Abstract
In this study, Sprague-Dawley rats were immobilized to a frame for 3 hours a day for 21 days to establish a model of chronic immobilization stress. The body weight and food intake of rats subjected to chronic immobilization stress were significantly decreased compared with the control group. Dual-labeling immunofluorescence revealed that the expression of leptin receptor and the co-localization coeffient in these leptic receptor neurons in the arcuate nucleus of the hypothalamus were both upregulated, while the number of neuropeptide Y neurons was decreased. Chronic immobilization stress induced high expression of leptin receptor in the arcuate nucleus and suppressed the synthesis and secretion of neuropeptide Y, thereby disrupting the pathways in the arcuate nucleus that regulate feeding behavior, resulting in diminished food intake and reduced body weight.
Keywords: neural regeneration, chronic immobilization stress, hypothalamus, arcuate nucleus, double immunofluorescence, neuropeptide Y, leptin receptor, feeding center, body weight, grants-supported paper, neuroregeneration
Research Highlights
(1) Leptin receptor expression was increased and neuropeptide Y neuron numbers were decreased in the hypothalamic arcuate nucleus of rats subjected to chronic immobilization stress.
(2) The regulatory networks affecting feeding behavior in the arcuate nucleus were disrupted in rats subjected to chronic immobilization stress, leading to a slow increase in body weight and lowered food intake.
INTRODUCTION
It is well-known that stress events profoundly affect ingestive behavior[1]. Chronic or repeated stress can result in reduction of food intake and body weight in rats[2,3,4,5]. Nuclei within the hypothalamus, such as the arcuate nucleus, ventromedial nucleus, paraventricular nucleus and lateral hypothalamic area, generate, integrate and regulate signals related to food intake, and form a complex appetite regulatory network[6,7,8]. In particular, the arcuate nucleus of the hypothalamus plays a critical role in the control of appetite and energy balance[9,10]. Neuropeptide Y is the most abundant neurotransmitter in the brain and is expressed in the hypothalamus mainly by neurons in the arcuate nucleus[11].
Leptin, derived from the periphery, crosses the blood-brain barrier and acts on the hypothalamus, to bind with leptin receptor to inhibit food intake and lower body weight[12]. Among the various brain areas, the arcuate nucleus has particularly high expression of the leptin receptor[13]. In the hypothalamus, the leptin receptor, is expressed by neuropeptide Y and proopiomelanocortin neurons, especially in the arcuate nucleus[14,15,16]. However, the role of leptin receptor and neuropeptide Y, as well as the interaction of these molecules, in the control of food intake and body weight in response to chronic stress is unknown. In this study, neuropeptide Y and leptin receptor in rat brain were labeled with double immunofluorescence for observing their expression in the hypothalamic arcuate nucleus of rats with chronic immobilization stress.
RESULTS
Quantitative analysis of experimental animals
A total of 40 Sprague-Dawley rats were used in this study and were divided into two groups: model group (n = 20; exposed to chronic immobilization stress for 21 days) and control group (n = 20; without chronic immobilization stress). Four rats were housed per cage, with five cages per group. Because of unsuitable immobilization, two rats in the model group died on the second day of the experiment. Therefore, 18 rats from the model group and 20 rats from the control group were included in the final analysis.
Effects of chronic immobilization stress on changes in body weight and food intake
Compared with control rats, the body weight and food intake of rats in the model group were significantly decreased (P < 0.01 or P < 0.05; Figure 1 and supplementary Tables 1 and 2 online).
Figure 1.
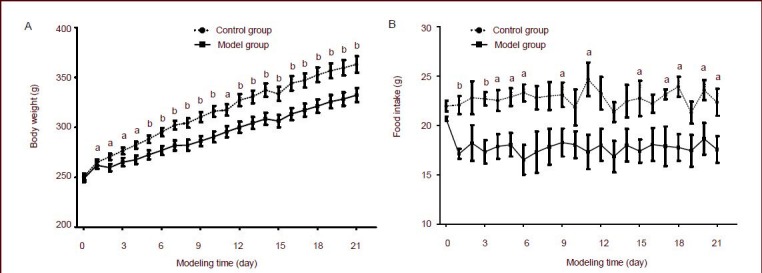
Effects of chronic immobilization stress on body weight (A) and food intake (B) in rats.
Data at each point are represented as mean ± SEM. The number of mice in control group and model group are 20 and 18, respectively. aP < 0.05, bP < 0.01, vs. model group using independent samples t-tests.
