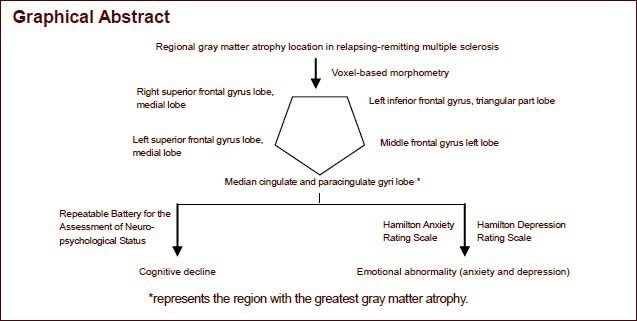
Keywords: neural regeneration, neurodegeneration, MRI, relapsing-remitting multiple sclerosis, gray matter atrophy, cognitive, mood, voxel-based morphometry, neuroregeneration
Abstract
In multiple sclerosis, gray matter atrophy is extensive, and cognitive deficits and mood disorders are frequently encountered. It has been conjectured that focal atrophy is associated with emotional decline. However, conventional MRI has revealed that the pathological characteristics cannot fully account for the mood disorders. Moreover, there is no correlation between cognitive disorders and MRI results in clinically isolated syndromes or in cases of definite multiple sclerosis. In this case-control study, voxel-based morphometric analysis was performed on 11 subjects with relapsing-remitting multiple sclerosis, and the results show that these patients exhibit gray matter atrophy. Moreover, the gray matter atrophy in the superior and middle gyri of the right frontal lobe in patients with multiple sclerosis was correlated with scores from the Hamilton Anxiety Rating Scale. The scores obtained with the Repeatable Battery for the Assessment of Neuropsychological Status were associated with gray matter atrophy in the middle gyrus of the left frontal lobe, the superior and middle gyrus of the right frontal lobe, the middle gyrus of the left cingulate, the superior and middle gyri of the left frontal lobe, and the triangular area of the left frontal lobe. However, there was no statistical significance. These findings suggest that the cingulate and frontal cortices of the nant hemisphere are the most severely atrophic regions of the brain, and this atrophy is correlated with cognitive decline and emotional abnormalities.
INTRODUCTION
Multiple sclerosis is a chronic inflammatory demyelinating disease of the central nervous system. It is widespread in western populations, but China is a low-incidence country[1]. The clinical and genetic features of multiple sclerosis are heterogeneous in the Eastern and Western populations. Focal cortical gray matter atrophy is present in all disease phenotypes, including progressive multiple sclerosis, “benign” multiple sclerosis and clinically isolated syndromes suggestive of multiple sclerosis[2,3,4,5]. Although cognitive deficits are frequently encountered in multiple sclerosis[2], they have a complex and multifactorial etiology that cannot be adequately explained by pathological features recorded on conventional MRI[6], and no significant association has been found between cognitive impairment and routine MRI measurements in clinically isolated syndromes or newly diagnosed multiple sclerosis patients[7,8]. Patients with low cognitive status exhibit a unique distribution of gray matter atrophy[3,9], which is most prominent in the frontal and temporal areas of the brain[10]. Mood disorders are often accompanied with multiple sclerosis[11,12]. Evidence indicates that the early focal atrophy may be associated with clinical progression and neuropsychological decline[13].
The effects of focal cortical gray matter atrophy on clinical presentation, including cognitive and emotional scores, have not yet been fully established. Therefore, additional research is required to clarify the relationship between them. Furthermore, there are very few reports examining whether gray matter atrophy is present in Chinese multiple sclerosis patients, and it is unclear whether it is extensive or focal. In recent years, the assessment of brain atrophy by MRI has been given great importance by foreign researchers interested in understanding the pathogenesis of the disease. The development of voxel-based morphometry has made it possible to accurately measure cerebral atrophy. This method has proven to be the most reliable and effective means of assessing gray matter atrophy.
For many years, motor and sensory disorders received widespread attention because they are easily identified[14,15]. However, non-motor deficits, such as cognitive impairment and abnormal emotion, are not only very common, but also seriously affect the patient's quality of life[16]. Therefore, it is not sufficient to evaluate the disability status of patients with multiple sclerosis using only the Expanded Disability Status Scale. Clinicians should also focus on the value of cognitive function in the understanding of multiple sclerosis. Indeed, greater attention has been given to cognitive function in recent years. Effective and active measures should be taken to lessen the cognitive functional decline. Early drug intervention and treatment in the progression of the disease have great potential in preventing or delaying the onset of cognitive impairment and emotional disorders[17].
In this study, we used the voxel-based morphometry method to accurately measure gray matter atrophy in eleven Chinese patients with multiple sclerosis to better understand the characteristics of gray matter atrophy. Furthermore, our study is the first to apply the Repeatable Battery for the Assessment of Neuropsychological Status scale in Chinese patients with relapsing-remitting multiple sclerosis to evaluate cognitive function. This study should provide insight into the relationship between gray matter atrophy and cognition and emotion impairments in relapsing-remitting multiple sclerosis.
