Abstract
OBJECTIVE:
To investigate the efficacy and safety of Chinese herbal medicines in the treatment of patients with vascular dementia.
DATA RETRIEVAL:
We retrieved publications from Cochrane Library (2004 to July 2011), PubMed (1966 to July 2011), the Chinese Science and Technique Journals Database (1977 to July 2011), the China National Knowledge Infrastructure (1979 to July 2011), Google Scholar (July 2011), and the Chinese Biomedical Database (1977 to July 2011) using the key words “Chinese medicine OR Chinese herbal medicine” and “vascular dementia OR mild cognition impair OR multi-infarct dementia OR small-vessel dementia OR strategic infarct dementia OR hypoperfusion dementia OR hemorrhagic dementia OR hereditary vascular dementia“.
SELECTION CRITERIA:
Randomized controlled trials comparing Chinese herbal medicines with placebo/western medicine in the treatment of patients with vascular dementia were included. Diagnostic standards included Diagnostic and Statistical Manual of Mental Disorders-IV, and National Institute of Neurological Disorders and Stroke and Association Internationale pour la Recherché et l’Enseignement en Neurosciences. Two participants independently conducted literature screening, quality evaluation and data extraction. The quality of each trial was assessed according to the Cochrane Reviewers’ Handbook 5.0.
MAIN OUTCOME MEASURES:
Effective rate, Mini-Mental State Examination scores, Hasegawa Dementia Scale scores, and incidence of adverse reactions.
RESULTS:
We identified 1 143 articles discussing the effects of Chinese medicine on vascular dementia. Thirty-one of these were included in the analysis. These studies involved a total of 2 868 participants (1 605 patients took Chinese medicine decoctions (treatment group); 1 263 patients took western medicine or placebo). The results of our meta-analysis revealed that Chinese herbal remedies in the treatment group were more efficacious than the control intervention (relative risk (RR) = 1.27; 95% confidence interval (CI): 1.18–1.38, P < 0.01). Mini-Mental State Examination scores were higher in patients taking Chinese herbal medicines than in those in the control group (weighted mean difference (WMD) = 2.83; 95%CI: 2.55–3.12, P < 0.01). Patients in the treatment group showed better disease amelioration than those in the control group (Hasegawa Dementia Scale scores; WMD = 2.41, 95%CI: 1.48–3.34, P < 0.01). There were also considerably fewer adverse reactions among those in the treatment group compared with those in the control group (RR = 0.20, 95%CI: 0.08–0.47, P < 0.01).
CONCLUSION:
Chinese herbal medicine appears to be safer and more effective than control measures in the treatment of vascular dementia. However, the included trials were generally low in quality. More well-designed, high-quality trials are needed to provide better evidence for the assessment of the efficacy and safety of Chinese medicines for vascular dementia.
Keywords: neural regeneration, Chinese herbal medicine, meta-analysis, vascular dementia, mild cognitive impairment, decoction, efficacy, Mini-Mental State Examination, Hasegawa Dementia Scale, adverse reaction, neurodegenerative disease, grants-supported paper, neuroregeneration
Research Highlights
(1) By performing a meta-analysis, we evaluated the efficacy and safety of Chinese herbal medicines for vascular dementia, using efficacy, Mini-Mental State Examination score, Hasegawa Dementia Scale score, and adverse reactions as evaluation indices.
(2) Our results suggested that Chinese herbal medicine appears to be safer and more effective than control measures in the treatment of vascular dementia. Chinese herbal medicines for vascular dementia exert characteristics of syndrome differentiation of traditional Chinese medicine, and have good potential in the clinic.
INTRODUCTION
Global dementia prevalence is predicted to double every 20 years to an expected 66 million in 2030, and 115 million in 2050. The burden of these diseases is considerable when taken together with the burden from the 15 million people worldwide who will suffer from stroke every year[1]. Aggarwal and Decarli[2] defined vascular dementia as “a term used to describe a constellation of cognitive and functional impairment that can be viewed as a subset of the larger syndrome of vascular cognitive impairment associated with cerebrovascular brain injury”. A common disorder among the elderly, it is also the second most common form of dementia after Alzheimer's disease in the United States[3]. There is still no definitive medical or surgical treatment available for vascular dementia in the Western world[3]. Chinese herbal medicine, which has been used for thousands of years in China, has long been considered an effective treatment for vascular dementia[4]. There are already meta-analyses of the effects of herbal extracts (ginkgo biloba and huperzine A) on vascular dementia[5]. However, there has been no systematic review of the efficacy and safety of Chinese herbal medicines for vascular dementia, despite its wide use in clinical practice. Therefore, we believe such a systematic review would be timely, and could provide better scientific evidence for the clinical treatment of vascular dementia.
