Abstract
A large number of studies have demonstrated that depression patients have cognitive dysfunction. With recently developed brain functional imaging, studies have focused on changes in brain function to investigate cognitive changes. However, there is still controversy regarding abnormalities in brain functions or correlation between cognitive impairment and brain function changes. Thus, it is important to design an emotion-related task for research into brain function changes. We selected positive, neutral, and negative pictures from the International Affective Picture System. Patients with major depressive disorder were asked to judge emotion pictures. In addition, functional MRI was performed to synchronously record behavior data and imaging data. Results showed that the total correct rate for recognizing pictures was lower in patients compared with normal controls. Moreover, the consistency for recognizing pictures for depressed patients was worse than normal controls, and they frequently recognized positive pictures as negative pictures. The consistency for recognizing pictures was negatively correlated with the Hamilton Depression Rating Scale. Functional MRI suggested that the activation of some areas in the frontal lobe, temporal lobe, parietal lobe, limbic lobe, and cerebellum was enhanced, but that the activation of some areas in the frontal lobe, parietal lobe and occipital lobe was weakened while the patients were watching positive and neutral pictures compared with normal controls. The activation of some areas in the frontal lobe, temporal lobe, parietal lobe, and limbic lobe was enhanced, but the activation of some areas in the occipital lobe were weakened while the patients were watching the negative pictures compared with normal controls. These findings indicate that patients with major depressive disorder have negative cognitive disorder and extensive brain dysfunction. Thus, reduced activation of the occipital lobe may be an initiating factor for cognitive disorder in depressed patients.
Keywords: neural regeneration, neuroimaging, major depressive disorder, cognitive function, functional MRI, occipital lobe, emotion, grants-supported paper, neuroregeneration
Research Highlights
(1) This study combined cognition tasks and functional MRI, and used multiple repeated event-related tasks to investigate brain functional characteristics in major depressive disorder.
(2) The number of error responses was calculated to identify bias of emotion recognition between patients with major depressive disorder and normal controls. Results showed that the depressed patients exhibited negative bias towards emotion task stimuli based on quantitative data.
(3) Using the International Affective Picture System-based event-related tasks, this study investigated brain imaging changes and cognitive dysfunction, as well as their relationship based on biased quantitative data. Results showed that the activation of the occipital lobe was attenuated in depressed patients when doing emotion tasks.
(4) Deficits in the occipital lobes may be an initiating factor for depression onset, which results in attention deficit disorder and cognitive dysfunction.
INTRODUCTION
Depressive disorder is a disease characterized by episodes of all-encompassing low mood accompanied by loss of interest or psychomotor inhibition. It has a high incidence rate and relapse rate and is associated with high suicide rate. In particular, major depressive disorder is a severe disease that adversely affects family, interpersonal relationships, work, school life, daily diet, sleeping, and physical functions[1]. The lifetime morbidity rate is 10–25% in females and 5–12% in males. Furthermore, 60% of people who commit suicide have major depressive disorder or other mood disorders[1]. However, the pathogenesis of depressive disorder remains uncertain. Brain function imaging can indicate functional changes during cerebral nerve activities using high resolution imaging to provide information on brain functions under different conditions, and provide insights into the etiopathogenesis of depression.
Clinical studies have demonstrated that emotional disorder and cognitive impairment are the main characteristics of depressive disorder[2,3,4]. Recovery from cognitive impairment is highly correlated with rehabilitation of depression and social function. Cognitive impairment is not restored during periods of reduced symptoms, indicating that the cognitive disorder may be a biological index of depressive disorder[5]. Previous studies showed that depressed people exhibited reduction in attention, memory, and execution abilities in emotion-related tasks[6,7,8]. Golinkoff and Sweeney[2] reported that the retention defect was found in depression, and they performed poorly in recalling and recognition tasks. Stip et al[3] designed sentence recognition tasks and found that the depressed patients showed significantly increased reaction time in emotion vocabulary stimulation. Results of a facial expression recognition task and an emotional categorization task demonstrated that depressed patients were significantly faster in recognizing facial expressions of fear than controls. They also showed significantly increased reaction time in recognizing negative personality characteristics in the categorization task[9].
To investigate the mechanism of cognitive impairment in depressive disorder, previous studies have designed emotion-related tasks and conducted brain functional imaging examinations, which indicated extensively abnormal activation in certain brain regions in depressed patients under resting and task conditions[10,11,12,13]. In particular, the abnormal brain functions involved activation of the hippocampus, amygdale, insula, and frontal lobe in the emotion task[14]. One study indicated that the abnormal activation of specific brain regions is linked to specific cognitive tasks[15]. Functional abnormality in brain regions provides novel insights into the pathomechanism of cognitive impairment in depressed patients and etiology of depressive disorder. A previous study demonstrated that the depressed individuals performed worse on the negative emotion semantic categorization, which is related to increased recruitment of the right amygdale and left inferior frontal gyrus, but brain functional abnormality resulted in damage in task-related behaviors[14]. An attenuated dynamic range of responses in the limbic-subcortical and extrastriate visual regions was evident in the depressed patients in happy facial stimuli, which were reversed following antidepressant treatment[16]. Amygdala hyperactivity was found in depressed patients in response to masked negative facial emotions, and positively associated with negative judgmental bias, disease severity, and course of disease[17]. A previous study from our group suggested that categorization, memory, and intelligence quotient of depressed patients were reduced, and a variety of items were negatively correlated with degree of depression[18]. A resting-state functional MRI study showed decreased regional homogeneity in the insula and cerebellum in depression[19].
