Abstract
Advanced glycation end products lead to cell apoptosis, and cause cell death by increasing endoplasmic reticulum stress. Advanced glycation end products alone may also directly cause damage to tissues and cells, but the precise mechanism remains unknown. This study used primary cultures of rat cerebral cortex neurons, and treated cells with different concentrations of glycation end products (50, 100, 200, 400 mg/L), and with an antibody for the receptor of advanced glycation end products before and after treatment with advanced glycation end products. The results showed that with increasing concentrations of glycation end products, free radical content increased in neurons, and the number of apoptotic cells increased in a dose-dependent manner. Before and after treatment of advanced glycation end products, the addition of the antibody against advanced glycation end-products markedly reduced hydroxyl free radicals, malondialdehyde levels, and inhibited cell apoptosis. This result indicated that the antibody for receptor of advanced glycation end-products in neurons from the rat cerebral cortex can reduce glycation end product-induced oxidative stress damage by suppressing glycation end product receptors. Overall, our study confirms that the advanced glycation end products-advanced glycation end products receptor pathway may be the main signaling pathway leading to neuronal damage.
Keywords: neural regeneration, brain injury, advanced glycation end products, advanced glycation end products receptor, antibody, pathway, cortical neurons, oxidative stress, oxidative stress injury, apoptosis, neuroregeneration
Research Highlights
-
(1)
This paper confirmed that advanced glycation end products act as toxic substances. The toxic effect is directly proportional to the concentration of advanced glycation end products, which results in increased generation of free radicals that can cause apoptosis of neurons in the rat cerebral cortex.
-
(2)
The antibody for the receptor of advanced glycation end products can reduce neuronal damage caused by oxidative stress by suppressing glycation end product receptor activity. Therefore, the glycation end products-glycation end products receptor pathway may be the main signaling pathway that produces the toxic effect.
INTRODUCTION
Advanced glycation end products are proteins or lipids that become glycated after exposure to glucose, and become irreversible polymers after a series of molecular rearrangements[1]. Usually, advanced glycation end products generated in vivo can be degraded by macrophages, however, if increased, advanced glycation end products will constantly deposit in tissues and cells.
Alikhani et al[2] verified that advanced glycation end products can lead to histiocytic apoptosis. Recent studies found that advanced glycation end products caused a decrease in cognitive function[3,4]. A new study shows that advanced glycation end products increase endoplasmic reticulum stress and lead to cell death[5,6]. Advanced glycation end products alone can directly damage tissues and cells[5], and can also be used as ligands when combined with receptors for advanced glycation end products[7]. During different diseases, the mechanism of damage varies; previous studies concerning advanced glycation end products mainly focused on peripheral nerves, and seldom on the central nervous system[8,9]. Thus, the precise mechanism of advanced glycation end products-induced neuronal damage remains unclear.
Therefore, in this study, we successfully established in vitro preparations of advanced glycation end products with bovine serum albumin and cultured primary cortical neurons to explore the relationship between advanced glycation end products and neurons after neurons were treated with different concentrations of advanced glycation end products. We monitored injury and the mechanism of the advanced glycation end products-receptors for advanced glycation end products pathway in the nervous system following exposure to the antibody of receptor of advanced glycation end-products at different time points.
RESULTS
Evaluation of advanced glycation end products-bovine serum albumin
Using fluorescence spectrophotometry, the fluorescence value of advanced glycation end products was 138.8 arbitrary fluorescence units; the fluorescence value for bovine serum albumin was 18.42 arbitrary fluorescence units. Using ultraviolet spectrophotometry, the protein concentration of advanced glycation end products was 8.72 mg/L, indicating that advanced glycation end products were successfully prepared using bovine serum albumin.
Morphology of neurons in the rat cerebral cortex
Primary cultures of rat cerebral cortex neurons were prepared. At 2 days following culture, the cells had attached. At 7 days, neuron networks had formed. Under the confocal microscope, Cy3 fluorescence-labeled neuron-specific enolase identification showed that cortical cells were plump, with the presence of processes. Some processes intercrossed and formed a network. Staining showed that neurons grew well. More than 90% of neurons were visible under the confocal microscope (Figure 1).