RESULTS
Quantitative analysis of subjects
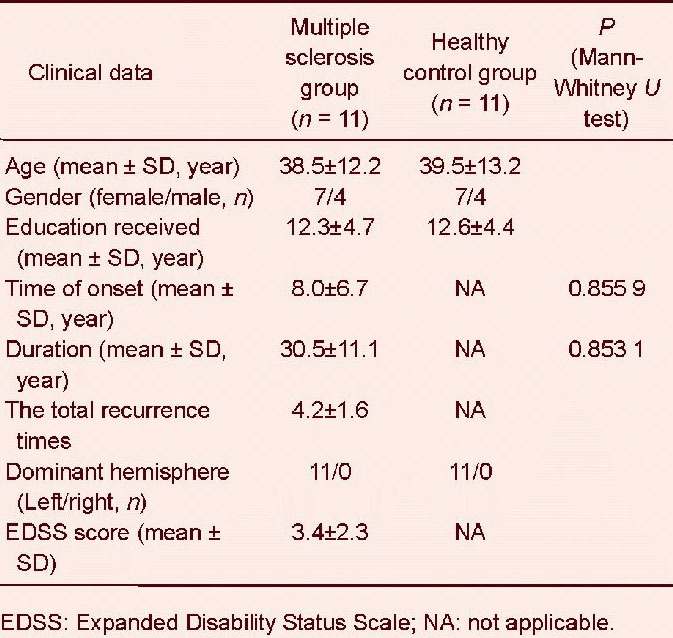
A total of 11 patients with relapsing-remitting multiple sclerosis and 11 healthy controls were enrolled and entered into the final analysis. Demographic and clinical data are displayed in Table 1.
Table 1.
Clinical data for the multiple sclerosis and healthy control groups
Gray matter atrophy in relapsing-remitting multiple sclerosis patients
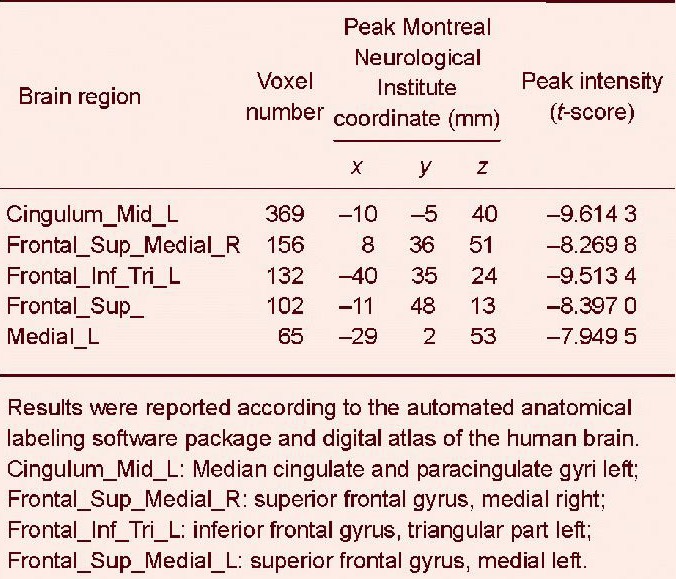
Voxel-based morphometric analysis showed that the volume of the gray matter in the multiple sclerosis group was significantly reduced compared with that in the healthy control group, most significantly in the cingulate and frontal cortices of the dominant hemisphere. The atrophic brain regions in the automated anatomical labeling template included the median cingulate and paracingulate gyri, the superior frontal gyrus, the medial inferior frontal gyrus, the triangular part, and the middle frontal gyrus (Figure 1, Table 2).
Figure 1.
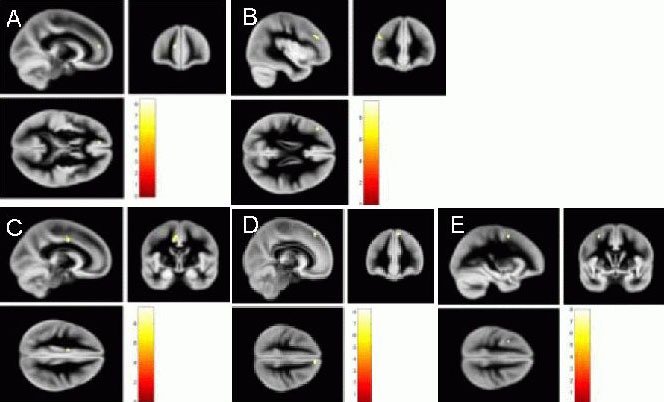
Gray matter atrophy in relapsing-remitting multiple sclerosis patients by MRI.