Effects of chronic immobilization stress on leptin receptor and neuropeptide Y expression in the arcuate nucleus
Expression of neuropeptide Y in neurons was displayed by red fluorescence, expression of leptin receptor was displayed by green fluorescence, and their co-localization was displayed by yellow fluorescence. There was substantial expression of neuropeptide Y and leptin receptor in the arcuate nucleus, and co-localization was also observed (Figure 2 and supplementary Figure 1 online). Quantitatively, the integrated absorbance for leptin receptor expression and the co-localization coefficient in these leptin receptor neurons was significantly increased in the model group compared with the control group (P < 0.01 or P < 0.05), while the number of neuropeptide Y neurons was substantially reduced (P < 0.01; Figure 3 and supplementary Table 3 online).
Figure 2.
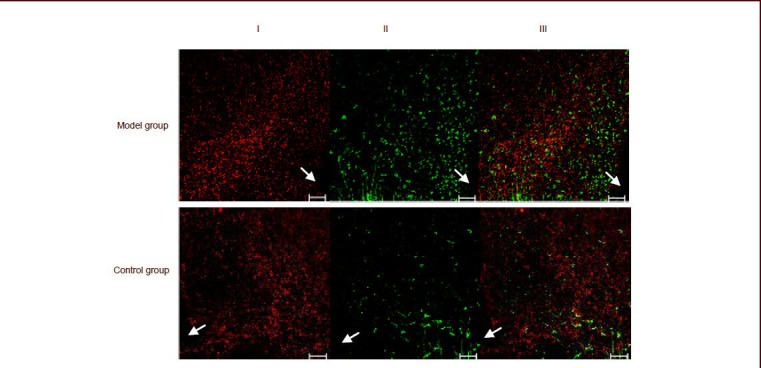
Double immunofluorescence labeling for neuropeptide Y and leptin receptor in the arcuate nucleus of rats by confocal imaging.
In each figure, leptin receptor was labeled with green fluorescence (II, labeled by Alexa Fluor 488), neuropeptide Y was labeled with red fluorescence (I, labeled by Alexa Fluor 594), and co-localization of neuropeptide Y and leptin receptor is represented by the yellow fluorescence (III).
Arrows indicate the third ventricle of the cerebrum. Scale bars: 50 μm.
Figure 3.
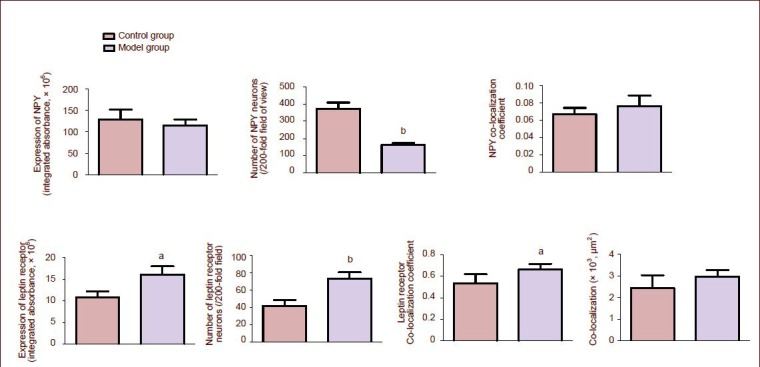
Effects of chronic immobilization stress on leptin receptor and neuropeptide Y (NPY) expression, and on their co-localization in the arcuate nucleus of rats.
The integrated absorbance, number and co-localization coefficients of NPY and leptin receptor, and the co-localization of NPY and leptin receptor were detected and analyzed with Zeiss LSM510 Meta System.
Co-localization refers to the area of NPY co-expressed with leptin receptor, that is the overlapping sites of green and red fluorescence in the images (μm2).
Data at each point were represented as mean ± SEM. aP < 0.05, bP < 0.01, vs. the control group using independent samples t-tests.
DISCUSSION
In this study, the body weight and food intake of rats subjected to chronic immobilization stress were significantly lower than in the control group, which is in agreement with results obtained by other laboratories[2,3,4,5] as well as previous studies conducted by our own research group[17,18].