However, because of differences in tasks, subjects, and size of samples, results were significantly varied. Moreover, most studies of depressive disorder used tasks to assess other mental disorders, such as schizophrenia, and mainly focused on recognition and recalling tasks using block design. Thus, the results mainly focused on reaction time, accuracy rate, and bias changes. Previous studies used recognition and recalling tasks to determine the abnormal activities of different brain regions, but little is known about characteristic symptom-related tasks and emotion tasks. The abnormal response to emotion has not been further investigated, and only one or two types of emotion tasks have been investigated, so the abnormal results in brain regions cannot comprehensively explain the pathomechanism. The present study used positive, neutral, and negative pictures in the International Affective Picture System to investigate bias of major depressive disorder patients in recognizing pictures based on event-related design and repetitive stimuli, resulting in increased novel index and recognition consistency. In addition, we conducted functional MRI to explore the emotion-related cognition functional changes and brain functional changes, as well as their possible relationship, in response to emotion picture stimuli to reveal the pathogenesis of depression.
RESULTS
Quantitative analysis of participants
A total of 49 depressed patients and 38 controls were enrolled, and two patients and one control individual participated in the pre-experiment. Because five patients and two controls could not complete the functional MRI test, 42 major depressive disorder patients and 35 controls were included in the analysis. The major depressive disorder patients comprised 16 males and 26 females, mean age 32.4 ± 10.1 years, with education duration of 12.5 ± 3.5 years. The control group included 16 males and 19 females, mean age 32.9 ± 9.2 years, with education duration of 13.7 ± 3.0 years. Average Hamilton Depression Rating Scale scores were 24.26 ± 6.47 in depressed patients and 1.74 ± 2.15 in controls. Test of normality showed normal distribution in age, education duration and gender in two groups, with no significant differences (age, t = –0.24, P = 0.811; education duration, t = –1.56, P = 0.122; gender, X2 = 0.456, P = 0.499).
Major depressive disorder patients exhibited poor recognition consistency for emotion pictures and negative judgmental bias
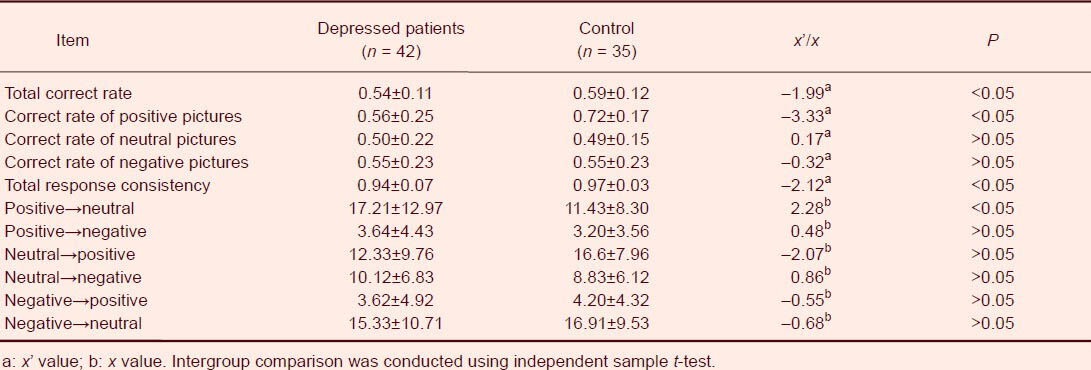
The total correct rate in recognizing emotion pictures was lower in major depressive disorder patients compared with normal controls (P < 0.05), and the recognition consistency was worse than the controls (P < 0.05), indicating that judgmental stability was worse in major depressive disorder patients. In addition, major depressive disorder patients misrecognized 17.21 ± 12.97 positive pictures as negative pictures, which was significantly higher than the control (11.43 ± 8.30, P < 0.05), demonstrating that major depressive disorder patients exhibited negative judgmental bias (Table 1).
Table 1.
Emotion picture recognition between major depressive disorder patients and normal controls
Hamilton Depresssion Rating Scale scores were negatively correlated with recognition consistency in major depressive disorder patients
The recognition consistency was negatively correlated with Hamilton Depresssion Rating Scale scores in major depressive disorder patients (r = –0.312, P < 0.05; Figure 1), indicating that the more severe the depression, the worse the consistency, resulting in instable evaluation.
Figure 1.