Figure 1.
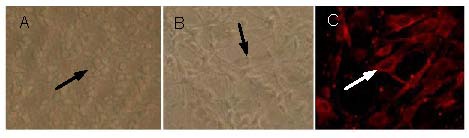
Morphology of neurons in the rat cerebral cortex following primary culture.
(A) Cortical neurons cultured for 2 days: adherent cells appeared plump (black arrow, × 100). (B) Cortical neurons cultured for 7 days: long and dense axons formed networks (black arrow, × 100). (C) Cy3-neuron-specific enolase fluorescent staining: neuronal bodies appeared plump, with the presence of processes, which interacted and formed a network as seen by confocal microscopy (white arrow, × 600).
Changes in the number of apoptotic neurons in the rat cerebral cortex before and after antibody of receptor of advanced glycation end-products exposure at different advanced glycation end product concentrations
This study contained three groups: an advanced glycation end product direct action group, an antibody of receptor of advanced glycation end-product pretreatment group, and an antibody of receptor of advanced glycation end-product posttreatment group. In each group, advanced glycation end product concentrations were 50, 100, 200, 400 mg/L and bovine serum albumin served as a control. Each group was further divided into five subgroups. In the above-mentioned groups, neurons were subjected to different concentrations of advanced glycation end products for 3 hours. In the antibody of receptor of advanced glycation end-product pretreatment group, and antibody of receptor of advanced glycation end-product posttreatment group, neurons were exposed to 50 mg/L antibody of receptor of advanced glycation end-products for 2 hours before and after using advanced glycation end products.
Following Hoechst fluorescent staining, chromatin from normal nuclei appeared uniform. Nuclei of apoptotic cells were darkly stained. Chromatin margination was visible. There were few apoptotic cells in normally cultured neurons. Compared with the bovine serum albumin control group, the number of apoptotic cells increased with increasing concentration of advanced glycation end products in the advanced glycation end product direct action group and antibody of receptor of advanced glycation end-product pretreatment group (P < 0.05), but the number of apoptotic cells in the antibody of receptor of advanced glycation end-product pretreatment group was lower than that in the advanced glycation end product direct action group (P < 0.01; Figure 2).
Figure 2.
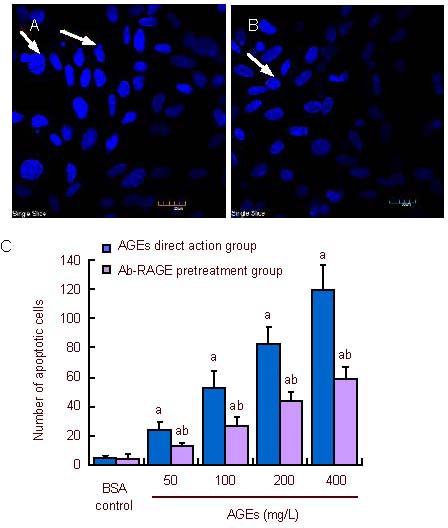
Effect of receptor of advanced glycation end-products antibody (Ab-RAGE) on apoptosis of neurons in the rat cerebral cortex after intervention with advanced glycation end products (AGEs).
Nuclei were blue after Hoechst fluorescence staining. (A) AGEs direct action group: as shown by arrows. Apoptotic nuclei were darkly stained, with the presence of chromatin margination. (B) Ab-RAGE pretreatment group: decreased number of apoptotic cells; arrow exhibits slight changes in neuronal morphology. (A, B) Hoechst fluorescent staining (confocal microscopy, × 600).
(C) The number of apoptotic cells with increasing concentration of AGEs. Neuronal apoptosis number in the AGEs direct action group and Ab-RAGE pretreatment group increased gradually. a P < 0.05, vs. bovine serum albumin (BSA) control group; b P < 0.01, vs. AGEs direct action group. Mean ± SD, n = 3, one-way analysis of variance and two sample t-test.