Voxel-based morphometric analysis showed that the volume of the gray matter in the multiple sclerosis group was significantly reduced compared with the healthy control group. Map of yellow dots represents significant parts of gray matter atrophy. The atrophy was most significant in the cingulate and frontal cortices of the dominant hemisphere. The atrophic brain regions in the automated anatomical labeling template included the following lobes: Cingulum_Mid_L (A): Median cingulate and paracingulate gyri left; Frontal_Sup_Medial_R (B): Superior frontal gyrus, medial right; Frontal_Inf_Tri_L (C): Inferior frontal gyrus, triangular part left; Frontal_Mid_L (D): Middle frontal gyrus left, and Frontal_Sup_Medial _L (E): Superior frontal gyrus, medial left.
Table 2.
Gray matter volume reduction in voxel-based morphometry based on T1 (False Discovery Rate correction q-value = 0.01)
Cognitive decline and emotional abnormality in relapsing-remitting multiple sclerosis patients
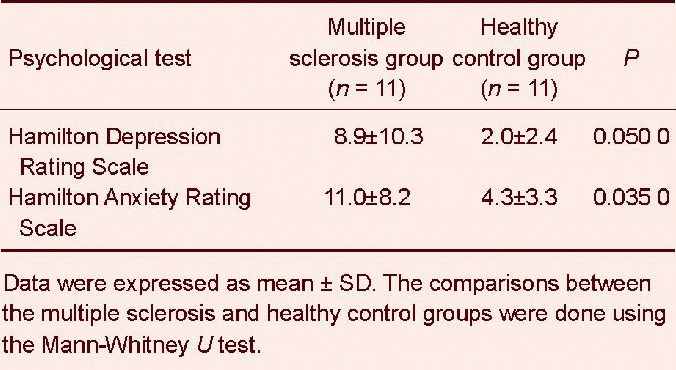
A statistically significant lower Repeatable Battery for the Assessment of Neuropsychological Status score was observed in the multiple sclerosis group compared with the healthy control group (Mann-Whitney U test, P = 0.001). The analysis of the items of the Repeatable Battery for the Assessment of Neuropsychological Status revealed that the multiple sclerosis patients had deficits in immediate memory, visuospatial skills and attention (Table 3). The existence of mood disorders in the multiple sclerosis patients was suggested by the higher scores in the Hamilton Anxiety Rating Scale (P = 0.050) and the Hamilton Depression Rating Scale (P = 0.035) compared with the healthy control group (Table 4).
Table 3.
Cognitive data from the Repeatable Battery for the Assessment of Neuropsychological Status items in multiple sclerosis patients and healthy controls
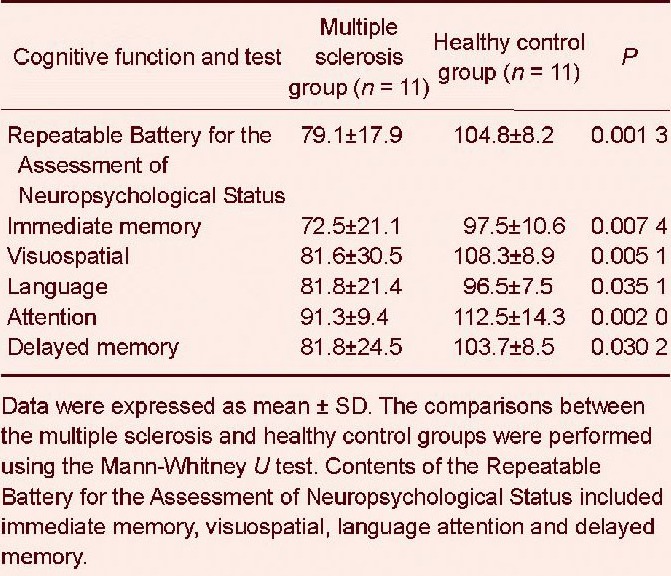
Table 4.
Psychological data for multiple sclerosis patients and healthy controls
Correlation between regional gray matter atrophy and cognitive decline/emotional abnormalities in patients with relapsing-remitting multiple sclerosis
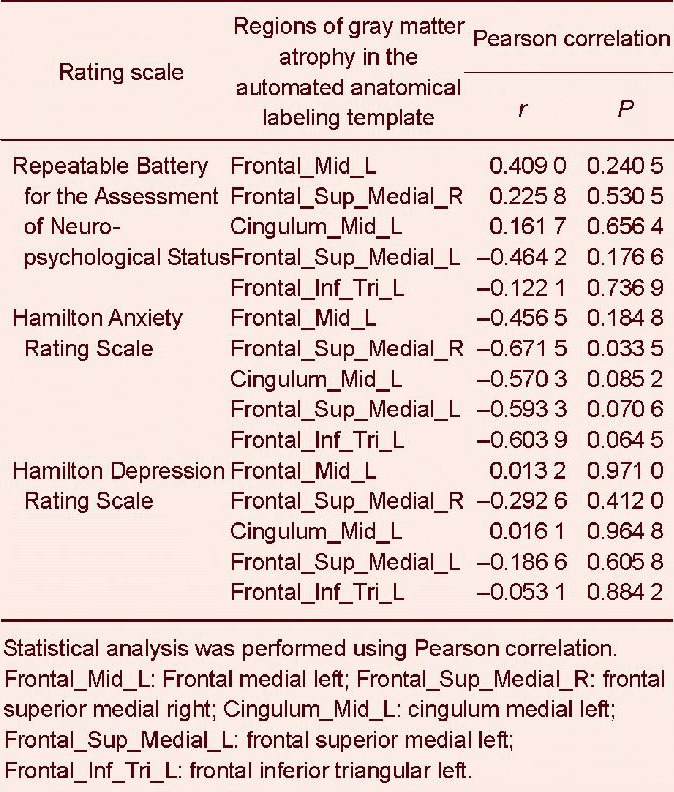
There was a significant association between gray matter atrophy in the superior frontal gyrus, medial right, and the scores in the Hamilton Anxiety Rating Scale (P = 0.033 5, Pearson correlation). Cognitive testing also revealed a trend towards a correlation between the Repeatable Battery for the Assessment of Neuropsychological Status items and regional atrophy of the gray matter, especially in the frontal medial left, frontal superior medial right, cingulum medial left, frontal superior medial left, and frontal inferior triangular left lobes, but without statistical significance (P = 0.06–0.65; Table 5).
