Abstract
Chronic activation of microglial cells endangers neuronal survival through the release of various proinflammatory and neurotoxic factors. The root of Paeonia lactiflora Pall has been considered useful for the treatment of various disorders in traditional oriental medicine. Paeonol, found in the root of Paeonia lactiflora Pall, has a wide range of pharmacological functions, including anti-oxidative, anti-inflammatory and neuroprotective activities. The objective of this study was to examine the efficacy of paeonol in the repression of inflammation-induced neurotoxicity and microglial cell activation. Organotypic hippocampal slice cultures and primary microglial cells from rat brain were stimulated with bacterial lipopolysaccharide. Paeonol pretreatment was performed for 30 minutes prior to lipopolysaccharide addition. Cell viability and nitrite (the production of nitric oxide), tumor necrosis factor-alpha and interleukin-1beta products were measured after lipopolysaccharide treatment. In organotypic hippocampal slice cultures, paeonol blocked lipopolysaccharide-related hippocampal cell death and inhibited the release of nitrite and interleukin-1beta. Paeonol was effective in inhibiting nitric oxide release from primary microglial cells. It also reduced the lipopolysaccharide-stimulated release of tumor necrosis factor-alpha and interleukin-1β from microglial cells. Paeonol possesses neuroprotective activity in a model of inflammation-induced neurotoxicity and reduces the release of neurotoxic and proinflammatory factors in activated microglial cells.
Keywords: neural regeneration, traditional Chinese medicine, brain inflammation, interleukin-1beta, microglia, neurotoxicity, neuroprotection, nitric oxide, organotypic hippocampal slice culture, Paeonia lactiflora Pall, paeonol, tumor necrosis factor-alpha, neuroregeneration
Research Highlights
(1) Inflammation and oxidative stress are pathological mechanisms underlying neurodegenerative diseases.
(2) Anti-inflammatory treatment is a new strategy for improving the prognosis of neurodegenerative diseases.
(3) In organotypic hippocampal slice cultures, paeonol blocked lipopolysaccharide-induced hippocampal neuronal death and release of proinflammatory mediators.
(4) Paeonol possesses neuroprotective activity in a model of inflammation-induced neurotoxicity and reduces the release of neurotoxic and proinflammatory factors in activated microglial cells.
INTRODUCTION
Of the different cell types in the central nervous system, microglial cells are resident macrophages that play important roles in immune and inflammatory responses. They are activated during neuropathological conditions to restore central nervous system homeostasis[1]. The activation of microglial cells involves their proliferation, migration to the injury site, increased expression of immunomodulators, and transformation into phagocytes capable of clearing damaged cells and debris[1,2]. Activated microglial cells can also promote neuronal injury through the release of proinflammatory and cytotoxic factors, including tumor necrosis factor-alpha, interleukin-1beta, interleukin-6, nitric oxide and reactive oxygen species[2]. Chronic microglial activation has been implicated in the neuronal destruction associated with various neurodegenerative diseases such as Alzheimer's disease and Parkinson's disease[3,4]. Thus, the activation of counter-regulatory mechanisms is essential to avoid the escalation of central nervous system inflammatory processes[5]. The identification of agents that target overactivated microglial cells is important for providing neuroprotection.
Paeonol (2’-hydroxy-4’-methoxyacetophenone) (Figure 1) is a major phenolic component of moutan cortex (Paeonia Suffruticosa Andrews) and the root of Paeonia lactiflora Pall, which have been used in traditional oriental medicines to alleviate various disorders[6].
Figure 1.
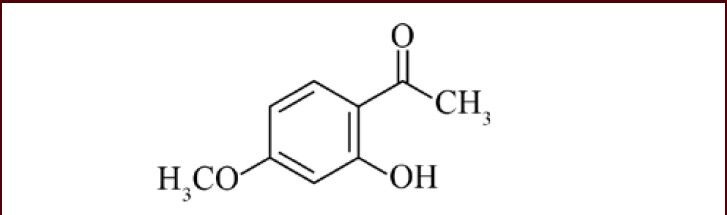
The chemical structure of paeonol.
