- [5].Xiang H, Fei XS, Lu QP, et al. Research on concentration-viscosity of PLGA/TCP for bone tissue engineering. Cailiao Daobao. 2004;18(7):93–95. [Google Scholar]
- [6].Zhang Y, Reddy VJ, Wong SY, et al. Enhanced biomineralization in osteoblasts on a novel electrospun biocomposite nanofibrous substrate of hydroxyapatite/collagen/ chitosan. Tissue Eng Part A. 2010;16(6):1949–1960. doi: 10.1089/ten.TEA.2009.0221. [DOI] [PubMed] [Google Scholar]
- [7].Lu JZ, Jiang JJ, Xu JG, et al. Effects of chitosan-collagen betamethasone dipropionate film on nerve scarring and regeneration of peripheral nerves in rats following injuries. Shenjing Sunshang yu Gongneng Chongjian. 2011;6(4):241–244. [Google Scholar]
- [8].Meng H, Li M, You F, et al. Assessment of processed human amniotic membrane as a protective barrier in rat model of sciatic nerve injury. Neurosci Lett. 2011;496(1):48–53. doi: 10.1016/j.neulet.2011.03.090. [DOI] [PubMed] [Google Scholar]
- [9].Cheng FC, Tai MH, Sheu ML, et al. Enhancement of regeneration with glia cell line-derived neurotrophic factor-transduced human amniotic fluid mesenchymal stem cells after sciatic nerve crush injury. J Neurosurg. 2010;112(4):868–879. doi: 10.3171/2009.8.JNS09850. [DOI] [PubMed] [Google Scholar]
- [10].Madurantakam PA, Cost CP, Simpson DG, et al. Science of nanofibrous scaffold fabrication: strategies for next generation tissue-engineering scaffolds. Nanomedicine (Lond) 2009;4(2):193–206. doi: 10.2217/17435889.4.2.193. [DOI] [PubMed] [Google Scholar]
- [11].Zhang P, Hong Z, Yu T, et al. In vivo mineralization and osteogenesis of nanocomposite scaffold of poly(lactide -co-glycolide) and hydroxyapatite surface-grafted with poly(L-lactide) Biomaterials. 2009;30(1):58–70. doi: 10.1016/j.biomaterials.2008.08.041. [DOI] [PubMed] [Google Scholar]
- [12].Yang YF, Zhao YH, Tang GW, et al. In vitro degradation of porous poly(l-lactide-co-glycolide)/β-tricalcium phosphate (PLGA/β-TCP) scaffolds under dynamic and static conditions. Polym Degrad Stab. 2008;93(8):1838–1845. [Google Scholar]
- [13].Prasad BR, Brook MA, Smith T, et al. Controlling cellular activity by manipulating silicone surface roughness. Colloids Surf B Biointerfaces. 2010;78(2):237–242. doi: 10.1016/j.colsurfb.2010.03.006. [DOI] [PubMed] [Google Scholar]
- [14].Petrie Aronin CE, Sadik KW, Lay AL, et al. Comparative effects of scaffold pore size, pore volume, and total void volume on cranial bone healing patterns using microsphere-based scaffolds. J Biomed Mater Res A. 2009;89(3):632–641. doi: 10.1002/jbm.a.32015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hu J, Feng K, Liu X, et al. Chondrogenic and osteogenic differentiations of human bone marrow-derived mesenchymal stem cells on a nanofibrous scaffold with designed pore network. Biomaterials. 2009;30(28):5061–5067. doi: 10.1016/j.biomaterials.2009.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kadam SS, Sudhakar M, Nair PD, et al. Reversal of experimental diabetes in mice by transplantation of neo-islets generated from human amnion-derived mesenchymal stromal cells using immuno-isolatory macrocapsules. Cytotherapy. 2010;12(8):982–991. doi: 10.3109/14653249.2010.509546. [DOI] [PubMed] [Google Scholar]
- [17].Chen Z, Lu XC, Shear DA, et al. Synergism of human amnion-derived multipotent progenitor (AMP) cells and a collagen scaffold in promoting brain wound recovery: pre-clinical studies in an experimental model of penetrating ballistic-like brain injury. Brain Res. 2011;1368:71–81. doi: 10.1016/j.brainres.2010.10.028. [DOI] [PubMed] [Google Scholar]