DATA AND METHODOLOGY
Data retrieval
In July 2011, we searched the following databases: Cochrane Library (2004 to July 2011), PubMed (1966 to July 2011), Google Scholar (July 2011), the Chinese Biomedical Database (1977 to July 2011), the Chinese Science and Technique Journals Database (VIP) (1977 to July 2011) and the China National Knowledge Infrastructure (1979 to July 2011). The key words for retrieval were “Chinese medicine OR Chinese herbal medicine” and “vascular dementia OR mild cognition impair OR multi-infarct dementia OR small-vessel dementia OR strategic infarct dementia OR hypoperfusion dementia OR hemorrhagic dementia OR hereditary vascular dementia”.
Inclusion and exclusion criteria
We selected randomized controlled clinical trials assessing the efficacy and safety of Chinese herbal medicines for vascular dementia. Patient participation requirement: all participants eligible for participation in the trials were diagnosed with vascular dementia according to internationally accepted diagnostic criteria[6]. Patients with serious heart, liver, kidney or hematopoietic system diseases were considered ineligible for participation. Moreover, patients suffering from intensive diabetes, active epilepsy and mental illnesses were excluded. Regarding the types of interventions, clinical trials with a minimum treatment duration of 4 weeks and a minimum of 20 participants for each group were eligible for inclusion. The control intervention was either placebo or western medicine. Studies in which the intervention was a combination of Chinese herbal medicine and western medicine or other forms of treatment were excluded. Trials without a control group were excluded, and those whose materials were incomplete or duplicate publications were excluded.
Data extraction and quality evaluation
Two authors independently examined the titles and abstracts of all of the articles identified in searches, for consideration for inclusion, basing their judgment on the selection criteria outlined above. The full texts of potentially relevant articles were then retrieved for a more detailed assessment. Any disagreement was resolved by further discussions or consulting a third party. Information on author, intervention, outcomes and patient number were collected independently by the two authors and entered into a pre-formulated form. The form was cross-checked. If necessary, we contacted the author of the original article for more details about the study.
After the selection process, two authors assessed the quality of the included studies independently. This was done by examining whether each included trial met the following internal validity criteria[7]: (1) using randomization; (2) adequate allocation concealment; (3) blinding of participants; (4) reporting patients lost to follow-up; (5) doing an intention-to-treat analysis. Studies meeting all of the standards outlined above had the least possibility of bias. Such studies were ranked grade A. If a study met some of these items or other items were not mentioned in the article, it was ranked grade B. If a study met only one or two of the standards outlined above, meaning that some quality control measures were not used or were incorrectly used, the study was considered to have a high risk of bias and was ranked grade C. Any disagreement was resolved by further discussion or consulting with a third party.
Outcome measures
The outcome measures were (1) effective rate, (2) Mini-Mental State Examination scores, (3) Hasegawa Dementia Scale scores, and (4) incidence of adverse reactions.
Statistical analysis
We used the software RevMan 5.0 provided by the Cochrane Collaborations (http://www.thecochranelibrary.com) for data analysis in this review. Results are presented using relative risk (RR) for dichotomous data and weighted mean difference (WMD) for continuous data, both with 95% confidence interval (CI). A standard chi-squared statistic or I2 statistic was used to test heterogeneity between groups. Data were analyzed with a fixed-effect model if no statistical heterogeneity was observed between subgroups (P > 0.1, I2 < 50%). In the presence of heterogeneity (P < 0.1, I2 > 50%), a random-effect model was used and the possible causes of heterogeneity were examined.
RESULTS
Data retrieval
A total of 1 143 articles discussing the effects of Chinese herbal medicine on vascular dementia were identified from our preliminary search. This number decreased to 123 after we excluded reviews, repetitions, experiential summaries, theoretical investigations and papers on acupuncture or experimental research. After reviewing the full text of each article, we excluded another 92 articles for the following reasons: 18 studies were not randomized and controlled. In one study, the memory score of the patients was the only outcome measure. In another six studies, participants in the treatment group received not only Chinese herbal remedies; 54 studies involved patients with special syndromes. The research purpose of 13 studies was divergent from the purpose of our systemic analysis. Finally, 31 studies were included in this systematic review.
General characteristics of the included studies
All of the clinical trials were described as randomized controlled trials, but none of them reported sample size calculations. The 31 included studies involved a total of 2 868 participants (1 605 patients took Chinese medicine decoctions; 1 263 patients took Western medicine or placebo), with the largest trial containing 300 participants and the smallest containing 40 participants. Patients ranged in age from 45 to 88 years. The trials lasted from 30 to 168 days.