Paeonol is a major phenolic compound in Paeonia species[6]. It has been suggested to readily traverse the blood-brain barrier under physiological conditions[7].
Paeonol has multiple pharmacological activities, including anti-oxidant, anti-atherosclerotic, anti-diabetic, anti-allergic, anti-inflammatory and anti-cancer effects[8,9,10,11,12,13]. For brain diseases in particular, paeonol has been reported to exert neuroprotective actions against cerebral ischemia and Alzheimer's disease in experimental models[14,15]. From these studies, paeonol has shown promise for the treatment of neuropathologies. However, the efficacy of paeonol in reducing inflammation-mediated neurotoxicity has not been determined. For this purpose, we employed organotypic hippocampal slice cultures, which provide a good model system for studying neuronal death and neuroprotective agents. Organotypic hippocampal slice cultures preserve the maturation of synapses, receptors, and intrinsic fiber pathways in vivo[16]. As organotypic hippocampal slice cultures also preserve physiological networks between neurons and glia, microglia-mediated neurotoxicity would be properly reflected in this system. We also examined whether paeonol could repress inflammatory responses in primary cultured microglial cells.
RESULTS
Paeonol protected against inflammation-mediated hippocampal cell damage
In the present study, hippocampal slice cultures exposed to lipopolysaccharide for 72 hours displayed strong propidium iodide uptake along the hippocampal layers in comparison with untreated control slices (Figure 2A). The increased propidium iodide uptake was markedly blocked by treatment with paeonol (Figures 2A, B), in parallel with the inhibition of lipopolysaccharide-induced production of proinflammatory mediators including nitric oxide and interleukin-1beta (Figures 2C, D).
Figure 2.
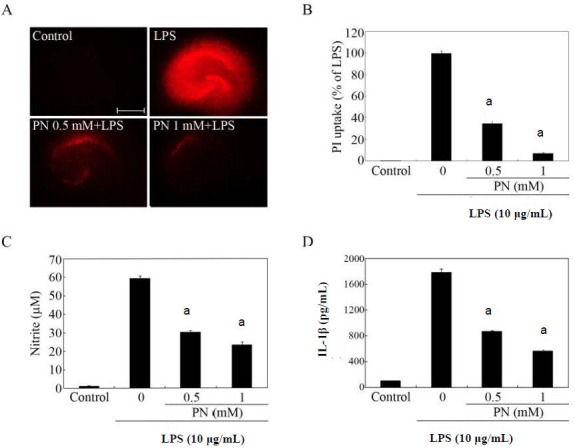
Effects of paeonol on lipopolysaccharide (LPS)-induced hippocampal cell death.
Organotypic hippocampal slice cultures were pretreated with paeonol (PN) at the indicated concentrations for 30 minutes before adding 10 μg/mL LPS. After stimulation with LPS for 72 hours, the culture medium was collected and subjected to nitrite and cytokine assays, and was subsequently replaced with fresh serum-free medium containing propidium iodide (PI). The red color is the PI fluorescence, which indicates cell membrane damage. (A) PI fluorescence images. Scale bar: 500 μm. (B) Quantification of hippocampal cell death. Data are expressed as the percentage of the LPS value (the fluorescence intensity of the LPS group was designated as 100%). (C, D) Determination of nitrite (production of nitric oxide) and interleukin-1beta in culture supernatants. (B–D) The data were expressed as mean ± SEM from triplicate assays. Ten to 15 hippocampal slices were used in each group. aP < 0.001, vs. LPS-only treated group (Student's paired t-test). Organotypic hippocampal slice cultures incubated in the absence of LPS for 72 hours served as controls. M: mol/L.