Neuropeptide Y and leptin receptor were expressed in the arcuate nucleus, and their co-localization was also observed. Statistical analysis showed that the number of neuropeptide Y neurons in the arcuate nucleus of rats subjected to chronic immobilization stress was decreased significantly compared with control rats, which is consistent with previous studies[19,20]. In contrast, the expression of leptin receptor in the arcuate nucleus of rats subjected to chronic immobilization stress was increased significantly compared with control rats. This is evidence that higher expression of leptin receptor can suppress secretion of neuropeptide Y, thereby disrupting signaling within the ingestion pathway in the arcuate nucleus of the hypothalamus; possibly one reason why body weight increased although food intake was reduced in rats subjected to chronic immobilization stress. However, some studies have shown that expression of neuropeptide Y mRNA is increased in the arcuate nucleus of rats with chronic stress, concomitant with diminished food intake[21,22]. This was probably associated with disrupted neuropeptide/neurotransmitter signaling resulting from the chronic stress. Whether elevated leptin/leptin receptor signaling activates other nerve pathways in the arcuate nucleus, such as the proopiomelanocortin system, is unknown. Furthermore, the interactions between the different pathways in the arcuate nucleus which control ingestive behavior and body weight after chronic immobilization stress are also unclear and require further study.
In summary, chronic immobilization stress modulates the expression of neuropeptide Y and leptin receptor in rats. Immunofluorescence for leptin receptor increased substantially in the arcuate nucleus of rats subjected to chronic immobilization stress compared with control rats, while neuropeptide Y expression decreased. The results show that higher expression of leptin receptor in the arcuate nucleus suppresses secretion of neuropeptide Y, likely contributing to reduced body weight and lowered food intake induced by chronic immobilization stress.
MATERIALS AND METHODS
Design
A randomized, controlled, animal experiment.
Time and setting
The experiment was performed at the Laboratory of Traditional Chinese Medicine Diagnosis in Beijing University of Chinese Medicine, China from October 2009 to May 2010.
Materials
All experiments were performed on male Sprague-Dawley rats (8 weeks old, weighing 180–210 g; Beijing Weitong Lihua Research Center for Experimental Animals, license No. SCXK (Jing) 2006-0009), and were carried out in accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals, formulated by the Ministry of Science and Technology of China[23]. A total of 40 rats were housed in a room with routine care at 21–22°C, at a relative humidity of 30–40%, under a 12-hour light-dark cycle, and were allowed free access to food and water for 1 week.
Methods
Establishment of chronic immobilization stress model
The rats were bound to the special binding frame (20 cm length × 10 cm width × 2.8 cm thickness, made of wood). The upper platform was 22 cm in length and 6.6 cm at its widest part. In the upper platform, there were two adaptable soft bands for fixing the rat's chest and waist) for 3 hours per day for 21 consecutive days, as described previously[24]. Binding time points were random. Body weight and food intake were monitored and recorded every day.
Sampling
Rats were anesthetized with chloral hydrate (350–400 mg/kg) and perfused transcardially via the ascending aorta. Following perfusion, all brains were removed quickly and postfixed for 12 hours in 4.0% paraformaldehyde, then immersed sequentially in 20% and 30% sucrose solution in 0.1 mol/L PBS (pH 7.4–7.6) at 4°C for dehydration. Brains were sectioned with a freezing microtome (Leica-CM 1900, Heidelberger, Germany) at a thickness of 30 μm in the coronal plane, and sections containing the arcuate nucleus were selected[25].
Double immunofluorescence detection of neuropeptide Y and leptin receptor expression in the arcuate nucleus of the hypothalamus
The primary and secondary antibodies were diluted in 0.05 mol/L Tris buffered saline containing 0.5% Triton X-100 and 2% donkey serum (Millipore Corporation, Billerica, MA, USA). Sections were rinsed in 0.05 mol/L Tris buffered saline, then incubated for 1 hour at 37°C in 0.05 mol/L Tris buffered saline containing 0.5% Triton X-100. Thereafter sections were rinsed and incubated for 1 hour in 0.05 mol/L Tris buffered saline containing 0.5% Triton X-100 and 10% donkey serum at room temperature to block nonspecific binding. After drawing off the blocking solution, sections were incubated with rabbit anti-neuropeptide Y polyclonal antibody (1:500; Millipore Corporation) overnight at 4°C in the refrigerator. Then, sections were rinsed with 0.05 mol/L Tris buffered saline containing 0.5% Triton X-100 and 2% donkey serum. Subsequently, sections were incubated with Alexa Fluor 594 donkey anti-rabbit IgG (1:200; Invitrogen, California, CA, USA) for 4 hours at room temperature away from light. Then, sections were rinsed and incubated with diluted goat anti-leptin receptor polyclonal antibody (1:50; Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) for 40 hours at 4°C in the dark. Then sections were incubated with Alexa Fluor 488 donkey anti-goat IgG (1:200; Invitrogen) for 4 hours at room temperature away from light. Then, sections were rinsed and mounted onto glass slides and coverslipped with antifade mounting medium (Vector H-1500). In each group, nine or ten sections were examined with a ZEISS LSM510 META confocal imaging system (Zeiss, Germany) to determine integrated absorbance, neuron numbers, co-localization coefficients of neuropeptide Y and leptin receptor and the area of neuropeptide Y and leptin receptor colocalization. Colocalization coefficient represents the relative number of colocalizing pixels in channel 1 or 2, compared to the total number of pixels above threshold.