In the 31 included studies, all patients in the treatment group took Chinese herbal medicine. Patients in the control groups of 30 studies took Western medicine, and those in another study took placebo. With regard to outcome measures, effective rates were reported in 25 studies, Mini-Mental State Examination scores in 26 studies, incidence of adverse reactions in 17 studies, Hasegawa Dementia Scale scores in 15 studies, activities of daily living scores in eight studies, traditional Chinese medicine symptom scores in four studies and Alzheimer's Disease Assessment Scale-cognitive subscale scores in one study. Basic information about each study included in this systematic analysis is shown in Table 1.
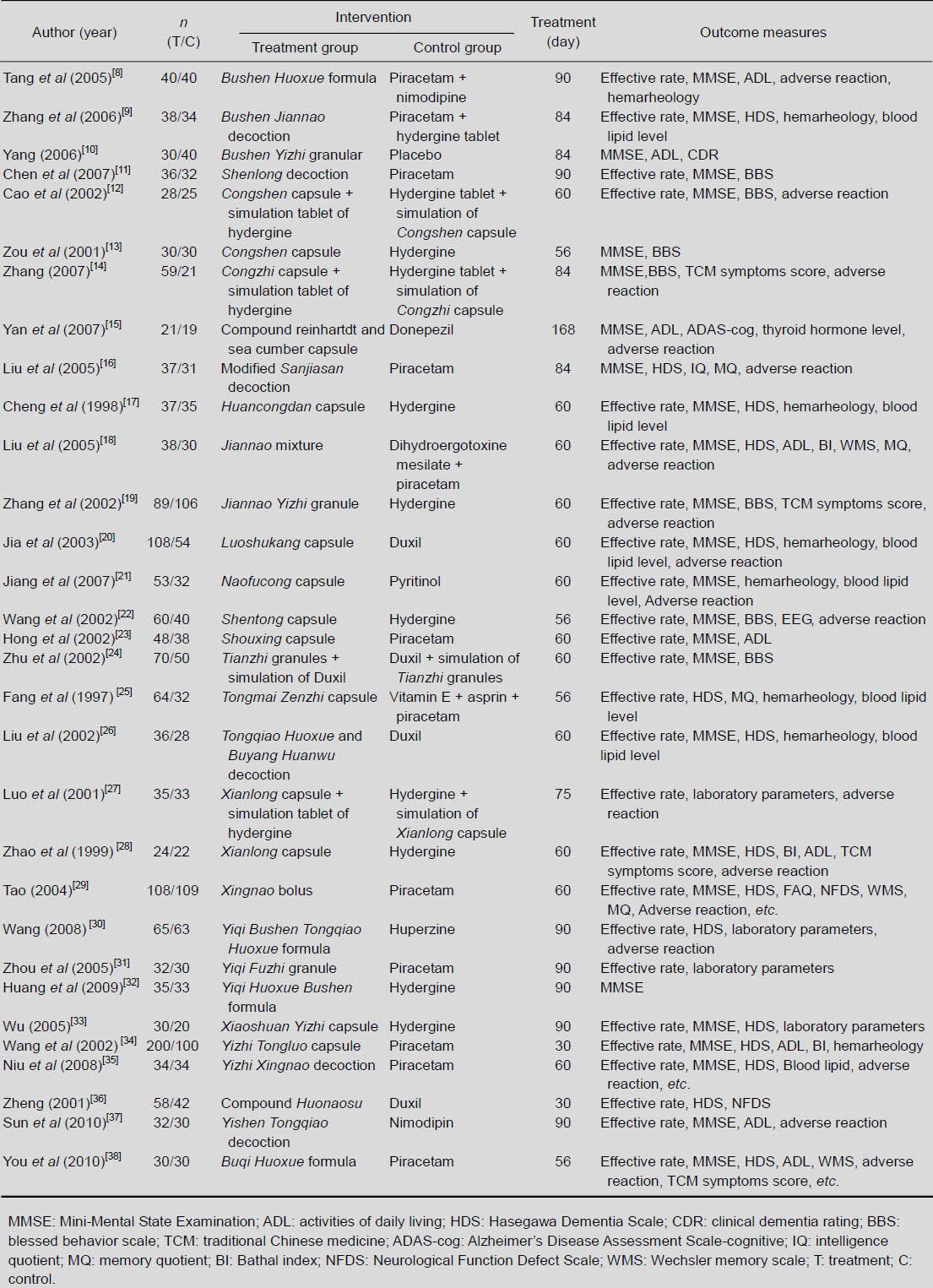
Table 1.
Detailed information on the studies included
Quality of included studies
As detailed in Table 2, differences in the methodological quality between the individual studies included in our review were quite evident[6].
Table 2.