Inhibition of lipopolysaccharide-induced nitric oxide release from primary microglial cells by paeonol
Pretreatment with paeonol suppressed lipopolysaccharide-induced nitrite release from primary microglial cells (Figure 3A). Lipopolysaccharide has been reported to cause activation-induced cell death in microglia[17]. Thus, cell viability, as measured using the methyl thiazolyl tetrazolium (MTT) assay, was often reduced in the presence of lipopolysaccharide (Figure 3B). Based on MTT assay, paeonol did not reduce the viability of activated microglia at 0.5 and 1 mM (Figure 3B). However, paeonol showed cytotoxicity to lipopolysaccharide-activated microglial cells at 2.5 and 5 mM (Figure 3B). When we checked if paeonol itself triggers nitrite generation from microglial cells, we found that paeonol alone did not affect the basal level of nitrite (Figure 3C). Taken together, the optimal dose range of paeonol that was not toxic to the activated microglial cells appeared to be between 0.5 and 1 mM. Thus, these doses were selected for the other assays performed in this study.
Figure 3.
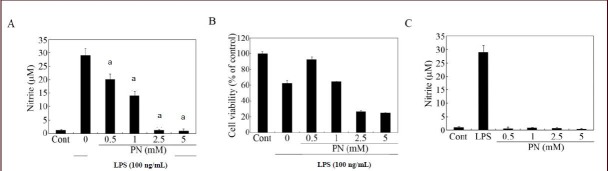
Effects of paeonol on nitrite production in lipopolysaccharide (LPS)-stimulated microglial cells.
Primary microglial cells were incubated in the absence (control; Cont) or presence of LPS (100 ng/mL). The cells were pretreated (A, B) with the indicated amounts of paeonol and LPS was added 30 minutes later. After 24 hours, the cultures were subjected to a nitrite assay (A) and a cell viability assay (B). As a reference, cells were treated with LPS or paeonol only for 24 hours, and the cultures were subjected to nitrite quantification (C). Data are expressed as mean ± SEM from triplicate assays. aP < 0.001, vs. the LPS-only treated group (Student's paired t-test). Microglial cells incubated in the absence of LPS for 24 hours served as Cont. M: mol/L.
Inhibition of lipopolysaccharide-induced secretion of proinflammatory cytokines from microglial cells by paeonol
To test the effects of paeonol on the production of proinflammatory molecules, the amounts of tumor necrosis factor-alpha and interleukin-1beta in the culture medium of primary microglial cells were measured by enzyme-linked immunosorbent assay (ELISA). Paeonol considerably reduced the lipopolysaccharide-induced production of these mediators (Figure 4).
Figure 4.
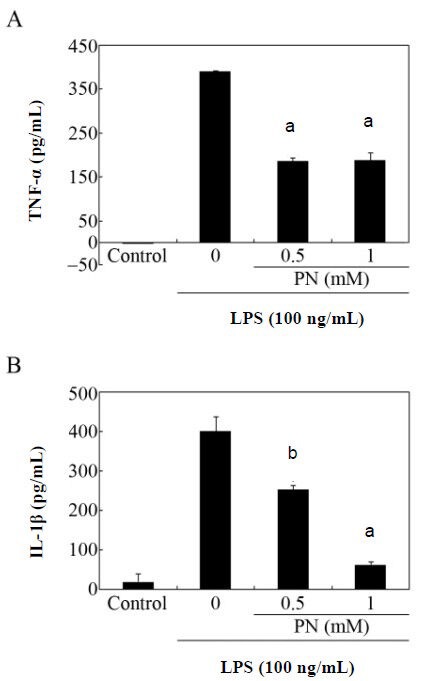
Effects of paeonol on the secretion of tumor necrosis factor-alpha (TNF-α) and interleukin-1 beta (IL-1β) by primary microglial cells.
Cultures were stimulated with lipopolysaccharide (LPS; 100 ng/mL) with or without pretreatment with the indicated amounts of paeonol (PN). After 24 hours of incubation, the culture supernatants were assayed by enzyme-linked immunosorbent assay (ELISA) for TNF-α (A) and IL-1β (B). Data are expressed as mean ± SEM from triplicate assays. aP < 0.001, bP < 0.05, vs. the LPS-only treated group (Student's paired t-test). The microglial cells incubated in the absence of LPS for 24 hours served as controls. M: mol/L.