Table 5.
Correlation between cognitive/emotional scores and gray matter atrophy in multiple sclerosis patients
DISCUSSION
Previous reports have conclusively established that there is a relationship between regional gray matter atrophy and cognitive impairment[2,8,9]. However, relapsing-remitting multiple sclerosis has been less investigated as it has the lowest rate of gray matter atrophy of all multiple sclerosis types. The results of this study not only confirm the presence of gray matter atrophy in relapsing-remitting multiple sclerosis, but suggest, for the first time, that the cingulate and frontal cortices of the dominant hemisphere are the most severely atrophied regions of the brain. Earlier research on gray matter atrophy in multiple sclerosis only included the frontal and temporal lobes and several deep gray matter foci[9,18,19]. Moreover, the present study demonstrates that gray matter atrophy in the cingulate and frontal cortices of the dominant hemisphere correlates with cognitive decline and emotional abnormalities. Previous studies have also shown a relationship between mood disorders and gray matter atrophy. Koolschijn's study of early-onset depression suggested that prefrontal and limbic system atrophy is a neural hallmark of senile depression[20]. The medial temporal lobe, prefrontal cortex and anterior cingulate cortex participate in emotional regulation[21].
In the present study, we found that there is no relationship between selective gray matter atrophy and loss of motor function in relapsing-remitting multiple sclerosis, suggesting that gray matter atrophy is not correlated with atrophy of critical white matter tracts or functional damage[20,21,22,23,24]. Longitudinal studies are now warranted to determine how white matter and gray matter atrophy evolve and to clarify their relationship with the progression of the disease[20,21,22,23,24]. Although white matter and gray matter atrophy can both occur in the forebrain and deep nuclei, the gray matter atrophy spectrum analysis showed that gray matter loss affects mainly the anterior portions of the brain, whereas white matter atrophy is also present in the brainstem and cerebellum, where gray matter loss is not seen[8,9,25]. The demonstration of a discrepancy between gray matter and white matter loss in infratentorial regions of patients with relapsing-remitting multiple sclerosis is in line with the pathological findings in these patients, which reveals relative axonal and neuronal preservation in the affected cerebellar cortical regions[26].
Previous studies show that multiple sclerosis patients exhibit deficits in memory, attention, information processing and executive function[27], but that memory recall and language comprehension are preserved[28]. This suggests that cognitive impairment in multiple sclerosis is not uniform and that the etiology is complex. Previous studies have suggested that diffuse subtle brain damage results in overall cognitive decline, whereas focal lesions are responsible for the specific cognitive impairments. Among the various types of multiple sclerosis, relapsing- remitting multiple sclerosis presents with the mildest cognitive decline, suggesting that its anatomical basis differs from the progressive forms of the disease. Our study suggests that the selective frontal and cingulate atrophic changes in the dominant hemisphere are likely the anatomical basis of the cognitive decline and emotional deficits in relapsing-remitting multiple sclerosis. In comparison, greater diffuse gray matter damage could be involved in primary progressive and secondary progressive multiple sclerosis. However, whether unique regional distribution patterns can be used to identify different clinical types of multiple sclerosis and neuromyelitis optica remains unclear. The present study also shows that there is no correlation between gray matter atrophy and disease course or the frequency of relapses. Gray matter atrophy may be present during the entire course of the disease, even at the early stage. This observation indicates the importance of early intervention for cognitive impairment in multiple sclerosis. As suggested by previous studies, treatment with donepezil and selective serotonin reuptake inhibitors[29,31], immunotherapeutic approaches[32,33] and disease-modifying drugs[28] seem to be advisable for preventing or delaying the development of cognitive and emotional impairment in multiple sclerosis.