Statistical analysis
Statistical analysis was performed with SPSS 17.0 software (SPSS, Chicago, IL, USA). Comparison of two groups was evaluated by independent samples t-tests. P value less than 0.05 was considered a significant difference.
Footnotes
Shaoxian Wang, M.D., Lecturer.
Funding: This research was supported by the National Natural Science Foundation of China, No. 30672578, 81072756 and 81202644; China National Funds for Distinguished Young Scientists, No. 30825046; Program for Innovative Research Team in Beijing University of Chinese Medicine, No. 2011CXTD-07; Program for University Key Teacher of Hebei Medical University; Specialized Research Fund for the Doctoral Program of Higher Education, No. 20121323120016.
Conflicts of interest: None declared.
Ethical approval: The study was approved by the Animal Ethics Committee of Beijing University of Chinese Medicine in China.
Supplementary information: Supplementary data associated with this article can be found, in the online version, by visiting www.nrronline.org.
(Reviewed by Patel B, Norman C, Yuan TF, Gu P)
(Edited by Yu J, Yang Y, Li CH, Song LP)
REFERENCES
- [1].Fryer S, Waller G, Kroese BS. Stress, coping, and disturbed eating attitudes in teenage girls. Int J Eat Disord. 1997;22(4):427–436. doi: 10.1002/(sici)1098-108x(199712)22:4<427::aid-eat8>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- [2].Monteiro F, Abraham ME, Sahakari SD, et al. Effect of immobilization stress on food intake, body weight and weights of various organs in rat. Indian J Physiol Pharmacol. 1989;33(3):186–190. [PubMed] [Google Scholar]
- [3].Rybkin II, Zhou Y, Volaufova J, et al. Effect of restraint stress on food intake and body weight is determined by time of day. Am J Physiol. 1997;273(5 Pt 2):R1612–1622. doi: 10.1152/ajpregu.1997.273.5.R1612. [DOI] [PubMed] [Google Scholar]
- [4].Vallès A, Martí O, García A, et al. Single exposure to stressors causes long-lasting, stress-dependent reduction of food intake in rats. Am J Physiol Regul Integr Comp Physiol. 2000;279(3):R1138–1144. doi: 10.1152/ajpregu.2000.279.3.R1138. [DOI] [PubMed] [Google Scholar]
- [5].Alterman A, Mathison R, Coronel CE, et al. Functional and proteomic analysis of submandibular saliva in rats exposed to chronic stress by immobilization or constant light. Arch Oral Biol. 2012;57(6):663–669. doi: 10.1016/j.archoralbio.2011.12.008. [DOI] [PubMed] [Google Scholar]
- [6].Kalra SP, Dube MG, Pu S, et al. Interacting appetite-regulating pathways in the hypothalamic regulation of body weight. Endocr Rev. 1999;20(1):68–100. doi: 10.1210/edrv.20.1.0357. [DOI] [PubMed] [Google Scholar]
- [7].Schwartz MW, Woods SC, Porte D, Jr, et al. Central nervous system control of food intake. Nature. 2000;404(6778):661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- [8].Lenard NR, Berthoud HR. Central and peripheral regulation of food intake and physical activity: pathways and genes. Obesity (Silver Spring) 2008;16(Suppl 3):S11–22. doi: 10.1038/oby.2008.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Minokoshi Y. Brain mechanism underlying food intake regulation. Brain Nerve. 2011;63(6):597–604. [PubMed] [Google Scholar]
- [10].Suzuki K, Simpson KA, Minnion JS, et al. The role of gut hormones and the hypothalamus in appetite regulation. Endocr J. 2010;57(5):359–372. doi: 10.1507/endocrj.k10e-077. [DOI] [PubMed] [Google Scholar]