DISCUSSION
In an effort to develop neuroprotective drugs, inhibition of the microglial inflammatory response is considered promising for the treatment of many neuropathologies[5,18]. We demonstrated a direct effect of paeonol against inflammation-induced neurotoxicity in hippocampal slice cultures in this study. We also showed the suppressive effect of paeonol on the production of nitric oxide and proinflammatory cytokines by primary microglial cells. Overall, our results broaden the range of pharmacological properties of paeonol for the treatment of neuropathologies.
We have established an experimental model to determine inflammation-induced neurotoxicity by lipopolysaccharide exposure to organotypic hippocampal slice cultures[19]. Organotypic hippocampal slice cultures preserve the maturation of synapses, receptors and intrinsic fiber pathways in vivo, thus providing a good model system in which to study neuronal cell death, neuroprotection and synaptic plasticity[20]. The results shown in Figure 2 indicate that paeonol has protective effects against inflammation-induced neurotoxicity.
Considering the results presented in Figure 3A and B together, it is intriguing that paeonol decreases cell viability further at higher concentrations but prevents lipopolysaccharide-induced production of nitric oxide in lipopolysaccharide-treated microglia. Although the precise mechanisms underlying suppression of microglial activation by paeonol need further investigation, it may work in a similar way to the p38 kinase inhibitor, SB239063, in activated microglia. In a recent report, SB239063 was shown to decrease the inflammatory response and the number of activated microglia in a model of oxygen-glucose-deprived neuronal death[21].
Among the diverse biological activities of paeonol, anti-inflammatory properties have been demonstrated in the peripheral system, including macrophages, synoviocytes and endothelial cells[22,23,24]. According to a very recent report, paeonol attenuates microglial inflammation in the BV2 cell line, which is generally consistent with the results of our study[25]. However, the range of effective doses was lower in the data reported by Himaya's group than in our study[25]. The apparent discrepancy in effective concentrations of the drug might be attributable to differences in the nature of the cultured cells. Nevertheless, both studies are consistent with the work by Hsieh et al[14], which indicates the anti-inflammatory effect of paeonol by reduced microglial activation and interleukin-1beta production in the brain of a rodent model of cerebral ischemia/reperfusion.
Besides inhibitory effects on brain inflammation, the direct neuroprotective potential of paeonol has been tested in vivo and in vitro. In a rat model of Alzheimer's disease, paeonol improved learning behavior and increased the expression of cytochrome oxidase in the hippocampus when injected with amyloid-beta[15]. In a model of ischemic brain damage, paeonol reduced cerebral infarction and improved neurological outcome[14]. In hippocampal neuron cultures exposed to oxygen-glucose deprivation, paeonol reversed the overload of intracellular calcium ions and inhibited the activity of N-methyl-D-aspartate receptors, which consequently enhanced cell survival[26]. Taken together with our results, paeonol possibly exerts multiple beneficial roles in controlling neuronal survival and microglial activation in the central nervous system. Exploration of the exact role of paeonol in neuronal survival and anti-inflammation is warranted in a wider spectrum of neuropathological conditions.
Recently, considerable attention has been focused on dietary and medicinal phytochemicals as sources of neuroprotective components. In particular, a list of polyphenols from natural products has been reported to be effective in controlling microglial activation. These include resveratrol, green tea polyphenols, wogonin from the root of the medicinal plant, Scutellaria baicalensis, and curcumin from Curcuma longa[7,27,28,29]. Our results support the neuroprotective potential of paeonol in targeting neurotoxicity induced by microglial inflammatory mediators. After administration into rats, paeonol has been detected in plasma and diverse tissues including brain[30], which indicates the permeability of paeonol to the blood-brain barrier. Although safety should be persistently estimated for clinical applications, the result is in favor of considering paeonol as a valuable candidate for neuroprotective drug development. Thus, the present study invites further study on the molecular targets and working mechanisms of paeonol in central nervous system cell types for precise estimation of its action in controlling the brain immune response.
MATERIALS AND METHODS
Design
A comparative observation, tissue and cell culture experiment.
Time and setting
The experiments were performed at the Graduate School of East-West Medical Science, Kyung Hee University, Korea, from September 2011 to August 2012.