Effects of antibody of receptor of advanced glycation end-product pretreatment and posttreatment on the ability of inhibiting hydroxyl free radicals in advanced glycation end products-treated neurons of the rat cerebral cortex
With the increasing concentrations of advanced glycation end products, the ability of inhibiting hydroxyl free radicals gradually reduced in each group (P < 0.05). The ability of inhibiting hydroxyl free radicals gradually elevated in the antibody of receptor of advanced glycation end-product pretreatment and posttreatment groups (P < 0.05, P < 0.05). Moreover, this effect was greater in the antibody of receptor of advanced glycation end-product pretreatment group than in the antibody of receptor of advanced glycation end-product posttreatment group (P < 0.05; Figures 3, 4). Results showed that the ability of inhibiting hydroxyl free radicals was strongest in the antibody of receptor of advanced glycation end-product pretreatment group, followed by the antibody of receptor of advanced glycation end-product posttreatment group. The ability was weakest in the advanced glycation end product direct action group.
Figure 3.
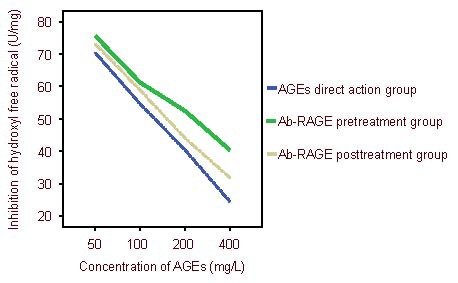
Inhibition of hydroxyl free radicals after pretreatment and posttreatment with antibody of receptor of advanced glycation end-products (Ab-RAGE).
Results show that the ability of inhibiting hydroxyl free radicals was strongest in the Ab-RAGE pretreatment group, followed by Ab-RAGE posttreatment group. The ability was weakest in the advanced glycation end products (AGEs) direct action group.
Figure 4.

Changes in the ability to inhibit hydroxyl free radicals after pretreatment and posttreatment with receptor of advanced glycation end-products antibody (Ab-RAGE).
With increasing concentrations of advanced glycation end-products (AGEs), the ability to inhibit hydroxyl free radicals gradually reduced in each group. a P < 0.05, vs. bovine serum albumin (BSA) control group; bP < 0.05, vs. Ab-RAGE pretreatment group; c P < 0.05, vs. AGEs direct action group; mean ± SD, n = 3, one-way analysis of variance and two sample t-test.
Effects of antibody of receptor of advanced glycation end-product pretreatment and posttreatment on malondialdehyde content of advanced glycation end products-treated neurons in the rat cerebral cortex
Compared with the bovine serum albumin control group, increasing concentrations of advanced glycation end products resulted in a gradual increase in malondialdehyde content (P < 0.05). Malondialdehyde content was highest in the advanced glycation end product direct action group (P < 0.05), followed by the antibody of receptor of advanced glycation end-product posttreatment group (P < 0.05). Malondialdehyde content was lowest in the antibody of receptor of advanced glycation end-product pretreatment group (P < 0.05; Figures 5, 6).
Figure 5.
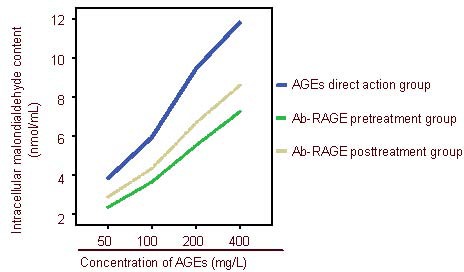
Malondialdehyde content in cells after pretreatment and posttreatment with antibody of receptor of advanced glycation end-products (Ab-RAGE).
Malondialdehyde content was highest in the advanced glycation end products (AGEs) direct action group, followed by Ab-RAGE posttreatment group. Malondialdehyde content was lowest in the Ab-RAGE pretreatment group.
Figure 6.

Changes in malondialdehyde content in cells after pretreatment and posttreatment with antibody of receptor of advanced glycation end-products (Ab-RAGE).
a P < 0.05, vs. bovine serum albumin (BSA) control group; bP < 0.05, vs. Ab-RAGE pretreatment group; c P < 0.05, vs. AGEs direct action group; mean ± SD, n = 3, one-way analysis of variance and two sample t-test.