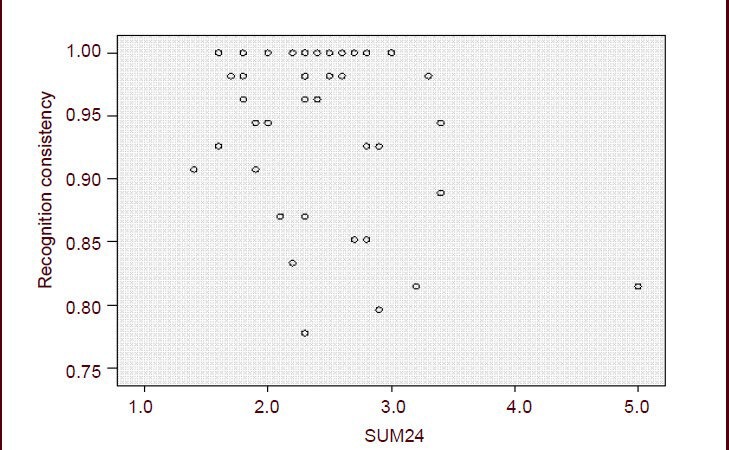
Correlation between Hamilton Depression Rating Scale (HAMD) scores and emotion picture recognition in depressed patients.
Recognition consistency was negatively correlated with HAMD scores in patients with major depressive disorder (r= –0.312, P < 0.05). HAMD scores between 8 and 20 represent normal, 20–35 possible depression, > 35 depression. SUM24 represents total scores of 24 items of HAMD.
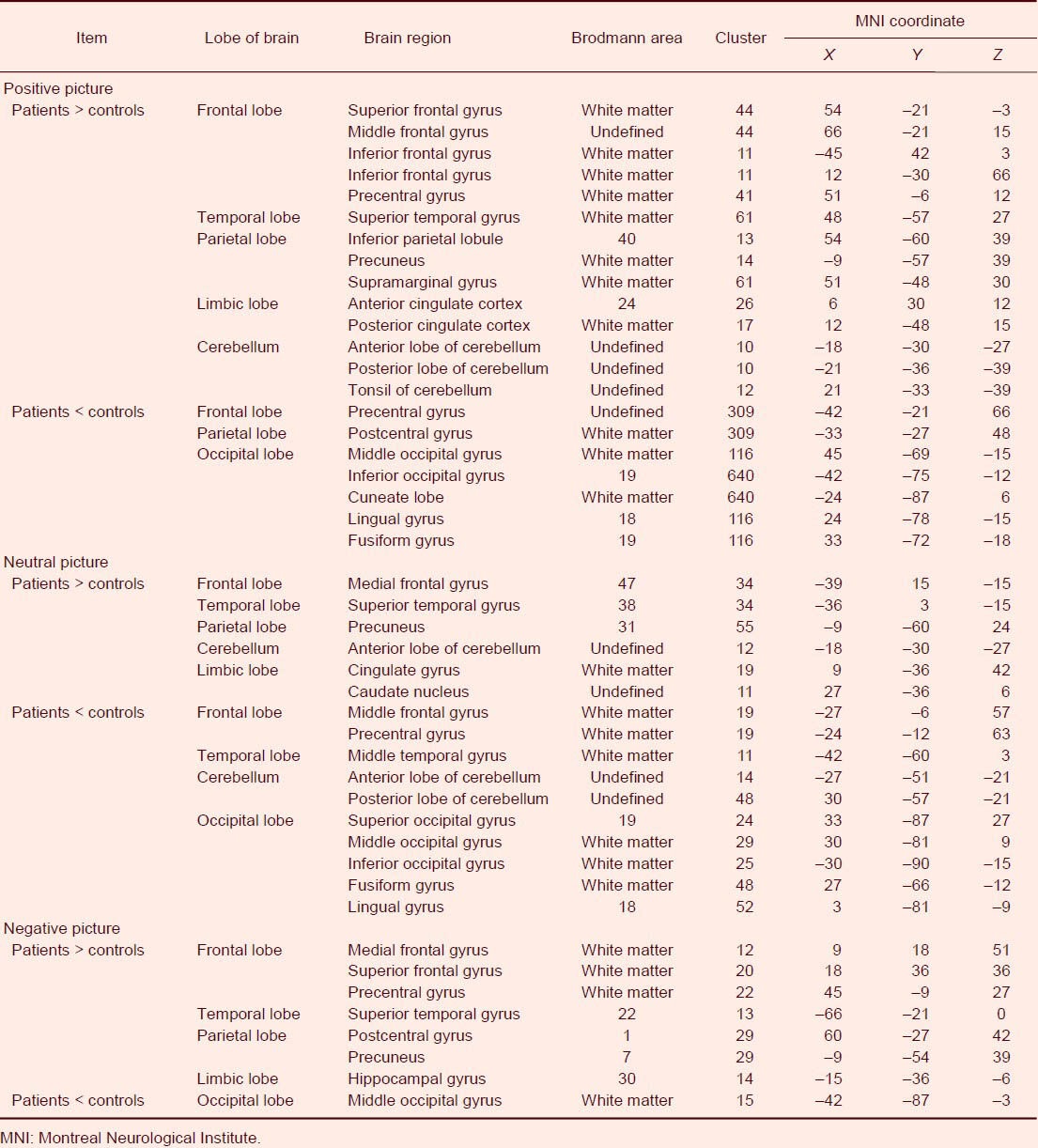
Abnormal activity of different brain regions in major depressive disorder patients
Functional MRI showed that activation of a number of brain regions was enhanced, and that some brain regions were attenuated when major depressive disorder patients were recognizing positive, neutral, and negative pictures compared with normal controls (Figures 2, 3). The activation of some regions in the frontal lobe, temporal lobe, parietal lobe, limbic lobe and cerebellum was enhanced, but some regions in the frontal lobe, parietal lobe and occipital lobe were weakened when the patients were watching the positive and neutral pictures compared with normal controls.