Materials
The 7-day-old and 1-day-old rats used for extraction of hippocampal slices and culturing brain microglial cells, respectively, were maintained in accordance with the Institutional Animal Care and Use Committee guidelines of Kyung Hee University in Korea. All rats were provided by Orient, Kyunggi-Do, Korea.
All cell culture products were purchased from Invitrogen (Carlsbad, CA, USA). Escherichia coli lipopolysaccharide (L2637) and other chemicals were purchased from Sigma (St. Louis, MO, USA).
Paeonol (H35803) was purchased from Sigma. The molecular formula of paeonol is C9H10O3, with a molecular weight of 166.18. Its chemical name is phenanthro[1,2-b]furan-10,11-dione, 6, 7, 8, 9-tetrahydro-1, 6, 6 -trimethyl (CAS No. 552-41-0, EINECS No. 209-012-2). Paeonol is a white or light yellow glossy needle crystal.
Methods
Organotypic hippocampal slice cultures
Organotypic hippocampal slice cultures were prepared according to the method of Stoppini et al[31]. Briefly, the hippocampus was isolated and cut transversely at a thickness of 350 μm with a McIlwain Tissue Chopper (Mickle Laboratory Engineering, Surrey, UK). The slices were placed on membrane inserts (Millicell-CM, PICM03050; Millipore, Bedford, MA, USA) in 6-well plates. Each well contained 1 mL of culture medium composed of 50% MEM, 25% Hank's balanced salt solution and 25% horse serum. The slices were cultured in an incubator at 36°C in the presence of 5% CO2 for 12–14 days, and the medium was changed every 2 or 3 days.
Lipopolysaccharide treatment and assessment of neuronal damage
Neurotoxicity was evaluated by the uptake of the fluorescent dye, propidium iodide, as previously described[32]. Briefly, lipopolysaccharide (10 μg/mL) was applied to organotypic hippocampal slice cultures with or without pretreatment with paeonol (0.5 and 1 mmol/L). After lipopolysaccharide treatment for 72 hours, the culture medium was collected and subjected to the nitrite assay, and the medium was subsequently replaced with fresh serum-free medium containing 5 μg/mL propidium iodide (Sigma). Organotypic hippocampal slice cultures incubated in the absence of lipopolysaccharide for 72 hours served as controls. Neuronal death was observed within 30–60 minutes after addition of propidium iodide. Propidium iodide-stained images were captured using a laser scanning microscope (LSM 510; Carl Zeiss, Mannheim, Germany) and the observed propidium iodide uptake areas were measured by confocal microscopy with LSM 510 software (release 3.2; Carl Zeiss). All data were background subtracted using the fluorescence emission originating from a region on the insert containing no tissue.
Cell culture and treatment
Primary microglial cells were prepared from cerebral cortices of 1-day-old rat pups (Orient, Kyunggi-Do, Korea) as described previously[33,34]. After the cells reached 80–90% confluence at 12 to 14 days, the flasks were shaken to remove the microglia. The detached microglial cells were incubated for 1 hour and the non-adherent cells were removed. The adherent microglial cells were cultured for 24 hours, and the purity of the cultures was judged by immunostaining with a rat anti-mouse OX-42 antibody (CBL1512; 1:100 dilution; Chemicon, Temecula, CA, USA) followed by tetraethyl rhodamine isothiocyanate-conjugated anti-mouse antibody (Zymed Laboratories, San Francisco, CA, USA) as described in our previous work[35]. The cells were pretreated with paeonol in fresh medium containing 0.1% fetal bovine serum (Invitrogen) for 30 minutes before adding lipopolysaccharide to 100 ng/mL for 24 hours. A dose range of 0.5–5 mmol/L of paeonol was applied.
Nitrite assay
The nitrite in microglial culture supernatants was measured as an indicator of nitric oxide production. An aliquot of the culture supernatant was mixed with a volume of Griess reagent (G7921; Molecular Probes, Eugene, OR, USA) and the absorbance at 570 nm was determined using a microplate reader. Sodium nitrite at 0–100 μM was used as a standard to assess nitrite concentrations.