DISCUSSION
The results show that advanced glycation end products have a neurotoxic effect, which can cause oxidative damage to neurons, namely an increase in free radicals and an increase in apoptotic cell number, which is consistent with a previous study[10]. Lecleire-Collet et al[10] found that advanced glycation end products can increase glial fibrillary acidic protein content, cause retinal nerve cell apoptosis, and result in degenerative change, but they did not investigate the mechanism of this toxic effect.
Advanced glycation end products can cause an increase in receptors for advanced glycation end products in tissue membranes; advanced glycation end products combined with receptors for advanced glycation end products can cause a serial reaction[11], and finally result in cell damage. To confirm that advanced glycation end products can cause toxic effects or when associated with their specific receptor, 50 mg/L anti-receptors for advanced glycation end products antibody was used to pretreat neurons for 2 hours. Advanced glycation end products under the same conditions were used to treat neurons. Our results revealed that neuronal damage decreased, indicating an increased ability to inhibit hydroxyl free radical production, and that malondialdehyde content and cell apoptosis number reduced. These results confirmed that the antibody of receptors for advanced glycation end products inhibited the damaging effects of advanced glycation end products on neurons. To better explain this situation, the above-described different concentrations of advanced glycation end products were used to treat neurons for 3 hours, and then 50 mg/L antibody of receptors for advanced glycation end products was added to posttreat neurons for 2 hours. This experiment revealed that oxidative damage of neurons reduced, but that the degree of protection was less than the antibody of receptor of advanced glycation end-product pretreatment group.
Our experimental results suggest that the toxic effects of advanced glycation end products on neurons are mainly exerted by triggering oxidative stress after combining with receptors for advanced glycation end products on neuron membranes, which are identical to previously published results[12]. The increase of free radicals can promote advanced glycation end products and form a vicious cycle[13], finally resulting in mitochondrial permeability increase, DNA fracture and cell apoptosis. The reason for the different degrees of neuronal damage before and after pretreatment with antibody of receptor of advanced glycation end-products is as follows: the use of antibody of receptor of advanced glycation end-products prior to the use of advanced glycation end products inhibited receptors for advanced glycation end products on the cell membrane to a certain degree, preventing the combination of advanced glycation end products and receptors for advanced glycation end products, and blocking signal conduction of the advanced glycation end products-receptors for advanced glycation end products pathway[14], thus reducing free radical generation, and exerting a protective effect. However, in the posttreatment group, because advanced glycation end products combined with membrane receptors for advanced glycation end products, antibody of receptor of advanced glycation end-products only partially inhibited membrane receptors for advanced glycation end products. Thus, its protective effect was weaker than that of the pretreatment group.
Results from this study demonstrated that the toxic effect of advanced glycation end products on neurons was mainly due to the combination of cell membrane receptors for advanced glycation end products and advanced glycation end products, leading to oxidative damage, free radical increase, mitochondrial permeability increase, and finally neuronal apoptosis. Therefore, receptors for advanced glycation end products may act as a therapeutic target to reduce tissue and cell damage[15]. However, the precise mechanism of how advanced glycation end products cause the generation of apoptosis and free radicals requires further investigation.
MATERIALS AND METHODS
Design
A contrast observation, in vitro experiment.
Time and setting
The experiment was performed at the Laboratory of the College of Public Health, Jilin University, China from May 2008 to June 2009.
Materials
A total of six clean newborn Wistar rats (within 24 hours), weighing 10–20 g, were supplied by the Animal Experiment Center, Basic Medical Sciences, Jilin University, China (SCXK (Ji) 2007-0003). The rats were housed at 20 ± 2°C, with a relative humidity of 40–50% and natural illumination. The protocols were conducted in accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals, issued by the Ministry of Science and Technology of China[16].