Although gray matter atrophy is widespread in multiple sclerosis, conventional MRI techniques are not sensitive enough to detect mild or early gray matter atrophy. The voxel-based morphometry technology quantitatively detects the density differences of the brain tissue and allows the dynamic monitoring of gray matter atrophy during the course of the disease. In addition, the voxel-based morphometry method uses the Diffeomorphic Anatomical Registration Through Exponentiated Lie algebra algorithm, which improves data registration and provides an accurate localization of the structural damage[34]. It also encompasses an automated lesion-filling technique, which minimizes the effect of focal lesions on tissue segmentation[8,35]. Because brain atrophy detected by voxel-based morphometric analysis may be associated with manifestations of multiple sclerosis, the method may be used as a tool for assisting early prediction of the clinical features and prognosis of the disease.
Because of the limited number of cases and differences in some parameters between the healthy control and multiple sclerosis groups, the correlations between regional gray matter atrophy and cognitive decline did not exhibit statistical significance. Nonetheless, obvious trends towards correlations were observed. However, the pathophysiological basis of gray matter atrophy in multiple sclerosis remains unclear. Further studies with larger sample sizes are required to unravel the mechanisms underlying the selective gray matter atrophy in relapsing-remitting multiple sclerosis.
In conclusion, the present study provides important neuroimaging evidence for regional gray matter atrophy in relapsing-remitting multiple sclerosis patients, which is associated with cognitive and emotional abnormalities, even at the early stage of the disease. These findings also suggest that monitoring the evolution of gray matter damage is important for the prediction of disease course, response to therapy and prognosis in multiple sclerosis.
SUBJECTS AND METHODS
Design
A case-control imaging study.
Time and setting
The study was performed in the Department of Neurology and Imaging, the First Affiliated Hospital of Fujian Medical University in China from January to June 2011.
Subjects
We recruited patients and volunteers by word of mouth, and then they signed informed consent. Multiple sclerosis patients were enrolled from the Outpatient Department of the First Affiliated Hospital of Fujian Medical University in China from 2010 to 2011. Normal controls were the local residents. Study participants included eleven healthy volunteers and eleven relapsing-remitting multiple sclerosis patients. They were matched for sex, age and years of education. Inclusion criteria for patients were a diagnosis of relapsing-remitting multiple sclerosis (McDonald criteria 2010 revised)[36], aged 21–59 years and a disease duration of 2–24 years. Exclusion criteria included poor cooperativity and abnormal lesions on the T2WI in the control group. Subjects underwent regular follow-up visits, and would undergo detailed clinical and neuropsychological testing and a comprehensive MRI examination with a 3.0T magnet. Demographic and clinical data recorded included age, gender, age at disease onset, disease duration, and treatment. Disability was measured with the Expanded Disability Status Scale. In patients with relapsing-remitting multiple sclerosis, we also assessed the annualized relapse rate as a measure of clinical disease activity. Relapses were defined as the appearance or reappearance of at least one neurological symptom or the worsening of an old symptom attributed to multiple sclerosis that lasted for at least 24 hours and that was preceded by a relatively stable or improving neurological state of at least 30 days[36]. All patients with multiple sclerosis received therapy consisting of high-dose methylprednisolone of 1 000 mg daily for 3 days in the relapse phase, then the dosage was gradually reduced to a full stop in the following month. Two of the patients received a treatment course with interferon beta-1a (Rebif) therapy (44 μg injected subcutaneously three times a week; Merck Serono, Geneva, Switzerland) for 12–34 months in the stable phase of the disease. None of the patients received neuropsychological therapy.
Methods
Neuropsychological testing
All subjects underwent neuropsychological assessment and Expanded Disability Status Scale scoring[37] within a time frame of 1 month. Each subject underwent (1) 3T MRI screening to rule out the presence of active lesions or incidental abnormal findings in the healthy volunteers; (2) Evaluation of cognitive function with the Repeatable Battery for the Assessment of Neuropsychological Status[38] scale and evaluation of emotional status with the Hamilton Anxiety Rating Scale and the Hamilton Depression Rating Scale[39,40].
The Repeatable Battery for the Assessment of Neuropsychological Status, which is a brief neurocognitive battery with four alternate forms measuring immediate and delayed memory, attention, language and visuospatial skills, was administered to each subject. The testing session lasted approximately 25 minutes. Technically, the Repeatable Battery for the Assessment of Neuropsychological Status is a “pencil-and-paper” test, with only a stimulus booklet and record form being necessary for its administration and scoring. It is broadly used for clinical diagnostic purposes and is increasingly employed as an endpoint in clinical trials of medication that may affect neurocognitive status. The Chinese translated and validated Repeatable Battery for the Assessment of Neuropsychological Status, Hamilton Anxiety Rating Scale and Hamilton Depression Rating Scale tests were conducted by two neurologists. A quiet, sunny room was provided, and a neat desk was placed for testing. The Repeatable Battery for the Assessment of Neuropsychological Status test was carried out according to the instructions. The whole evaluation process took 25–30 minutes. The majority of participants completed the test in one session. The Hamilton Anxiety Rating Scale and the Hamilton Depression Rating Scale were used to assess the anxiety and depression status of the subjects by the two neurologists.