Figure 2.
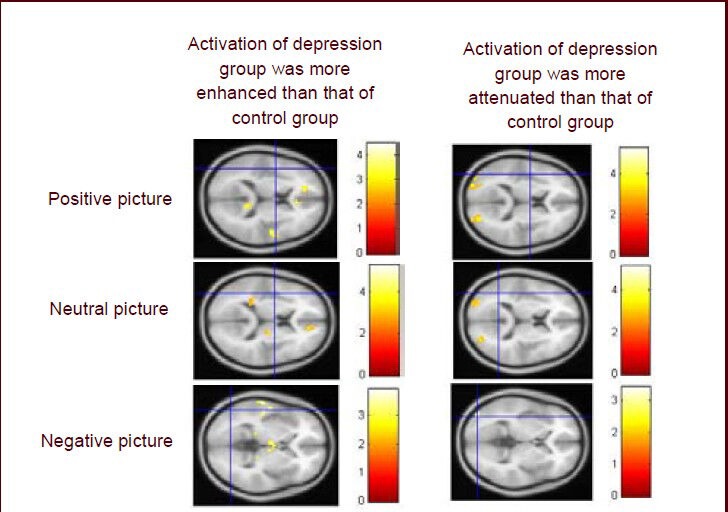
Functional MRI of activation of brain regions of patients with major depressive disorder.
The activation of some regions in the frontal lobe, temporal lobe, parietal lobe, limbic lobe and cerebellum was enhanced, but activation of some regions in the frontal lobe, parietal lobe and occipital lobe was weakened when the patients were watching positive and neutral pictures compared with normal controls. The activation of some regions in the frontal lobe, temporal lobe, parietal lobe, and limbic lobe was enhanced but activation of some regions in the occipital lobe was weakened when the patients were watching the negative pictures compared with normal controls. Colored bars reflect T scores for each analysis.
Figure 3.
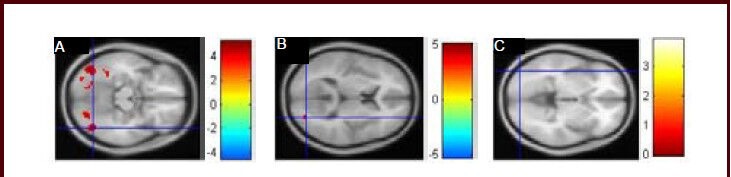
Functional MRI of activation of the occipital lobe was weakened when patients with major depressive disorder were recognizing positive (A), neutral (B), and negative (C) pictures.
Activation of the occipital lobe was weakened when major depressive disorder patients were recognizing pictures, and the activation was significantly reduced when recognizing positive pictures. Colored bars reflect T scores for each analysis: higher T values represent higher intensity.
The activation of some regions in the frontal lobe, temporal lobe, parietal lobe, and limbic lobe was enhanced but activation of some regions in the occipital lobe was weakened when the patients were watching negative pictures compared with normal controls. This is the first time it has been shown that activation of the occipital lobe was weakened when major depressive disorder patients were recognizing pictures, and activation was significantly reduced when recognizing positive pictures (Figure 3). These results indicate that more activated brain regions are required for major depressive disorder patients to complete the tasks, and the activity of some brain regions is weak in the task state (Table 2).
Table 2.
Abnormally activated brain regions in patients with major depressive disorder
DISCUSSION
The present study selected pictures from the International Affective Picture System and analyzed the accuracy, bias, error tendency, and consistency using event-related design and repeated stimuli. Compared with previous block design, this method more accurately and objectively reflected emotion-related cognitive function. Results from this study showed that major depressive disorder patients could not correctly recognize positive stimuli well, and exhibited negative bias. Moreover, the recognition consistency was worse than the control group, and the consistency was negatively correlated with severity of depression. A previous study showed that depressed adolescents exhibited impaired sustained attention[20]. Depressed males tended to make less advantageous choices, and there was an inverse pattern of correlations between depressive symptom counts and reaction time to affective stimuli[20]. Segrave and Gotlib[21] reported that depressed patients exhibited negative bias while recognizing faces with expression, and depressed participants were slower to disengage from sad stimuli[21], consistent with results from the above studies that major depressive disorder patients exhibited negative judgmental bias[20,21].
After we concluded the negative bias of major depressive disorder patients, we further analyzed error data.
Statistical analysis of error tendency data and error response rate showed that major depressive disorder patients exhibited evident negative bias compared with normal controls. This further demonstrates that negative bias in emotion tasks based on quantitative data is consistent with negative cognition and negatively biased cognition dysfunction in depressive disorder[22,23].