Methods
Preparation of advanced glycation end products-bovine serum albumin
Bovine serum albumin (1 g; Sigma, St, Louis, MO, USA), 9.9 g glucose, 1 mM ethylenediamine tetraacetic acid, 100 U/mL penicillin, and 100 U/mL streptomycin were dissolved in PBS (pH 7.2), stirred at room temperature for 3 hours, filtered with a 0.22 μm-pore diameter filter, and incubated in a thermostat at 37°C for 3 months. advanced glycation end products-bovine serum albumin was prepared following 48 hours of dialysis[17]. In the bovine serum albumin control group under the same conditions, glucose was not added, and the above-mentioned operations were repeated. According to the fluorescence characteristics and special absorption spectrum characteristics of advanced glycation end products, arbitrary fluorescence units were determined using a fluorescence spectrophotometer (F-4010, Hitachi, Shanghai, China) with an excitation wavelength of 370 nm, emission wavelength of 440 nm, and a slit of 3 nm. Compared with the control group, the ratio of fluorescence units (> 5 times) was significant. PBS (pH 7.2–7.4) was used to dissolve glucose for approximately 2 days. After filtration sterilization with a 0.22-μm filter, protein concentration was measured using an ultraviolet spectrophotometer (U-3410, Hitachi). The specimens were stored at –20°C.
In vitro culture and identification of neurons in the rat cerebral cortex
According to previously published methods[18,19], the rat cerebral cortex was obtained, cut into pieces, washed three times in PBS, incubated in polylysine-coated plates, and digested in 0.25% (w/v) trypsin, at 37°C for 10–15 minutes. After digestion was terminated, the specimens were triturated into single cells, centrifuged at 800 r/min for 5 minutes, and then the supernatant was abandoned. Cells were resuspended, and incubated in 24-well plates at a density of 1 ×105, at 37°C, 95% O2, 5% CO2 for 48 hours. The medium was replaced. Cytosine arabinoside (5 μM; Pharmacia Italia S.P.A, Via Robert Koch, 1.2-20152, Milan, Italy) was added. From then on, half the medium was replaced every 3 days. Seven days later, cell growth was observed under a microscope. Well-grown cells were collected, and the medium was discarded. After washing in PBS, cells were fixed in 4% (w/v) paraformaldehyde for 10 minutes, washed in PBS 3 × 5 minutes, treated with 3% (v/v) H2O2-methanol for 30 minutes, incubated in 0.25% (v/v) TritonX-100 (Gen view, FL, USA) for 10 minutes, and blocked in 5% (v/v) bovine serum albumin (Shanghai Bohan, Shanghai, China) at room temperature for 20 minutes. Surplus liquid was removed. Cells were incubated in rabbit anti-rat neuron-specific enolase polyclonal antibody (1:50; Boster, Wuhan, China) at 37°C for 1 hour, and then left overnight at 4°C overnight. Cells were washed in PBS 3 × 5 minutes, incubated in Cy3-labeled goat anti-rabbit IgG (1:400; Boster) at 37°C for 30 minutes, and washed in PBS 3 × 5 minutes. Cell morphology was observed under a confocal microscope (FV1000; Olympus, Tokyo, Japan). Cells were counted under 600-fold visual field and photographed.
Apoptotic cell death between advanced glycation end products direct action neurons and those pretreated with antibody of receptor of advanced glycation end-products
To confirm the extent of damage following advanced glycation end products exposure on neurons, in accordance with previously published methods[20,21], advanced glycation end products-bovine serum albumin was diluted into 50, 100, 200, 400 mg/L. Advanced glycation end products-bovine serum albumin (20 μL) was separately added in petri dishes to each subgroup. Bovine serum albumin (20 μL) served as a control. Cells were left to incubate for 3 hours. According to the apoptosis kit instructions (BiYunTian Biological Technology, Shanghai, China), fixative was added to the cells of each group for 10 minutes. Cells were stained with 0.5 mL Hoechest 33528 for 5 minutes, washed, treated with anti-fluorescence quenching and observed under the confocal microscope. The excitation wavelength was adjusted to 370 nm, and the emission wavelength was about 480 nm. Changes to the nuclei were observed. The number of positive cells was recorded in each specimen from five different visual fields. The average value was obtained and cells were photographed. Neurons were initially incubated in 50 mg/L Ab-RAG (Abcam PIC. 332 Cambridge Science Park, Cambridge, CB4 0FW, UK) for 2 hours, and then in advanced glycation end products as the antibody of receptor of advanced glycation end-product pretreatment group, followed by treatment with different concentrations of advanced glycation end products for 3 hours. Cell changes were observed and cells were photographed using the same method.