MRI data acquisition
MRI data were collected using a Siemens Verio 3T system (Siemens Healthcare, Erlangen, Germany). Sagittal T1-weighted images (repetition time/echo time = 2 000 ms/ 2.98 ms, flip angle = 9°, matrix = 240 × 256, field of view = 24 × 24 cm2, slice thickness/gap = 1 mm/0 mm, 176 slices covered the whole brain) were acquired.
Data preprocessing
Data preprocessing was partly carried out using the statistical parametric mapping software (SPM8, http://www.fil.ion.ucl.ac.uk/spm). Each 3D T1-weighted image was segmented using the Diffeomorphic Anatomical Registration Through Exponentiated Lie algebra SPM 8 toolbox[41]. The T1-weighted images were first segmented into gray matter, white matter and cerebrospinal fluid. The gray and white matter images were then imported into Diffeomorphic Anatomical Registration Through Exponentiated Lie algebra space for preprocessing. The segmented gray matter images of each participant were used to create a study-specific template for warping and normalization. The images underwent a non-linear transformation using a diffeomorphic registration algorithm[41] and were then transformed into Montreal Neurological Institute stereotactic space using the default International Consortium for Brain Mapping template. Prior to the statistical computations, the images were smoothed with an 8 mm full width at half maximum Gaussian filter to minimize individual anatomical variability and reduce the chance of false positives[42]. All images were reviewed before statistical analysis to ensure the quality of the segmentation process. The preprocessed images were used for voxel-wise statistical comparison.
Data analysis
The differences between multiple sclerosis patients and healthy controls in gray matter were evaluated using voxel-level random-effects analysis (two-sample t-tests controlling potentially confounding variables, such as age, gender, white matter and cerebrospinal fluid). Each parametric map was statistically assessed at a threshold P value < 0.01 with a threshold extent of 20 voxels and with false discovery rate corrected for multiple comparisons. The Mann-Whitney U test was used to assess whether two independent samples of observations have equally large values. Pearson correlation was used to analyze the correlation between the parameters.
Research background: Cognitive dysfunction and emotional abnormalities appear during the early stages of multiple sclerosis. They may be used for disease surveillance and prognostic assessment, but the underlying mechanisms are unclear. Numerous international studies have suggested that gray matter atrophy may be involved in the development of cognitive and emotional impairment. The atrophic regions include the frontal lobe, temporal lobe and deep nuclei.
Research frontiers: This study examined the presence of gray matter atrophy and the relationship between neuropsychological and clinical characteristics in Chinese relapsing-remitting multiple sclerosis patients. It is the first study to use the Repeatable Battery for the Assessment of Neuropsychological Status scale to evaluate cognitive status in relapsing-remitting multiple sclerosis. Voxel-based morphometric analysis was performed to evaluate gray matter atrophy. The findings indicate that the regions with the greatest gray matter atrophy are the cingulate and frontal cortices of the dominant hemisphere. Furthermore, the regional gray matter atrophy was significantly associated with emotional abnormalities.
Clinical significance: This study examines gray matter atrophy in patients with relapsing-remitting multiple sclerosis to provide insight into the relationship between the cognitive and affective disorders and the neuroanatomical lesions, and to further our understanding of the neuropathological basis of the deficits.
Academic terminology: Voxel-based morphometry is a comprehensive, objective, unbiased brain structural imaging analysis technique, which can quantitatively analyze small changes in brain structure and find occult brain structural damage. It has been widely used in clinical research into brain damage, including the relationship between gray matter volume reduction and intelligence quotient in heart failure patients, brain structural research in Parkinson disease, cerebellar volume asymmetry in handedness, age and gender effects on brain anatomy, brain structure in multiple system atrophy and brain structural research in amyotrophic lateral sclerosis.
Peer review: The magnetic resonance imaging analysis and neuropsychological assessment demonstrate that regional gray matter atrophy is present in relapsing-remitting multiple sclerosis, and that it is significantly correlated with emotional disorder. This study has strong clinical significance.
Acknowledgments
We wish to thank Dr. Wu GR, from the University of Chengdu Electronic Science and Technology in China, for data processing, and Dr. Zhuang ZS, from the Department of Psychology, the First Affiliated Hospital of Fujian Medical University in China, for assistance in psychological evaluation.
Footnotes
Conflict of Interest: None declared.
Ethical approval: The study was approved by the Ethics Committee of Fujian Medical University in China and was in accordance with the Declaration of Helsinki. All participants provided informed written consent.