In the present study, each picture was shown randomly three times. Judgmental consistency was further analyzed, which showed that the recognition consistency was lower in major depressive disorder patients compared with the normal control, and the reduced consistency was positively correlated with Hamilton Depression Rating Scale scores, indicating that the more severe the depression, the worse the participant's operation stability, further demonstrating cognitive impairment in major depressive disorder patients. A previous neurocognitive function study showed that in a memory task, depressed people recalled fewer working memory items, and memory accuracy and stability were reduced[24]. Our results additionally demonstrated that in emotion-related tasks, the judgmental stability of major depressive disorder patients decreased, and judgmental stability was positively correlated with severity of depression, indicating general executive control deficits in depressed patients.
It is believed that the activation of the cortex-limbic system and abnormal regulation exists in depressive disorder patients. For example, cortical function is reduced, limbic system activation is enhanced, and function of the cortex to regulate the limbic system is attenuated in depressed patients[25,26,27]. Other studies reported abnormal activation in the temporal lobe, occipital lobe, parietal lobe and cerebellum of patients with depressive disorder[28,29]. Beauregard et al[30] found that transient negative stimuli produced significant activation in the frontal lobe, temporal lobe, cerebellum, and the caudate in both depressed and normal subjects, but hyperactivity was observed in the cingulate gyrus and prefrontal cortices in depressed patients than in normal control subjects. Another study indicated that the activation was attenuated in the frontal lobe, but enhanced in the limbic system in depressed patients[31]. Results from the present study showed increased activation in the frontal lobe, temporal lobe, parietal lobe, limbic lobe and cerebellum in major depressive disorder patients in response to positive, neutral, and negative pictures compared with normal controls, in agreement with the results of some studies[30,31].
The occipital lobe contains most of the anatomical region of the visual cortex and contributes to visual information processing and communication with the cerebral cortex. It is believed that the function of the occipital lobe is reduced in depressed patients. Resting-state functional MRI showed that the homogeneity of the occipital lobe was decreased in depressed patients[32]. In addition, cortical atrophy tended to appear in the occipital lobes in patients with affective disorder[33], and a subtle gray-matter decrease was also found in the occipital lobes[34]. In the present study, the activation of the occipital lobes was attenuated in depressed patients, consistent with the above results. Deficits in the occipital lobes may contribute to low levels and imbalance of neurotransmitters[35]. Because the occipital lobes are responsible for visual perception and processing, reduced attention of depressed patients may be associated with attenuated function of the occipital lobes. Deficit in the occipital lobes may be an initiating factor for depression onset, which may result in attention deficit disorder and cognitive dysfunction.
In summary, major depressive disorder patients displayed deficits in positive emotion processing and abnormal activation in the frontal-temporal-parietal lobe in emotion facial processing. The reduction in attention of depressed patients may be associated with functional decrease in the occipital lobes.
SUBJECTS AND METHODS
Design
A case controlled neuroimaging study.
Time and setting
The experiments were conducted in the Department of Mental Health, First Hospital of Shanxi Medical University, China from May 2010 to March 2012.
Subjects
Depressed patients admitted to the Department of Mental Health, First Hospital of Shanxi Medical University were enrolled.
Inclusion criteria
Patients were included if they were diagnosed with characteristics of major depressive disorder according to the Structured Clinical Interview for DSM-IV-TR Axis I Disorders-Patient Edition[36], they had scores more than 17 in Hamilton Depression Rating Scale 24[18], they lived in Shanxi Province, China, were aged 18–50 years, were right-handed, and could cooperate during the examination.
Exclusion criteria
Patients who were lactating or were pregnant, or had severe somatic disease or other mental disorders, or had attempted suicide, were excluded from the study. Informed consent was obtained from the patients and their families.
A total of 35 controls were recruited from unrelated patient relatives and community volunteers from the same region, who were free of mental disorder, were aged 18–50 years, right-handed, and had Hamilton Depression Rating Scale 24 scores < 7. Informed consent was obtained from the controls. The experimental procedures were conducted in accordance with the Administrative Regulations on Medical Institutions, issued by the State Council of China[37].
Methods
Baseline data collection
Age, gender, education level and Hamilton Depression Rating Scale of all participants were recorded. In Hamilton Depression Rating Scale, scores of 8–20 represent normal, 20–35 represent possible depression, and > 35 represent depression[38].
Recognition of different emotion expressions
Picture source: all the pictures were selected from the photo center of the International Affective Picture System[39], including 19 positive, 18 neutral, 17 negative, and one baseline (+) picture; 55 in total. Each emotion picture and baseline picture was shown three times. The valence standard scores were 7–9 for neutral-positive pictures, 4–6 for neutral pictures, and 1–3 for negative pictures; arousal standard scores were around 4.5[32]. Number of positive pictures: 1 340, 1 440, 1 463, 1 721, 1 750, 1 920, 2 071, 2 091, 2 165, 2 311, 2 340, 2 341, 2 391, 2 395, 4 603, 5 831, 7 480, 8 120, 8 497; neutral pictures: 1 121, 1 240, 1 850, 1 945, 2 005, 2 025, 2 222, 2 372, 2 410, 2 600, 2 635, 4 503, 4 535, 5 900, 6 930, 7 211, 7 320, 7 620; negative pictures: 2 110, 2 141, 2 205, 2 276, 2 278, 2 455, 2 750, 2 900, 3 301, 6 311, 9 041, 9 220, 9 265, 9 290, 9 415, 9 561, 9 830 (supplementary Figure 1 online).