- [18].Wolford LM, Rodrigues DB. Autogenous grafts/allografts/ conduits for bridging peripheral trigeminal nerve gaps. Atlas Oral Maxillofac Surg Clin North Am. 2011;19(1):91–107. doi: 10.1016/j.cxom.2010.11.008. [DOI] [PubMed] [Google Scholar]
- [19].Karcher DM, Fleming-Waddell JN, Applegate TJ. Developmental changes in insulin-like growth factor (IGF)-I and -II mRNA abundance in extra-embryonic membranes and small intestine of avian embryos. Growth Horm IGF Res. 2009;19(1):31–42. doi: 10.1016/j.ghir.2008.05.003. [DOI] [PubMed] [Google Scholar]
- [20].Dong W, Chen H, Yang X, et al. Treatment of intracerebral haemorrhage in rats with intraventricular transplantation of human amniotic epithelial cells. Cell Biol Int. 2010;34(6):573–577. doi: 10.1042/CBI20090248. [DOI] [PubMed] [Google Scholar]
- [21].O’Neill AC, Randolph MA, Bujold KE, et al. Preparation and integration of human amnion nerve conduits using a light-activated technique. Plast Reconstr Surg. 2009;124(2):428–437. doi: 10.1097/PRS.0b013e3181af010c. [DOI] [PubMed] [Google Scholar]
- [22].Ferreira-da-Silva FW, da Silva-Alves KS, Lemos-Dos-Santos M, et al. n5-STZ diabetic model develops alterations in sciatic nerve and dorsal root ganglia neurons of Wistar rats. ISRN Endocrinol 2013. 2013 doi: 10.1155/2013/638028. 638028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Vigneswara V, Berry M, Logan A, et al. Caspase-2 is upregulated after sciatic nerve transection and its inhibition protects dorsal root ganglion neurons from apoptosis after serum withdrawal. PLoS One. 2013;8(2):e57861. doi: 10.1371/journal.pone.0057861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Li S, Liu Q, Wang Y, et al. Differential gene expression profiling and biological process analysis in proximal nerve segments after sciatic nerve transection. PLoS One. 2013;8(2):e57000. doi: 10.1371/journal.pone.0057000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Sertoz N, Deniz MN, Ayanoglu HO. Relationship between glycosylated hemoglobin level and sciatic nerve block performance in diabetic patients. Foot Ankle Int. 2013;34(1):85–90. doi: 10.1177/1071100712460366. [DOI] [PubMed] [Google Scholar]
- [26].Wu J, Huang DY, Cheng J, et al. Effects of electrical stimulation of sciatic nerve on synaptic plasticity of spinal dorsal horn, hippocampal CA1 region and spinal c-fos, hippocampal CA1 region expression in neonatal rats. Zhonghua Yi Xue Za Zhi. 2012;92(41):2938–2942. [PubMed] [Google Scholar]
- [27].Kyriacou S, Pastides PS, Singh VK, et al. Exploration and neurolysis for the treatment of neuropathic pain in patients with a sciatic nerve palsy after total hip replacement. Bone Joint J. 2013;95-B(1):20–22. doi: 10.1302/0301-620X.95B1.29740. [DOI] [PubMed] [Google Scholar]
- [28].Ugrenovic SZ, Jovanovic ID, Kovacevic P, et al. Similarities and dissimilarities of the blood supplies of the human sciatic, tibial, and common peroneal nerves. Clin Anat. doi: 10.1002/ca.22135. in press. [DOI] [PubMed] [Google Scholar]
- [29].Zamir M, Twynstra J, Vercnocke AJ, et al. Intrinsic microvasculature of the sciatic nerve in the rat. J Peripher Nerv Syst. 2012;17(4):377–384. doi: 10.1111/j.1529-8027.2012.00435.x. [DOI] [PubMed] [Google Scholar]
- [30].Sun G, Li Z, Wang X, et al. Modulation of MAPK and Akt signaling pathways in proximal segment of injured sciatic nerves. Neurosci Lett. 2013;534:205–210. doi: 10.1016/j.neulet.2012.12.019. [DOI] [PubMed] [Google Scholar]
- [31].Fitó F, Casbas JL, Barbal F, et al. The “breathing nerve” sign. Subepineural puncture of the sciatic nerve in the popliteal fossa. Rev Esp Anestesiol Reanim. doi: 10.1016/j.redar.2012.11.005. in press. [DOI] [PubMed] [Google Scholar]