Detection of hydroxyl free radicals and malondialdehyde content in the advanced glycation end product direct action, antibody of receptor of advanced glycation end-product pretreatment and posttreatment groups
Cells were exposed to the above-described concentrations of advanced glycation end products-bovine serum albumin for 3 hours. According to the free radical kit instructions (Jiancheng Biological Engineering Institute, Nanjing China), absorbance values in each group were measured using the ultraviolet spectrophotometer. Neurons were pretreated with 50 mg/L antibody of receptor of advanced glycation end-products for 2 hours, and treated with various concentrations of advanced glycation end products for 3 hours, and posttreated with 50 mg/L antibody of receptor of advanced glycation end-products for 2 hours. Absorbance values in each group were measured by the same method. The ability of inhibiting hydroxyl free radicals was calculated. The absorbance of standard wells was 8.824 μM. According to the malondialdehyde kit instructions, neurons were homogenated and malondialdehyde content was measured using the micromethod.
Statistical analysis
Measurement data were expressed as mean ± SD and analyzed using SPSS 17.0 software package (SPSS, Chicago, IL, USA). Intergroup differences were compared using a two sample t-test and one-way analysis of variance. A value of P < 0.05 was considered statistically significant.
Acknowledgments
We thank Professor Xinrui Wang from the Pathology Teaching and Research Section, Agriculture Department, Jilin University, China for technical support.
Footnotes
Conflicts of interest: None declared.
Ethical approval: This study received permission from the Animal Ethics Committee of Jilin University, China.
(Reviewed by Diwakarla S, Stow A, Liu Q, Cao YP)
(Edited by Wang J, Qiu Y, Li CH, Song LP)
REFERENCES
- [1].Rees MD, Kennett EC, Whitelock JM, et al. Oxidative damage to extracellular matrix and its role in human pathologies. Free Radic Biol Med. 2008;44(12):1973–2001. doi: 10.1016/j.freeradbiomed.2008.03.016. [DOI] [PubMed] [Google Scholar]
- [2].Alikhani Z, Alikhani M, Boyd C, et al. Advanced glycation endproducts enhance expression of pro-apoptotic genes and stimulate fibroblast apoptosis through cytoplasmic and mitochondrial pathways. J Biol Chem. 2005;280(13):12087–12095. doi: 10.1074/jbc.M406313200. [DOI] [PubMed] [Google Scholar]
- [3].Wang T, Fu F, Han B, et al. Danshensu ameliorates the cognitive decline in streptozotocin-induced diabetic mice by attenuating advanced glycation end product-mediated neuroinflammation. J Neuroimmunol. 2012;45(1-2):79–86. doi: 10.1016/j.jneuroim.2012.02.008. [DOI] [PubMed] [Google Scholar]
- [4].Wang L, Yu CJ, Liu W, et al. Rosiglitazone protects neuroblastoma cells against advanced glycation end products-induced injury. Acta Pharmacol Sin. 2011;32(8):991–988. doi: 10.1038/aps.2011.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Nitti M, Furfaro AL, Traverso N, et al. PKC delta and NADPH oxidase in AGE-induced neuronal death. Neurosci Lett. 2007;416(3):261–265. doi: 10.1016/j.neulet.2007.02.013. [DOI] [PubMed] [Google Scholar]