Test performance: the subjects were asked to focus on the pictures on the rear projection mirror, and gave responses under identical guidance. First, the subjects were trained to be familiar with the key and response time. They wore earplugs and earphones to attenuate interference from machine noise. Glasses could also be worn if necessary. The lighting was turned off at the time of scanning. Unified instructions were as follows: press key 1 if the picture makes you feel happy, glad, satisfied, pleased, relaxed or other positive feelings; press key 3 if the picture makes you feel sad, angry, hateful, uneasy, uncomfortable, or other negative feelings; press key 2 if you cannot definitely identify your feelings, negative or positive. Baseline picture was a “+” in the center of the screen for 2 seconds. During the test, the subjects were presented with emotion pictures and made a judgment. The emotion pictures were presented for 2 seconds, during which the subject was asked to stare at the picture, followed by baseline picture “+” for 2 seconds. The emotion pictures and baseline picture were presented randomly, and each picture was shown three times. The number of emotion pictures and baseline picture was equal. When the subject saw colored pictures, he/she could judge the feeling. Input equipment was connected to the machine, i.e. a handle with keys 1, 2, 3, connected to a computer with task software. The task window was opened, the keypad was started, and the task pictures were rear projected to the front-upper site of the face of the subjects. Emotion stimuli program was edited using psychology presentation software (Eprime Software, http://www.pstnet.com/e-prime) and played automatically by computer. Behavior data of each type of pictures were output from an E-data file, automatically generated by computer, to calculate the correct rate of individual to pictures, number of consistency, and bias.
Functional MRI detection of changes in different brain regions
Functional MRI was conducted while the subject was doing the task. Magnetom Trio 3 T whole body MRI system (Siemens China Branch, Shanghai, China), 32-sliced, axial view, for 486 scans, was used with the following scan parameters: repetition time = 2 000 ms; echo time = 30 ms; slice thickness = 3 mm, slice gap = 3.99 mm (3 × 33%), acquisition matrix = 64, 0, 0, 64, pixel spacing = 3.75 × 3.75, field of view = 240 × 240 mm, resolution = 64 × 64, flip angle = 90°, average: 1. Image data were pre-treated using DPARSF software (http://www.Restfmri.net/forum/DPARSF), and raw data were transformed into NIfTI format. Ten time-point data were eliminated because the initial signals were not stable, followed by slice timing, realignment, normalization and Gaussian smoothing. Individual analysis and group analysis were conducted using SPM8 (http://www.fil.ion.ucl.ac.uk/spm). P value was set as 0.001 (uncorrected), and cluster > 10, i.e. brain regions with continuous activation voxel more than these values were statistically significant. xjview plug-in (http://www.alivelearn.net/xjview) was used to observe the size of the voxel with statistical significance, to localize the MNI coordinate in the brain, and to record cluster and intensity of activation.
Statistical analysis
Measurement data were expressed as mean ± SD. The age of the two groups was subjected to an independent sample t-test; gender and education level were subjected to multiple-group chi-square test. Correct rate represents the correct rate of response to all pictures, to positive, neutral, and negative pictures, respectively. Consistency was obtained by the number of pictures with identical response after three-time presentation (more than two times) in two groups/total number of pictures (n = 54). Bias was quantified, i.e. to calculate the number of pictures with neutral response to positive pictures, negative response to positive pictures, positive response to neutral pictures, negative response to neutral pictures, positive response to negative pictures, and neutral response to negative pictures, followed by two-group independent sample t-test. Data of correct rate and consistency not following normal distribution were subjected to subduplicate arcsine transformation, x’ = arctan(x)x1/2, (x: correct rate or consistency), followed by two-group independent sample t-test. Activation intensity obtained by functional MRI was analyzed using t-test, and results were presented by T value: the greater T value represents a higher intensity. Pearson correlation analysis was performed between Hamilton Depression Rating Scale scores and consistency, and correlation coefficient was calculated to identify whether there was a linear correlation between them.
Acknowledgments:
We thank all the subjects for participating in this study and the staff from the MRI Room, Shanxi People's Hospital, China for their help.
Footnotes
Funding: The study was supported by the National Natural Science Foundation of China, No. 30971054, 30770770, 81171290.
Conflicts of interest: None declared.
Ethical approval: The study was approved by Medical Ethics Committee of First Hospital, Shanxi Medical University, China.
Supplementary information: Supplementary data associated with this article can be found, in the online version, by visiting www.nrronline.org.