Cell viability assay
For the cell viability assay, microglial cultures were incubated in MTT solution (M5655; 1 mg/mL; Sigma) in two volumes of culture medium for 1 hour at 37°C. The MTT solution was then removed, the cells were dissolved in dimethyl sulfoxide (150 μL; D8418; Sigma), and the absorbance of the samples was measured at 570 nm using a microplate reader (Molecular Probes).
Cytokine assays
At the end of each treatment, the culture medium was collected in microcentrifuge tubes (Bio-Rad, Hercules, CA, USA). After centrifugation at 10 000 × g for 10 minutes, the culture supernatants were assayed for secreted mediators using tumor necrosis factor-alpha and interleukin-1beta immunoassay kits (RTA00 and RLB00; R&D Systems, Minneapolis, MN, USA) according to the manufacturer's instructions.
Statistical analysis
Data were expressed as mean ± SEM from at least three independent experiments. Student's paired t-test using SPSS 15.0 for Windows (SPSS, Chicago, IL, USA) was used for statistical analyses and P-values < 0.05 were reported as statistically significant.
Footnotes
Kyong Hyon Nam, Ph.D., Post-doctoral Fellow, Graduate School of East-West Medical Science, Kyung Hee University, Yongin-si 446-701, Republic of Korea
Conflicts of interest: None declared.
Ethical approval: All animal protocols used in this study were approved by the Animal Ethics Committee of Kyung Hee University, Republic of Korea, in accordance with the 14th article of the Korean Animal Protection Law.
(Reviewed by Lodge A, Wysong S, Bariskaner H, Lee EJ)
(Edited by Li CH, Song LP)
REFERENCES
- [1].Kaur G, Han SJ, Yang I, et al. Microglia and central nervous system immunity. Neurosurg Clin N Am. 2010;21:43–51. doi: 10.1016/j.nec.2009.08.009. [DOI] [PubMed] [Google Scholar]
- [2].Graeber MB, Streit WJ. Microglia: biology and pathology. Acta Neuropathol. 2010;119:89–105. doi: 10.1007/s00401-009-0622-0. [DOI] [PubMed] [Google Scholar]
- [3].Frank-Cannon TC, Alto LT, McAlpine FE, et al. Does neuroinflammation fan the flame in neurodegenerative disease? Mol Neurodegener. 2009;4:47. doi: 10.1186/1750-1326-4-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Sugama S, Takenouchi T, Cho BP, et al. Possible roles of microglial cells for neurotoxicity in clinical neurodegenerative diseases and experimental animal models. Inflamm Allergy Drug Targets. 2009;8:277–284. doi: 10.2174/187152809789352249. [DOI] [PubMed] [Google Scholar]
- [5].McCarty MF. Down-regulation of microglial activation may represent a practical strategy for combating neurodegenerative disorders. Med Hypotheses. 2006;67:251–269. doi: 10.1016/j.mehy.2006.01.013. [DOI] [PubMed] [Google Scholar]
- [6].Wu SH, Wu DG, Chen YW. Chemical constituents and bioactivities of plants from the genus Paeonia. Chem Biodivers. 2010;7:90–104. doi: 10.1002/cbdv.200800148. [DOI] [PubMed] [Google Scholar]
- [7].Jung KK, Lee HS, Cho JY, et al. Inhibitory effect of curcumin on nitric oxide production from lipopolysaccharide-activated primary microglia. Life Sci. 2006;79:2022–2031. doi: 10.1016/j.lfs.2006.06.048. [DOI] [PubMed] [Google Scholar]
- [8].Okubo T, Nagai F, Seto T, et al. The inhibition of phenylhydroquinone-induced oxidative DNA cleavage by constituents of Moutan Cortex and Paeoniae Radix. Biol Pharm Bull. 2000;23:199–203. doi: 10.1248/bpb.23.199. [DOI] [PubMed] [Google Scholar]