Detailed information for assessing the risk of bias in the included studies
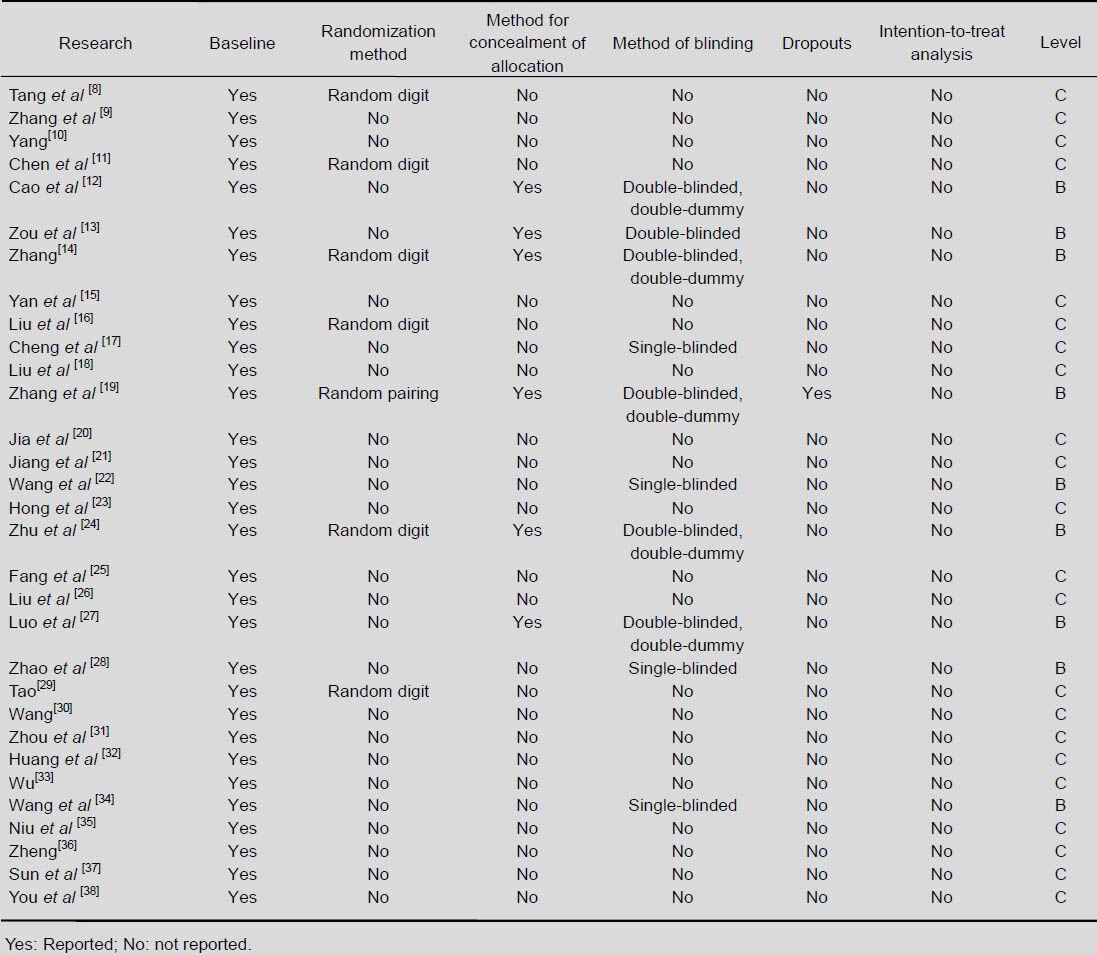
(1) Randomization method: All 31 studies described themselves as randomized, but only seven of them specified the randomization method.
(2) Allocation concealment: Six studies mentioned the method for concealment of allocation.
(3) Method of blinding: Five studies claimed to be double-blinded and double-dummy. One study was described as double-blinded, and the remaining four studies were single-blinded.
(4) Dropouts and intention-to-treat analysis: Only one study mentioned the number of patients who withdrew from the trial, but no intention-to-treat analysis was carried out.
(5) Baseline: 31 studies included a comparison of baseline information between the treatment group and the control group. It seemed that both groups shared similar baseline age, gender, disease duration and severity of disease.
Meta-analysis results
Effective rate
Twenty-five studies reported the therapeutically effective rate, and 16 of them used the same evaluation criteria, i.e., Clinical Research Guiding Principle for the New Chinese Herbal Medicine[39]. As there was no heterogeneity between groups in the 16 studies (x2 = 18.69, df = 15, P = 0.23, I2 = 20%), a fixed-effect model was applied. Meta-analysis of the 16 studies indicated that Chinese herbal remedies in the treatment group were more efficacious than the control intervention (RR = 1.27; 95%CI: 1.18–1.38, P < 0.01; Figure 1).
Figure 1.