(Reviewed by Bijur G, de Souza M, Zuo XN, Xiang J)
(Edited by Yu J, Su LL, Li CH, Song LP)
REFERENCES
- [1].Shen YC. 4th ed. Beijing: People's Medical Publishing House; 2007. Psychiatry. [Google Scholar]
- [2].Golinkoff M, Sweeney JA. Cognitive impairments in depression. J Affect Disord. 1989;17(2):105–112. doi: 10.1016/0165-0327(89)90032-3. [DOI] [PubMed] [Google Scholar]
- [3].Stip E, Lecours AR, Chertkow H, et al. Influence of affective words on lexical decision task in major depression. J Psychiatry Neurosci. 1994;19(3):202–207. [PMC free article] [PubMed] [Google Scholar]
- [4].Le Masurier M, Cowen PJ, Harmer CJ. Emotional bias and waking salivary cortisol in relatives of patients with major depression. Psychol Med. 2007;37(3):403–410. doi: 10.1017/S0033291706009184. [DOI] [PubMed] [Google Scholar]
- [5].Chen C, Li ZY, Gao CG, et al. Study of congnitive function injury of major depression patients. Shiyong Yiji Zazhi. 2008;15(10):9–11. [Google Scholar]
- [6].Hammar A, Strand M, Ardal G, et al. Testing the cognitive effort hypothesis of cognitive impairment in major depression. Nord J Psychiatry. 2011;65(1):74–80. doi: 10.3109/08039488.2010.494311. [DOI] [PubMed] [Google Scholar]
- [7].Hammar A, Isaksen L, Schmid M, et al. Patients with major depression show intact memory performance-given optimal conditions. Appl Neuropsychol. 2011;18(3):191–196. doi: 10.1080/09084282.2011.595445. [DOI] [PubMed] [Google Scholar]
- [8].Hammar A, Sørensen L, Ardal G, et al. Enduring cognitive dysfunction in unipolar major depression: a test-retest study using the Stroop paradigm. Scand J Psychol. 2010;51(4):304–308. doi: 10.1111/j.1467-9450.2009.00765.x. [DOI] [PubMed] [Google Scholar]
- [9].Veer IM, Beckmann CF, van Tol MJ, et al. Whole brain resting-state analysis reveals decreased functional connectivity in major depression. Front Syst Neurosci. 2010:4. doi: 10.3389/fnsys.2010.00041. pii: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wyckoff N, Kumar A, Gupta RC, et al. Magnetization transfer imaging and magnetic resonance spectroscopy of normal-appearing white matter in late-life major depression. J Magn Reson Imaging. 2003;18(5):537–543. doi: 10.1002/jmri.10400. [DOI] [PubMed] [Google Scholar]
- [11].Pillay SS, Renshaw PF, Bonello CM, et al. A quantitative magnetic resonance imaging study of caudate and lenticular nucleus gray matter volume in primary unipolar major depression: relationship to treatment response and clinical severity. Psychiatry Res. 1998;84(2-3):61–74. doi: 10.1016/s0925-4927(98)00048-1. [DOI] [PubMed] [Google Scholar]
- [12].Vakili K, Pillay SS, Lafer B, et al. Hippocampal volume in primary unipolar major depression: a magnetic resonance imaging study. Biol Psychiatry. 2000;47(12):1087–1090. doi: 10.1016/s0006-3223(99)00296-6. [DOI] [PubMed] [Google Scholar]
- [13].Wagner V, Müller JL, Sommer M, et al. Changes in the emotional processing in depressive patients: a study with functional magnetoresonance tomography under the employment of pictures with affective contents. Psychiatr Prax. 2004;31(Suppl 1):S70–72. doi: 10.1055/s-2004-828410. [DOI] [PubMed] [Google Scholar]
- [14].van Wingen GA, van Eijndhoven P, Tendolkar I, et al. Neural basis of emotion recognition deficits in first-episode major depression. Psychol Med. 2011;41(7):1397–1405. doi: 10.1017/S0033291710002084. [DOI] [PubMed] [Google Scholar]
- [15].Schöning S, Zwitserlood P, Engelien A, et al. Working-memory fMRI reveals cingulate hyperactivation in euthymic major depression. Hum Brain Mapp. 2009;30(9):2746–2756. doi: 10.1002/hbm.20702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Fu CH, Williams SC, Brammer MJ, et al. Neural responses to happy facial expressions in major depression following antidepressant treatment. Am J Psychiatry. 2007;164(4):599–607. doi: 10.1176/ajp.2007.164.4.599. [DOI] [PubMed] [Google Scholar]
- [17].Dannlowski U, Ohrmann P, Bauer J, et al. Amygdala reactivity to masked negative faces is associated with automatic judgmental bias in major depression: a 3 T fMRI study. J Psychiatry Neurosci. 2007;32(6):423–429. [PMC free article] [PubMed] [Google Scholar]
- [18].Yang CH, Shen WY, Du QR, et al. Cognitive function in first-episode patients with major depressive disorder. Shanghai Jingshen Yixue. 2009;21(3):143–146. [Google Scholar]
- [19].Liu Z, Xu C, Xu Y, et al. Decreased regional homogeneity in insula and cerebellum: a resting-state fMRI study in patients with major depression and subjects at high risk for major depression. Psychiatry Res. 2010;182(3):211–215. doi: 10.1016/j.pscychresns.2010.03.004. [DOI] [PubMed] [Google Scholar]