- [32].Yu B, Zhou S, Hu W, et al. Altered long noncoding RNA expressions in dorsal root ganglion after rat sciatic nerve injury. Neurosci Lett. 2013;534:117–122. doi: 10.1016/j.neulet.2012.12.014. [DOI] [PubMed] [Google Scholar]
- [33].Zhang ZJ, Zhang JP, Li N, et al. Control of PLGA on the morphology and mechanical property of zein nanofiber membrane. Zhejiang Ligong Daxue Xuebao. 2010;27(3):343–347. [Google Scholar]
- [34].Peng CG, Zhang Q, Yang Q, et al. Strain and stress variations in the human amniotic membrane and fresh corpse autologous sciatic nerve anastomosis in a model of sciatic nerve injury. Neural Regen Res. 2012;7(23):1779–1785. doi: 10.3969/j.issn.1673-5374.2012.23.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Hou SY, Zhang HY, Quan DP, et al. Tissue-engineered peripheral nerve grafting by differentiated bone marrow stromal cells. Neuroscience. 2006;140(1):101–110. doi: 10.1016/j.neuroscience.2006.01.066. [DOI] [PubMed] [Google Scholar]
- [36].Schakenraad JM, Nieuwenhuis P, Molenaar I, et al. In vivo and in vitro degradation of glycine/DL-lactic acid copolymers. J Biomed Mater Res. 1989;23(11):1271–1288. doi: 10.1002/jbm.820231105. [DOI] [PubMed] [Google Scholar]
- [37].Hu LM, Piao ZX, Wang Q, et al. Mechanism of rat sciatic nerve regeneration induced by human hair keratin. Nan Fang Yi Ke Da Xue Xue Bao. 2008;28(7):1136–1140. [PubMed] [Google Scholar]
- [38].Li SY, Wang SD, Zhang YS, et al. The Structure and biomechanical properties of the electrospun PLGA tubular scaffold. Hecheng Xianwei. 2009;29(11):22–30. [Google Scholar]
- [39].Zhao L, He CG, Gao YJ, et al. Study on influence of copolymer compositions of PLGA on properties of scaffolds. Zhongguo Shengwu Gongcheng Zazhi. 2008;28(5):22–28. [Google Scholar]
- [40].Zhang JF, Sun X. Mechanical properties of poly(lactic acid)/starch composites compatibilized by maleic anhydride. Biomacromolecules. 2004;5(4):1446–1151. doi: 10.1021/bm0400022. [DOI] [PubMed] [Google Scholar]
- [41].Li SY. Prospect of PLGA scaffolds. Guowai Sichou. 2009;23(2):29–31. [Google Scholar]
- [42].Stitzel J, Liu J, Lee SJ, et al. Controlled fabrication of a biological vascular substitute. Biomaterials. 2006;27(7):1088–1094. doi: 10.1016/j.biomaterials.2005.07.048. [DOI] [PubMed] [Google Scholar]
- [43].Zhang JX, Xu ZW, Chang F. Experimental research of tissue-engineerring artificial bone in treating bone defect. Zhongguo Jiaoxing Waike Zazhi. 2009;17(16):1258–1261. [Google Scholar]
- [44].Hollister SJ. Porous scaffold design for tissue engineering. Nat Mater. 2005;4(7):518–524. doi: 10.1038/nmat1421. [DOI] [PubMed] [Google Scholar]
- [45].Li XK, Cai SX, Liu B, et al. Characteristics of PLGA-gelatin complex as potential artificial nerve scaffold. Colloids Surf B Biointerfaces. 2007;57(2):198–203. doi: 10.1016/j.colsurfb.2007.02.010. [DOI] [PubMed] [Google Scholar]
- [46].Hu X, Shen H, Yang F, et al. Preparation and cell affinity of microtubular orientation-structured PLGA(70/30) blood vessel scaffold. Biomaterials. 2008;29(21):3128–3136. doi: 10.1016/j.biomaterials.2008.04.010. [DOI] [PubMed] [Google Scholar]
- [47].Pan H, Jiang H, Chen W. The biodegradability of electrospun Dextran/PLGA scaffold in a fibroblast/macrophage co-culture. Biomaterials. 2008;29(11):1583–1592. doi: 10.1016/j.biomaterials.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Cheng HP, He GB, Liu ZJ, et al. Axial biomechanical properties of the rat scistic nerve in vivo. Disan Junyi Daxue Xuebao. 1995;17(3):239–240. [Google Scholar]
- [49].Yu B, Gao M, Li XY. Creep properties of the left coronary artery in aging spontaneously hypertensive rats versus Sprague Dawley rats. Zhongguo Zuzhi Gongcheng Yanjiu yu Linchuang Kangfu. 2012;16(37):6902–6905. [Google Scholar]