- [6].Yin QQ, Dong CF, Dong SQ, et al. AGEs induce cell death via oxidative and endoplasmic reticulum stresses in both human SH-SY5Y neuroblastoma cells and rat cortical neurons. Cell Mol Neurobiol. 2012;32(8):1299–1309. doi: 10.1007/s10571-012-9856-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Yan SF, Ramasamy R, Schmidt AM. The receptor for advanced glycation endproducts (RAGE) and cardiovascular disease. Expert Rev Mol Med. 2009;11:e9. doi: 10.1017/S146239940900101X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Vincent AM, Perrone L, Sullivan KA, et al. Receptor for advanced glycation end products activation injuries primary sensory neurons via oxidative stress. Endocrinology. 2007;148(2):548–558. doi: 10.1210/en.2006-0073. [DOI] [PubMed] [Google Scholar]
- [9].Zochodne DW, Ramji N, Toth C. Neuronal targeting in diabetes mellitus: a story of sensory neurons and motor neurons. Neuroscientist. 2008;14(4):311–318. doi: 10.1177/1073858408316175. [DOI] [PubMed] [Google Scholar]
- [10].Lecleire-Collet A, Tessier LH, Massin P, et al. Advanced glycation end products can induce glial reaction and neuronal degeneration in retinal explants. Br J Ophthalmol. 2005;89(12):1631–1633. doi: 10.1136/bjo.2005.079491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lue LF, Yan SD, Stern DM, et al. Preventing activation of receptor for advanced glycation endproducts in Alzheimer's disease. Curr Drug Targets CNS Neurol Disord. 2005;4(3):249–266. doi: 10.2174/1568007054038210. [DOI] [PubMed] [Google Scholar]
- [12].Tan AL, Forbes JM, Cooper ME. AGE, RAGE, and ROS in diabetic nephropathy. Semin Nephrol. 2007;27(2):130–143. doi: 10.1016/j.semnephrol.2007.01.006. [DOI] [PubMed] [Google Scholar]
- [13].Menini S, Iacobini C, Ricci C. Ablation of the gene encoding p66Shc protects mice against AGE-induced glomerulopathy by preventing oxidant-dependent tissue injury and further AGE accumulation. Diabetologia. 2007;50(9):1997–2007. doi: 10.1007/s00125-007-0728-7. [DOI] [PubMed] [Google Scholar]
- [14].Zong H, Madden A, Ward M, et al. Homodimerization is essential for the receptor for advanced glycation end products (RAGE)-mediated signal transduction. J Biol Chem. 2010;285(30):23137–23146. doi: 10.1074/jbc.M110.133827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Askarova S, Yang X, Sheng W, et al. Role of Aβ-receptor for advanced glycation endproducts interaction in oxidative stress and cytosolic phospholipase A2 activation in astrocytes and cerebral endothelial cells. Neuroscience. 2011;29(199):375–385. doi: 10.1016/j.neuroscience.2011.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals 2006-09-30 [Google Scholar]
- [17].Yamagishi S, Amano S, Inagaki Y, et al. Advanced glycation end products-induced apoptosis and overexpression of vascular endothelial growth factor in bovine retinal pericytes. Biochem Biophys Res Commun. 2002;290(3):973–978. doi: 10.1006/bbrc.2001.6312. [DOI] [PubMed] [Google Scholar]
- [18].Zhang XH, Yu HL, Xiao R, et al. Neurotoxicity of beta-amyloid peptide 31-35 and 25-35 to cultured rat cortical neurons. Zhonghua Yu Fang Yi Xue Za Zhi. 2009;43(12):1081–1085. [PubMed] [Google Scholar]
- [19].Meberg PJ, Miller M. Culturing hippocampal and cortical neurons. Methods Cell Biol. 2003;71:111–127. doi: 10.1016/s0091-679x(03)01007-0. [DOI] [PubMed] [Google Scholar]
- [20].Gangoiti MV, Cortizo AM, Arnol V. Opposing effects of bisphosphonates and advanced glycation end-products on osteoblastic cells. Eur J Pharmacol. 2008;600(1-3):140–147. doi: 10.1016/j.ejphar.2008.10.031. [DOI] [PubMed] [Google Scholar]
- [21].Jin H, Liu NF, Tang R. Effects of advanced glycosylation end products on prolifer ation and cytosolic free calcium in cultured rat aortic smooth muscle cells. Zhongguo Yao Li Xue Bao. 1997;18(5):422–425. [PubMed] [Google Scholar]