- [20].Han G, Klimes-Dougan B, Jepsen S, et al. Selective neurocognitive impairments in adolescents with major depressive disorder. J Adolesc. 2012;35(1):11–20. doi: 10.1016/j.adolescence.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Levens SM, Gotlib IH. Updating positive and negative stimuli in working memory in depression. J Exp Psychol Gen. 2010;139(4):654–664. doi: 10.1037/a0020283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Liao CJ, Feng ZZ. Mechanism of affective and cognitive-control brain regions in depression. Xinli Kexue Jinzhan. 2010;18(2):92–97. [Google Scholar]
- [23].Liu MF, Huang RZ, Tu YL, et al. Inhibition function of emotional words in depressed patients. Zhongguo Linchuang Xinli Xue Zazhi. 2007;15(2):55–57. [Google Scholar]
- [24].Doumas M, Smolders C, Brunfaut E, et al. Dual task performance of working memory and postural control in major depressive disorder. Neuropsychology. 2012;26(1):110–118. doi: 10.1037/a0026181. [DOI] [PubMed] [Google Scholar]
- [25].Schlösser RG, Wagner G, Koch K, et al. Fronto-cingulate effective connectivity in major depression: a study with fMRI and dynamic causal modeling. Neuroimage. 2008;43(3):645–655. doi: 10.1016/j.neuroimage.2008.08.002. [DOI] [PubMed] [Google Scholar]
- [26].Bär KJ, Wagner G, Koschke M, et al. Increased prefrontal activation during pain perception in major depression. Biol Psychiatry. 2007;62(11):1281–1287. doi: 10.1016/j.biopsych.2007.02.011. [DOI] [PubMed] [Google Scholar]
- [27].Holmes AJ, Pizzagalli DA. Response conflict and frontocingulate dysfunction in unmedicated participants with major depression. Neuropsychologia. 2008;46(12):2904–2913. doi: 10.1016/j.neuropsychologia.2008.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Biver F, Wikler D, Lotstra F, et al. Serotonin 5-HT2 receptor imaging in major depression: focal changes in orbito-insular cortex. Br J Psychiatry. 1997;171:444–448. doi: 10.1192/bjp.171.5.444. [DOI] [PubMed] [Google Scholar]
- [29].Naismith SL, Lagopoulos J, Ward PB, et al. Fronto-striatal correlates of impaired implicit sequence learning in major depression: an fMRI study. J Affect Disord. 2010;125(1-3):256–261. doi: 10.1016/j.jad.2010.02.114. [DOI] [PubMed] [Google Scholar]
- [30].Beauregard M, Leroux JM, Bergman S, et al. The functional neuroanatomy of major depression: an fMRI study using an emotional activation paradigm. Neuroreport. 1998;9(14):3253–3258. doi: 10.1097/00001756-199810050-00022. [DOI] [PubMed] [Google Scholar]
- [31].Moses-Kolko EL, Perlman SB, Wisner KL, et al. Abnormally reduced dorsomedial prefrontal cortical activity and effective connectivity with amygdala in response to negative emotional faces in postpartum depression. Am J Psychiatry. 2010;167(11):1373–1380. doi: 10.1176/appi.ajp.2010.09081235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Peng DH, Jiang KD, Fang YR, et al. Decreased regional homogeneity in major depression as revealed by resting-state functional magnetic resonance imaging. Chin Med J (Engl) 2011;124(3):369–373. [PubMed] [Google Scholar]
- [33].Tanaka Y, Hazama H, Fukuhara T, et al. Computerized tomography of the brain in manic-depressive patients--a controlled study. Folia Psychiatr Neurol Jpn. 1982;36(2):137–143. doi: 10.1111/j.1440-1819.1982.tb00264.x. [DOI] [PubMed] [Google Scholar]
- [34].Lai CH, Hsu YY. A subtle grey-matter increase in first-episode, drug-naive major depressive disorder with panic disorder after 6 weeks’ duloxetine therapy. Int J Neuropsychopharmacol. 2011;14(2):225–235. doi: 10.1017/S1461145710000829. [DOI] [PubMed] [Google Scholar]
- [35].Maciag D, Hughes J, O’Dwyer G, et al. Reduced density of calbindin immunoreactive GABAergic neurons in the occipital cortex in major depression: relevance to neuroimaging studies. Biol Psychiatry. 2010;67(5):465–470. doi: 10.1016/j.biopsych.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Li T, Zhou RY, Hu JM, et al. Chengdu: Institute of Mental Health, West China Hospital of Sichuan University; 2004. DSM-IV-TR axis I disorders Structured Clinical examination: Patient Edition (SCID-I/P) [Google Scholar]
- [37].State Council of the People's Republic of China. Administrative Regulations on Medical Institution. 1994 Sep 01; [Google Scholar]
- [38].Zhang ZJ. Beijing: Chinese Medical Multimedia Press; 2005. Behavioral Medicine Scale Manual. [Google Scholar]
- [39].Lang PJ, Bradley MM, Cuthbert BN. Center for Research in Psychophysiology, University of Florida; 2005. International affective picture system (IAPS): Instruction manual and affective ratings. [Google Scholar]