- [11].Boguszewski CL, Paz-Filho G, Velloso LA. Neuroendocrine body weight regulation: integration between fat tissue, gastrointestinal tract, and the brain. Endokrynol Pol. 2010;61(2):194–206. [PubMed] [Google Scholar]
- [12].Mantzoros CS. The role of leptin in human obesity and disease: a review of current evidence. Ann Intern Med. 1999;130(8):671–680. doi: 10.7326/0003-4819-130-8-199904200-00014. [DOI] [PubMed] [Google Scholar]
- [13].Morton GJ, Niswender KD, Rhodes CJ, et al. Arcuate nucleus-specific leptin receptor gene therapy attenuates the obesity phenotype of Koletsky (fa(k)/fa(k)) rats. Endocrinology. 2003;144(5):2016–2024. doi: 10.1210/en.2002-0115. [DOI] [PubMed] [Google Scholar]
- [14].Baskin DG, Schwartz MW, Seeley RJ, et al. Leptin receptor long-form splice-variant protein expression in neuron cell bodies of the brain and co-localization with neuropeptide Y mRNA in the arcuate nucleus. J Histochem Cytochem. 1999;47(3):353–362. doi: 10.1177/002215549904700309. [DOI] [PubMed] [Google Scholar]
- [15].Hâkansson ML, Brown H, Ghilardi N, et al. Leptin receptor immunoreactivity in chemically defined target neurons of the hypothalamus. J Neurosci. 1998;18(1):559–572. doi: 10.1523/JNEUROSCI.18-01-00559.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Baskin DG, Hahn TM, Schwartz MW. Leptin sensitive neurons in the hypothalamus. Horm Metab Res. 1999;31(5):345–350. doi: 10.1055/s-2007-978751. [DOI] [PubMed] [Google Scholar]
- [17].Chen JX, Li W, Zhao X, et al. Effects of the Chinese traditional prescription Xiaoyaosan decoction on chronic immobilization stress-induced changes in behavior and brain BDNF, TrkB, and NT-3 in rats. Cell Mol Neurobiol. 2008;28(5):745–755. doi: 10.1007/s10571-007-9169-6. [DOI] [PubMed] [Google Scholar]
- [18].Yue LF, Ding J, Chen JX, et al. Establishment and review of rat model of syndrome of liver depression with spleen insufficiency. Beijing Zhongyiyao Daxue Xuebao. 2008;31(6):396–400. [Google Scholar]
- [19].Kim H, Whang WW, Kim HT, et al. Expression of neuropeptide Y and cholecystokinin in the rat brain by chronic mild stress. Brain Res. 2003;983(1-2):201–208. doi: 10.1016/s0006-8993(03)03087-7. [DOI] [PubMed] [Google Scholar]
- [20].Rao HM. Beijing: Beijing University of Chinese Medicine; 2007. Changes on behavior and NPY, CCK in immobilization stress rat and Its relations with liver function of smoothing qi flow. [Google Scholar]
- [21].Sergeyev V, Fetissov S, Mathé AA, et al. Neuropeptide expression in rats exposed to chronic mild stresses. Psychopharmacology (Berl) 2005;178(2-3):115–124. doi: 10.1007/s00213-004-2015-3. [DOI] [PubMed] [Google Scholar]
- [22].Makino S, Baker RA, Smith MA, et al. Differential regulation of neuropeptide Y mRNA expression in the arcuate nucleus and locus coeruleus by stress and antidepressants. J Neuroendocrinol. 2000;12(5):387–395. doi: 10.1046/j.1365-2826.2000.00451.x. [DOI] [PubMed] [Google Scholar]
- [23].The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006 Sep 30; [Google Scholar]
- [24].Chen JX, Tang YT, Yang JX. Changes of glucocorticoid receptor and levels of CRF mRNA, POMC mRNA in brain of chronic immobilization stress rats. Cell Mol Neurobiol. 2008;28(2):237–244. doi: 10.1007/s10571-007-9170-0. [DOI] [PubMed] [Google Scholar]
- [25].Paxinos G, Watson C. 5th ed. London: Academic Press; 2005. The Rat Brain in Stereotaxic Coordinates. [Google Scholar]