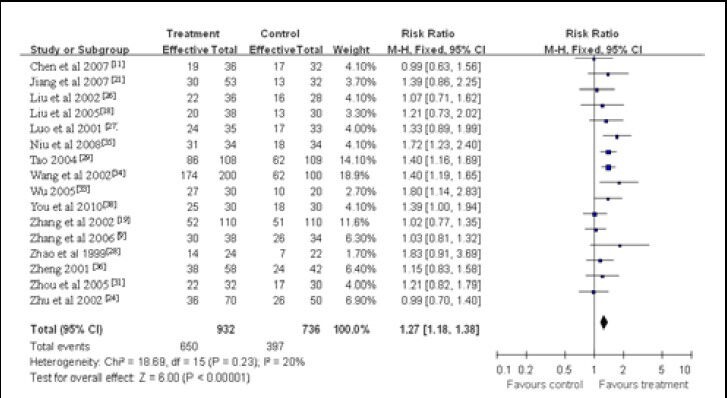
Meta-analysis of the effective rate in vascular dementia patients treated with Chinese herbal medicines.
Mini-Mental State Examination scores after treatment
An aggregate of 26 studies reported Mini-Mental State Examination scores of the patients with vascular dementia. A random-effect model applied as obvious heterogeneity was observed between groups in these studies (x2 = 331.74, df = 25, P < 0.000 01, I2 = 92%). Meta-analysis of the 26 studies indicated that Mini-Mental State Examination scores were higher in patients taking Chinese herbal medicines than in those in the control group (WMD = 2.83; 95%CI: 2.55–3.12, P < 0.01; Figure 2).
Figure 2.
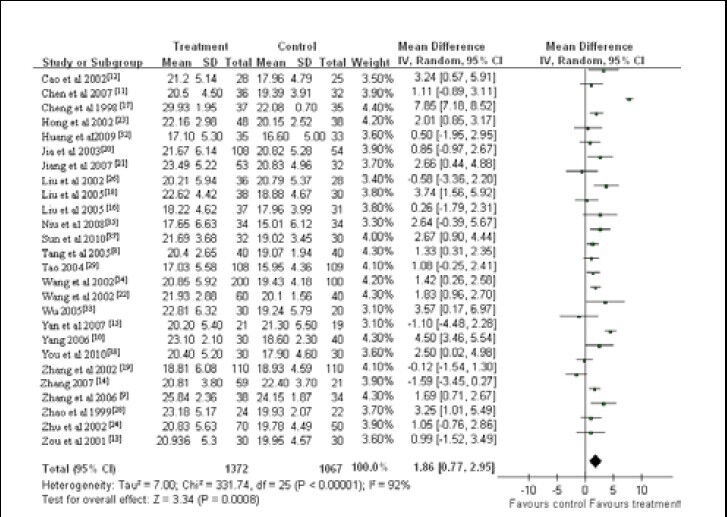
Meta-analysis of the Mini-Mental State Examination scores of vascular dementia patients treated with Chinese herbal medicines.
A high mini-mental state examination score indicates good cognitive ability.
Hasegawa Dementia Scale scores after treatment
A total of 15 studies reported Hasegawa Dementia Scale scores of the clinical trial participants. As there was heterogeneity between groups in these studies (x2 = 106.28, df = 14, P < 0.000 01, I2 = 87%), a random-effect model was used. Meta-analysis indicated that patients in the treatment group showed better disease amelioration than those in the control group (WMD = 2.41, 95%CI: 1.48–3.34, P < 0.01; Figure 3).
Figure 3.
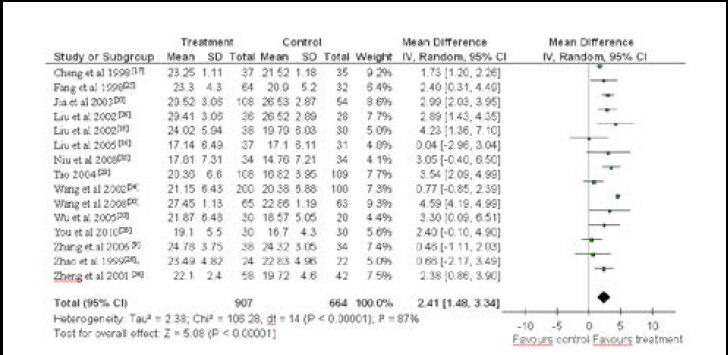
Meta-analysis of the Hasegawa Dementia Scale scores of vascular dementia patients treated with Chinese herbal medicines.
A high Hasegawa Dementia Scale score indicates good cognitive ability.