(Reviewed by Patel B, Haase R, Li L, Wang P)
(Edited by Yu J, Qiu Y, Li CH, Song LP, Liu WJ, Zhao M)
REFERENCES
- [1].Wasay M, Khatri IA, Khealani B, et al. MS in Asian countries. Int MS J. 2006;13(2):58–65. [PubMed] [Google Scholar]
- [2].Feinstein A, Youl B, Ron M. Acute optic neuritis. A cognitive and magnetic resonance imaging study. Brain. 1992;115(Pt 5):1403–1415. doi: 10.1093/brain/115.5.1403. [DOI] [PubMed] [Google Scholar]
- [3].Giorgio A, De Stefano N. Cognition in multiple sclerosis: relevance of lesions, brain atrophy and proton MR spectroscopy. Neurol Sci. 2010;31(Suppl 2):S245–248. doi: 10.1007/s10072-010-0370-x. [DOI] [PubMed] [Google Scholar]
- [4].De Stefano N, Matthews PM, Filippi M, et al. Evidence of early cortical atrophy in MS: relevance to white matter changes and disability. Neurology. 2003;60(7):1157–1162. doi: 10.1212/01.wnl.0000055926.69643.03. [DOI] [PubMed] [Google Scholar]
- [5].Khalil M, Enzinger C, Langkammer C, et al. Cognitive impairment in relation to MRI metrics in patients with clinically isolated syndrome. Mult Scler. 2011;17(2):173–180. doi: 10.1177/1352458510384009. [DOI] [PubMed] [Google Scholar]
- [6].Swirsky-Sacchetti T, Mitchell DR, Seward J, et al. Neuropsychological and structural brain lesions in multiple sclerosis: a regional analysis. Neurology. 1992;42(7):1291–1295. doi: 10.1212/wnl.42.7.1291. [DOI] [PubMed] [Google Scholar]
- [7].Achiron A, Barak Y. Cognitive impairment in probable multiple sclerosis. J Neurol Neurosurg Psychiatry. 2003;74(4):443–446. doi: 10.1136/jnnp.74.4.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Glanz BI, Holland CM, Gauthier SA, et al. Cognitive dysfunction in patients with clinically isolated syndromes or newly diagnosed multiple sclerosis. Mult Scler. 2007;13(8):1004–1010. doi: 10.1177/1352458507077943. [DOI] [PubMed] [Google Scholar]
- [9].Prinster A, Quarantelli M, Orefice G, et al. Grey matter loss in relapsing-remitting multiple sclerosis: a voxel-based morphometry study. Neuroimage. 2006;29(3):859–867. doi: 10.1016/j.neuroimage.2005.08.034. [DOI] [PubMed] [Google Scholar]
- [10].Sailer M, Fischl B, Salat D, et al. Focal thinning of the cerebral cortex in multiple sclerosis. Brain. 2003;126(Pt 8):1734–1744. doi: 10.1093/brain/awg175. [DOI] [PubMed] [Google Scholar]
- [11].Pepper CM, Krupp LB, Friedberg F, et al. A comparison of neuropsychiatric characteristics in chronic fatigue syndrome, multiple sclerosis, and major depression. J Neuropsychiatry Clin Neurosci. 1993;5(2):200–205. doi: 10.1176/jnp.5.2.200. [DOI] [PubMed] [Google Scholar]
- [12].Sullivan MJ, Weinshenker B, Mikail S, et al. Depression before and after diagnosis of multiple sclerosis. Mult Scler. 1995;1(2):104–108. doi: 10.1177/135245859500100208. [DOI] [PubMed] [Google Scholar]
- [13].Sastre-Garriga J, Ingle GT, Chard DT, et al. Gray and white matter volume changes in early primary progressive multiple sclerosis: a longitudinal study. Brain. 2005;128(Pt 6):1454–1460. doi: 10.1093/brain/awh498. [DOI] [PubMed] [Google Scholar]
- [14].Li DQ, Luo BY. Depression and anxiety disorders in multiple sclerosis patients. Zhongguo Shenjing MIanyi Xue yu Shenjing Bing Xue Zazhi. 2010;17(1):16–18. [Google Scholar]
- [15].Karlińska I, Selmaj K. Cognitive impairment in multiple sclerosis. Neurol Neurochir Pol. 2005;39(2):125–133. [PubMed] [Google Scholar]
- [16].Patti F. Cognitive impairment in multiple sclerosis. Mult Scler. 2009;15(1):2–8. doi: 10.1177/1352458508096684. [DOI] [PubMed] [Google Scholar]
- [17].Mandler RN. Neuromyelitis optica – Devic's syndrome, update. Autoimmun Rev. 2006;5(8):537–543. doi: 10.1016/j.autrev.2006.02.008. [DOI] [PubMed] [Google Scholar]
- [18].Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- [19].Houtchens MK, Benedict RH, Killiany R, et al. Thalamic atrophy and cognition in multiple sclerosis. Neurology. 2007;69(12):1213–1223. doi: 10.1212/01.wnl.0000276992.17011.b5. [DOI] [PubMed] [Google Scholar]
- [20].Li HY, Zhang N, Zhang MY, et al. Comparison of brain atrophy patterns between first-episode late-onset depression and mild cognitive impairment. Zhonghua Laonian Xin Nao Xueguan Bing Zazhi. 2011;13(7):587–590. [Google Scholar]