- [9].Li H, Dai M, Jia W. Paeonol attenuates high-fat-diet-induced atherosclerosis in rabbits by anti-inflammatory activity. Planta Med. 2009;75:7–11. doi: 10.1055/s-0028-1088332. [DOI] [PubMed] [Google Scholar]
- [10].Lau CH, Chan CM, Chan YW, et al. Pharmacological investigations of the anti-diabetic effect of Cortex Moutan and its active component paeonol. Phytomedicine. 2007;14:778–784. doi: 10.1016/j.phymed.2007.01.007. [DOI] [PubMed] [Google Scholar]
- [11].Lee B, Shin YW, Bae EA, et al. Antiallergic effect of the root of Paeonia lactiflora and its constituents paeoniflorin and paeonol. Arch Pharm Res. 2008;31:445–450. doi: 10.1007/s12272-001-1177-6. [DOI] [PubMed] [Google Scholar]
- [12].Chou TC. Anti-inflammatory and analgesic effects of paeonol in carrageenan-evoked thermal hyperalgesia. Br J Pharmacol. 2003;139:1146–1152. doi: 10.1038/sj.bjp.0705360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Xu SP, Sun GP, Shen YX, et al. Antiproliferation and apoptosis induction of paeonol in HepG2 cells. World J Gastroenterol. 2007;13:250–256. doi: 10.3748/wjg.v13.i2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hsieh CL, Cheng CY, Tsai TH, et al. Paeonol reduced cerebral infarction involving the superoxide anion and microglia activation in ischemia-reperfusion injured rats. J Ethnopharmacol. 2006;106:208–215. doi: 10.1016/j.jep.2005.12.027. [DOI] [PubMed] [Google Scholar]
- [15].Zhou J, Zhou L, Hou D, et al. Paeonol increases levels of cortical cytochrome oxidase and vascular actin and improves behavior in a rat model of Alzheimer's disease. Brain Res. 2011;1388:141–147. doi: 10.1016/j.brainres.2011.02.064. [DOI] [PubMed] [Google Scholar]
- [16].Holopainen IE. Organotypic hippocampal slice cultures: a model system to study basic cellular and molecular mechanisms of neuronal death, neuroprotection, and synaptic plasticity. Neurochem Res. 2005;30:1521–1528. doi: 10.1007/s11064-005-8829-5. [DOI] [PubMed] [Google Scholar]
- [17].Lee P, Lee J, Kim S, et al. NO as an autocrine mediator in the apoptosis of activated microglial cells: correlation between activation and apoptosis of microglial cells. Brain Res. 2001;892:380–385. doi: 10.1016/s0006-8993(00)03257-1. [DOI] [PubMed] [Google Scholar]
- [18].Stoll G, Jander S. The role of microglia and macrophages in the pathophysiology of the CNS. Prog Neurobiol. 1999;58:233–247. doi: 10.1016/s0301-0082(98)00083-5. [DOI] [PubMed] [Google Scholar]
- [19].Nam KN, Son MS, Park JH, et al. Shikonins attenuate microglial inflammatory responses by inhibition of ERK, Akt, and NF-kappaB: neuroprotective implications. Neuropharmacology. 2008;55:819–825. doi: 10.1016/j.neuropharm.2008.06.065. [DOI] [PubMed] [Google Scholar]
- [20].Holopainen IE. Organotypic hippocampal slice cultures: a model system to study basic cellular and molecular mechanisms of neuronal cell death, neuroprotection, and synaptic plasticity. Neurochem Res. 2005;30:1521–1528. doi: 10.1007/s11064-005-8829-5. [DOI] [PubMed] [Google Scholar]
- [21].Strassburger M, Braun H, Reymann KG. Anti-inflammatory treatment with the p38 mitogen-activated protein kinase inhibitor SB239063 is neuroprotective, decreases the number of activated microglia and facilitates neurogenesis in oxygen-glucose-deprived hippocampal slice cultures. Eur J Pharmacol. 2008;592:55–61. doi: 10.1016/j.ejphar.2008.06.099. [DOI] [PubMed] [Google Scholar]