Adverse reactions
Seventeen studies reported on adverse effects occurring in patients from both the treatment group and the control group. No between-group heterogeneity was observed (x2 = 7.21, df = 4, P = 0.13, I2 = 45%), so a fixed-effect model was adopted. Meta-analysis showed that there were considerably fewer adverse reactions in the treatment group than in the control group (RR = 0.20, 95%CI: 0.08–0.47, P < 0.01). The main reported adverse reactions in the treatment group were nausea, diarrhea and pruritus (Figure 4).
Figure 4.
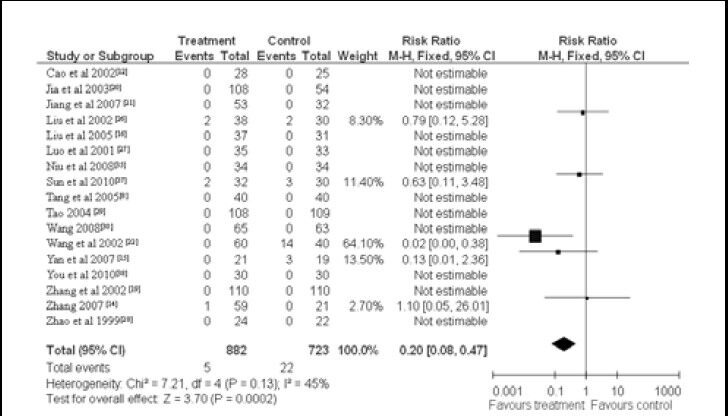
Meta-analysis of the incidence of adverse reactions in vascular dementia patients treated with Chinese herbal medicines.
DISCUSSION
Chinese herbal medicine is widely used in China to treat vascular dementia. We carried out a comprehensive systemic analysis to evaluate the efficacy and safety of Chinese herbal medicine (here we are mainly referring to Chinese herbal medicine decoctions) in clinical treatment of vascular dementia because no such systematic review has ever been performed. The results are promising; however, evidence provided in this study may not be reliable owing to the poor quality of the included trials.
For example, only one study assessed the improvement in the Alzheimer's Disease Assessment Scale-Cognitive subscale, a worldwide accepted cognitive testing instrument, to indicate a treatment effect of the medicine. Many studies reported Chinese medicine symptom scores, a measure that is not very well recognized internationally. As a result, we analyzed effective rate, Mini-Mental State Examination scores, Hasegawa Dementia Scale scores and incidence of adverse reactions in this meta-analysis. The results showed that Chinese herbal medicine has a better treatment effect, improves Mini-Mental State Examination and Hasegawa Dementia Scale scores by more, and causes fewer adverse reactions than the interventions in the control group.
Limitations of this study
(1) All of the included trials were conducted in China. (2) Only nine studies were blinded. Five of them were double-blinded, double-dummy studies. Even these studies did not detail the randomization method. Moreover, few of the studies used allocation concealment, and some did not report adverse events. For those studies without adequate explanation of quality control measures, we cannot rule out the possibility of a selective bias, implementation bias and measurement bias, which may lead to unreliable results. (3) In 16 studies, the effective rate was calculated according to a national standard, i.e., clinical research guiding principle for the new Chinese herbal medicine. We do not know to which extent this will reflect the real treatment effect of the medicine. (4) Most trials used Mini-Mental State Examination, Hasegawa Dementia Scale and other self-developed scales as tools for evaluating the clinical effects of the treatment. Only one of the 31 included studies used the Alzheimer's Disease Assessment Scale-cognitive subscale, an internationally recognized cognitive assessment tool. (5) The duration of the trials ranged from 30 to 168 days, and most of them lasted about 30 days. The average duration was relatively short. Therefore, endpoint outcomes such as mortality rate were seldom reported.
Implications for future research
We found evidence that Chinese herbal medicine outperforms Western medicine or placebo in the treatment of vascular dementia. However, confirmation of these conclusions in rigorously controlled, randomized trials is required before firm conclusions about traditional Chinese medicine therapy can be drawn. It is highly recommended that these trials were double-blinded and with a large sample size. They should have detailed descriptions of allocation concealment and the randomization method, as required by the CONSORT guidelines. Also, outcome measures and trial duration should be determined while taking the characteristics of the disease in question into consideration.
Acknowledgments:
We thank academician Boli Zhang from Tianjin University of Traditional Chinese Medicine in China who provided us with a very highly qualified team.
Footnotes
Xiude Qin, M.D., Associate researcher.
Funding: This work was supported by a Special Funding Project for the Chinese National Outstanding Ph.D. Thesis Author, No. 201082; the First Grade of China Postdoctoral Science Foundation, No. 20110490080; and the National Natural Science Foundation of China, No. 81202653.
Conflicts of interest: None declared.