- [21].Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4(6):215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- [22].Koolschijn PC, van Haren NE, Schnack HG, et al. Cortical thickness and voxel-based morphometry in depressed elderly. Eur Neuropsychopharmacol. 2010;20(6):398–404. doi: 10.1016/j.euroneuro.2010.02.010. [DOI] [PubMed] [Google Scholar]
- [23].Phan KL, Wager T, Taylor SF, et al. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002;16(2):331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- [24].Riccitelli G, Rocca MA, Pagani E, et al. Mapping regional grey and white matter atrophy in relapsing-remitting multiple sclerosis. Mult Scler. 2012;18(7):1027–1037. doi: 10.1177/1352458512439239. [DOI] [PubMed] [Google Scholar]
- [25].Ceccarelli A, Rocca MA, Pagani E, et al. A voxel-based morphometry study of grey matter loss in MS patients with different clinical phenotypes. Neuroimage. 2008;42(1):315–322. doi: 10.1016/j.neuroimage.2008.04.173. [DOI] [PubMed] [Google Scholar]
- [26].Kutzelnigg A, Faber-Rod JC, Bauer J, et al. Widespread demyelination in the cerebellar cortex in multiple sclerosis. Brain Pathol. 2007;17(1):38–44. doi: 10.1111/j.1750-3639.2006.00041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Beatty WW, Monson N. Problem solving by patients with multiple sclerosis: comparison of performance on the Wisconsin and California Card Sorting Tests. J Int Neuropsychol Soc. 1996;2(2):134–140. doi: 10.1017/s1355617700000989. [DOI] [PubMed] [Google Scholar]
- [28].Amato MP, Portaccio E, Goretti B, et al. Cognitive impairment in early stages of multiple sclerosis. Neurol Sci. 2010;31(Suppl 2):S211–214. doi: 10.1007/s10072-010-0376-4. [DOI] [PubMed] [Google Scholar]
- [29].Greene YM, Tariot PN, Wishart H, et al. A 12-week, open trial of donepezil hydrochloride in patients with multiple sclerosis and associated cognitive impairments. J Clin Psychopharmacol. 2000;20(3):350–356. doi: 10.1097/00004714-200006000-00010. [DOI] [PubMed] [Google Scholar]
- [30].Korostil M, Feinstein A. Anxiety disorders and their clinical correlates in multiple sclerosis patients. Mult Scler. 2007;13(1):67–72. doi: 10.1177/1352458506071161. [DOI] [PubMed] [Google Scholar]
- [31].Amato MP, Portaccio E, Zipoli V. Are there protective treatments for cognitive decline in MS? J Neurol Sci. 2006;245(1-2):183–186. doi: 10.1016/j.jns.2005.07.017. [DOI] [PubMed] [Google Scholar]
- [32].Bastianello S, Giugni E, Amato MP, et al. Changes in magnetic resonance imaging disease measures over 3 years in mildly disabled patients with relapsing-remitting multiple sclerosis receiving interferon β-1a in the COGnitive Impairment in MUltiple Sclerosis (COGIMUS) study. BMC Neurol. 2011;11:125. doi: 10.1186/1471-2377-11-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Lang C, Reiss C, Mäurer M. Natalizumab may improve cognition and mood in multiple sclerosis. Eur Neurol. 2012;67(3):162–166. doi: 10.1159/000334722. [DOI] [PubMed] [Google Scholar]
- [34].Chard DT, Jackson JS, Miller DH, et al. Reducing the impact of white matter lesions on automated measures of brain gray and white matter volumes. J Magn Reson Imaging. 2010;32(1):223–228. doi: 10.1002/jmri.22214. [DOI] [PubMed] [Google Scholar]
- [35].Battaglini M, Jenkinson M, De Stefano N. Evaluating and reducing the impact of white matter lesions on brain volume measurements. Hum Brain Mapp. 2012;33(9):2062–2071. doi: 10.1002/hbm.21344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69(2):292–302. doi: 10.1002/ana.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS) Neurology. 1983;33(11):1444–1452. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- [38].Yang GG, Tian J, Tan YL, et al. The application performance of the Repeatable Battery for the Assessment of Neuropsychological Status among normal persons in Beijing. Zhongguo Xinli Weisheng Zazhi. 2010;24(12):926–931. [Google Scholar]
- [39].Hamilton M. Rating depressive patients. J Clin Psychiatry. 1980;41(12 Pt 2):21–24. [PubMed] [Google Scholar]
- [40].Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32(1):50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- [41].Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38(1):95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- [42].Salmond CH, Ashburner J, Vargha-Khadem F, et al. The precision of anatomical normalization in the medial temporal lobe using spatial basis functions. Neuroimage. 2002;17(1):507–512. doi: 10.1006/nimg.2002.1191. [DOI] [PubMed] [Google Scholar]