- [22].Huang H, Chang EJ, Lee Y, et al. A genome-wide microarray analysis reveals anti-inflammatory target genes of paeonol in macrophages. Inflamm Res. 2008;57:189–198. doi: 10.1007/s00011-007-7190-3. [DOI] [PubMed] [Google Scholar]
- [23].Wu M, Gu Z. Screening of bioactive compounds from moutan cortex and their anti-inflammatory activities in rat synoviocytes. Evid Based Complement Alternat Med. 2009;6:57–63. doi: 10.1093/ecam/nem066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Nizamutdinova IT, Oh HM, Min YN, et al. Paeonol suppresses intercellular adhesion molecule-1 expression in tumor necrosis factor-alpha-stimulated human umbilical vein endothelial cells by blocking p38, ERK and nuclear factor-kappaB signaling pathways. Int Immunopharmacol. 2007;7:343–350. doi: 10.1016/j.intimp.2006.11.004. [DOI] [PubMed] [Google Scholar]
- [25].Himaya SW, Ryu B, Qian ZJ, et al. Paeonol from Hippocampus kuda Bleeler suppressed the neuro-inflammatory responses in vitro via NF-κB and MAPK signaling pathways. Toxicol In Vitro. 2012;26:878–887. doi: 10.1016/j.tiv.2012.04.022. [DOI] [PubMed] [Google Scholar]
- [26].Wu JB, Song NN, Wei XB, et al. Protective effects of paeonol on cultured rat hippocampal neurons against oxygen-glucose deprivation-induced injury. J Neurol Sci. 2008;264:50–55. doi: 10.1016/j.jns.2007.06.057. [DOI] [PubMed] [Google Scholar]
- [27].Zhang F, Kiu J, Shi JS. Anti-inflammatory activities of resveratrol in the brain: role of resveratrol in microglial activation. Eur J Pharmacol. 2010;636:1–7. doi: 10.1016/j.ejphar.2010.03.043. [DOI] [PubMed] [Google Scholar]
- [28].Li R, Huang YG, Fang D, et al. (-)-Epigallocatechin gallate inhibits lipopolysaccharide-induced microglial activation and protects against inflammation-mediated dopaminergic neuronal injury. J Neurosci Res. 2004;78:723–731. doi: 10.1002/jnr.20315. [DOI] [PubMed] [Google Scholar]
- [29].Lee H, Kim YO, Kim H, et al. Flavonoid wogonin from medicinal herb is neuroprotective by inhibiting inflammatory activation of microglia. FASEB J. 2003;17:1943–1944. doi: 10.1096/fj.03-0057fje. [DOI] [PubMed] [Google Scholar]
- [30].Li H, Wang S, Zhang B, et al. Influence of co-administered danshensu on pharmacokinetic fate and tissue distribution of paeonol in rats. Planta Med. 2012;78:135–140. doi: 10.1055/s-0031-1280269. [DOI] [PubMed] [Google Scholar]
- [31].Stoppini L, Buchs PA, Muller D. A simple method for organotypic cultures of nervous tissue. J Neurosci Methods. 1991;37:173–182. doi: 10.1016/0165-0270(91)90128-m. [DOI] [PubMed] [Google Scholar]
- [32].Vornov JJ, Tasker RC, Park J. Neurotoxicity of acute glutamate transport blockade depends on coactivation of both NMDA and AMPA/kainite receptors in organotypic hippocampal cultures. Exp Neurol. 1995;133:7–17. doi: 10.1006/exnr.1995.1002. [DOI] [PubMed] [Google Scholar]
- [33].Lee KH, Yun SJ, Nam KN, et al. Activation of microglial cells by ceruloplasmin. Brain Res. 2007;1171:1–8. doi: 10.1016/j.brainres.2007.07.053. [DOI] [PubMed] [Google Scholar]
- [34].McCarthy KD, de Vellis J. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J Cell Biol. 1980;85:890–902. doi: 10.1083/jcb.85.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Nam KN, Kim JH, Jung HJ, et al. Berberine inhibits inflammatory activation of rat brain microglia. Neural Regen Res. 2010;5:1384–1390. [Google Scholar]