(Reviewed by McGowan D, Hindle A, Wang YJ, Li LX)
(Edited by Wang LM, Qiu Y, Li CH, Song LP)
REFERENCES
- [1].Cumming T, Brodtmann A. Dementia and stroke: the present and future epidemic. Int J Stroke. 2010;5(6):453–454. doi: 10.1111/j.1747-4949.2010.00527.x. [DOI] [PubMed] [Google Scholar]
- [2].Aggarwal NT, Decarli C. Vascular dementia: emerging trends. Semin Neurol. 2007;27(1):66–77. doi: 10.1055/s-2006-956757. [DOI] [PubMed] [Google Scholar]
- [3].Hao Z, Liu M, Liu Z, et al. Huperzine A for vascular dementia. Cochrane Database Syst Rev. 2009;2:CD007365. doi: 10.1002/14651858.CD007365.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Luo ZG, Zou FX, Zhou WQ. The review of Chinese medicine on VD. Zhongguo Zhongyi Jichu Yixue Zazhi. 2001;7(5):76–77. [Google Scholar]
- [5].Birks J, Grimley Evans J. Ginkgo biloba for cognitive impairment and dementia. Cochrane Database Syst Rev. 2007;2:CD003120. doi: 10.1002/14651858.CD003120. [DOI] [PubMed] [Google Scholar]
- [6].Wiederkehr S, Simard M, Fortin C, et al. Validity of the clinical diagnostic criteria for vascular dementia: a critical review. Part II. J Neuropsychiatry Clin Neurosci. 2008;20(2):162–177. doi: 10.1176/jnp.2008.20.2.162. [DOI] [PubMed] [Google Scholar]
- [7].New York: John Wiley & Sons Inc; 2006. The Cochrane Collaboration. Cochrane Handbook for Systematic Reviews of Interventions 4.2.6. [Google Scholar]
- [8].Tang XJ, Lao YR, Yang ZM, et al. Clinical observation on modified Bushen Huoxie Decoction for the treatment of vascular dementia. Guangzhou Zhongyiyao Daxue Xuebao. 2005;22(6):426–429. [Google Scholar]
- [9].Zhang WY, Lu ZP. Clinical study on effect of Bushenjiannao Decoction in vascular dementia patients. Zhongguo Zhongxiyi Jiehe Xin Nao Xueguan Bing Zazhi. 2006;4(8):680–681. [Google Scholar]
- [10].Yang DD. Clinical observation on Bushen Yizhi granule treating light and midrange vascular dementia. Chengdu Zhongyiyao Daxue Xuebao. 2006;29(2):6–7. [Google Scholar]
- [11].Chen LP, Wang FW, Jia JJ, et al. Effects of Shenlong decoction on life quality of the aged patients with cerebral vascular dementia. Jiefangjun Yixue Zazhi. 2007;32(8):866–868. [Google Scholar]
- [12].Cao XL, Song XX, Hu ZQ, et al. Clinical study on treatment of vascular dementia with Congsheng capsule. Zhongguo Zhongyi Jizheng. 2002;11(2):80–81. [Google Scholar]
- [13].Zou YH, Xie YZ, Gao Y, et al. Clinical observation of 30 cases of vascular dementia treated with Congsheng capsules. Beijing Zhongyiyao Daxue Xuebao. 2001;24(6):54–57. [Google Scholar]
- [14].Zhang CR. Changchun: Jilin University; 2007. Clinical study of Congzhi granules on treatment of vascular dementia. [Google Scholar]
- [15].Yan YX, Liang LZ, Zhou ZL. Clinical study of combined treatment with compound Reinhartdt and Sea Cumber Capsule and donepezil for vascular dementia. Zhongguo Zhongxiyi Jiehe Zazhi. 2007;27(10):887–890. [PubMed] [Google Scholar]
- [16].Liu T, Wang CH, Yang J, et al. Modified Sanjiasan decoction in regulating intelligence state of patients with vascular dementia. Zhongguo Zhongxiyi Jiehe Zazhi. 2005;25(6):492–495. [PubMed] [Google Scholar]
- [17].Cheng WW, Zhou WQ, Chen K, et al. Clinical study on effect of Huancongdan capsule in treating senile vascular dementia. Zhongguo Zhongxiyi Jiehe Zazhi. 1998;18(2):81–83. [PubMed] [Google Scholar]
- [18].Liu DH, Zhou WQ, Xu ZZ, et al. Clinical study on Jiannao mixture for senile vascular dementia. Fujian Zhongyi Xueyuan Xuebao. 2005;12(2):4–7. [Google Scholar]
- [19].Zhang BL, Wang YY, Chen RX, et al. Clinical randomized double blinded study on treatment of vascular dementia by Jiannao Yizhi granule. Zhongguo Zhongxiyi Jiehe Zazhi. 2002;22(8):577–580. [PubMed] [Google Scholar]
- [20].Jia WH, Ding XN, Xu ZM, et al. Clinical study of Luoshukang capsules for the vascular dementia. Guangming Zhongyi. 2003;18(107):20–22. [Google Scholar]
- [21].Jiang CH, Ding AG, Wang BL, et al. Naofucong Capsule for vascular dementia. Zhongguo Zhongxiyi Jiehe Xin Nao Xueguan Bing Zazhi. 2007;5(5):396–398. [Google Scholar]
- [22].Wang YY, Lu H, Chen F, et al. Clinical and experimental studies on treatment of cerebrovascular dementia with Shen Tong capsules. Zhongyi Zazhi. 2002;43(6):437–439. [Google Scholar]
- [23].Hong ML, Hou GD, Hong LS, et al. Clinical study on vascular dementia treated by Shouxing capsule. Hubei Zhongyi Zazhi. 2002;24(4):3–5. [Google Scholar]
- [24].Zhu AH, Tian JZ, Zhong J, et al. A clinical study on a randomized, double-blind control of Tianzhi granules in treatment of the senile vascular dementia. Zhongguo Laonian Xue Zazhi. 2002;25(12):1435–1438. [Google Scholar]
- [25].Fang GY, Li JC, Lin ZT, et al. Clinical study of Tongmaizenzhi capsule in treating patients with vascular dementia. Henan Yike Daxue Xuebao. 1997;32(4):15–17. [Google Scholar]
- [26].Liu CZ, Zhou LH, Shui Z. Clinical research on the effect of Tongqiao Huoxue and Buyang Huanwu Decoction for vascular dementia: a report of 36 cases. Zhongyi Zazhi. 2002;43(7):526–527. [Google Scholar]
- [27].Luo ZG, Zhou WQ, Gao P, et al. Clinical observation on effect of Xianlong capsule in treating vascular dementia. Zhongguo Zhongxiyi Jiehe Zazhi. 2001;21(8):565–568. [PubMed] [Google Scholar]
- [28].Zhao Y, Zhou WQ, Gao P, et al. Clinical study on effect of Xianlong capsule on senile vascular dementia. Zhongguo Zhongxiyi Jiehe Zazhi. 1999;19(10):585–588. [PubMed] [Google Scholar]
- [29].Tao GY. Clinical research on the effect of Xingnao Bolus for vascular dementia. Shaanxi Zhongyi Xueyuan Xuebao. 2004;27(1):5–7. [Google Scholar]
- [30].Wang XZ. An observation of curative effect of Yiqi Bushen Tongqiao Huoxue formula for vascular dementia: a report of 65 cases. Sichuan Zhongyi Zazhi. 2008;26(11):71–73. [Google Scholar]
- [31].Zhou JY, Liu T, Sun CC. Clinical study on “Yiqi Fuzhi Granule” in treating vascular dementia. Shanghai Zhongyiyao Zazhi. 2005;39(3):11–13. [Google Scholar]
- [32].Huang XM, Zhang LP. An observation of curative effect of Yiqi Huoxue Bushen therapy for vascular dementia: a report of 35 cases. Zhejiang Zhongyi Zazhi. 2009;44(11):791–792. [Google Scholar]
- [33].Wu XH. Clinical study on the method for supplementing Qi and activating blood circulation for treatment of 30 cases of multiple infarctional dementia. Zhongyi Zazhi. 2005;46(4):267–269. [Google Scholar]
- [34].Wang FJ, Lv SJ, Dong Z. Clinical research on the effect of Yizhi Tongluo capsules in treatment for vascular dementia. Xiandai Zhongxiyi Jiehe Zazhi. 2002;11(4):295–297. [Google Scholar]
- [35].Niu JX, Li YM, Niu CB. Clinical observation on Yizhi Xingnao decoction for the treatment of vascular dementia. Xin Zhongyi. 2008;40(5):45–46. [Google Scholar]
- [36].Zheng CY. Compound Huonaosu capsules for vascular dementia: an clinical study of 58 cases. Zhongguo Zhongyiyao Xinxi Zazhi. 2001;8(1):75–76. [Google Scholar]
- [37].Sun Y, Chen M, Hou Q, et al. Yishen Tongqiao decoction treat vascular dementia. Zhejiang Zhongyiyao Daxue Xuebao. 2010;34(3):345–347. [Google Scholar]
- [38].You ZJ, Wang ZJ, Zhou SH, et al. The clinical study of “buqi huoxue prescription” in treatment of multiple infarct dementia. Liaoning Zhongyi Zazhi. 2010;37(1):102–104. [Google Scholar]
- [39].Zheng XY. Beijing: China Medical Science Press; 2002. Clinical Research Guiding Principle for the New Chinese Herbal Medicine. [Google Scholar]


