Abstract
Human umbilical mesenchymal stem cells from Wharton's jelly of the umbilical cord were induced to differentiate into oligodendrocyte precursor-like cells in vitro. Oligodendrocyte precursor cells were transplanted into contused rat spinal cords. Immunofluorescence double staining indicated that transplanted cells survived in injured spinal cord, and differentiated into mature and immature oligodendrocyte precursor cells. Biotinylated dextran amine tracing results showed that cell transplantation promoted a higher density of the corticospinal tract in the central and caudal parts of the injured spinal cord. Luxol fast blue and toluidine blue staining showed that the volume of residual myelin was significantly increased at 1 and 2 mm rostral and caudal to the lesion epicenter after cell transplantation. Furthermore, immunofluorescence staining verified that the newly regenerated myelin sheath was derived from the central nervous system. Basso, Beattie and Bresnahan testing showed an evident behavioral recovery. These results suggest that human umbilical mesenchymal stem cell-derived oligodendrocyte precursor cells promote the regeneration of spinal axons and myelin sheaths.
Keywords: neural regeneration, stem cells, spinal cord injury, Wharton's jelly, human umbilical mesenchymal stem cells, oligodendrocyte precursor-like cells, axon, myelin sheath, nerve repair, grants-supported paper, neuroregeneration
Research Highlights
-
(1)
We isolated human umbilical mesenchymal stem cells from Wharton's jelly of the umbilical cord, and these cells differentiated into oligodendrocyte precursor-like cells after induction with neurobasal medium and nerve factor.
-
(2)
Transplanted cells survived in injured spinal cord and differentiated into mature and immature oligodendrocyte precursor cells.
-
(3)
Oligodendrocyte precursor cells promoted the regeneration of spinal axons and myelin sheaths and improved neurological function in rats with spinal cord injury.
-
(4)
Human umbilical mesenchymal stem cells can be easily obtained from Wharton's jelly of the umbilical cord, proliferate rapidly in vitro, are not immunogenic, and are ideal cells for the treatment of spinal cord injury.
INTRODUCTION
The demyelination of intact axons and the disruption of action potential propagation resulting from the death of oligodendrocytes are important factors resulting in loss of function after spinal cord injury[1,2,3]. Therefore, an effective strategy may be to increase the extent of remyelination by transplanting central nervous system myelin-forming cells into the injured spinal cord. Oligodendrocyte precursor cells induced from embryonic brain and spinal cord have been identified as possible candidates for the treatment of spinal cord injury[4,5,6,7,8]. However, ethical considerations limit the practical use of embryonic tissue-derived cells and many researchers have therefore begun to explore other cell sources. Considerable interest is now focused on adult stem cells[9,10]. Bone marrow mesenchymal stem cells are the most commonly studied adult stem cells. However, the harvest of bone marrow mesenchymal stem cells is a highly invasive procedure[11]. Furthermore, the differentiation potential and the number of mesenchymal stem cells isolated from bone marrow decline with increasing age[12]. Thus, alternative sources from which to isolate mesenchymal stem cells should be investigated. Mesenchymal stem cells from Wharton's jelly of the umbilical cord can be obtained easily and proliferate rapidly in culture, and they are immunologically compatible and amenable to stable transfection[13,14,15]. Thus, human umbilical mesenchymal stem cells may serve as an alternative source of pluripotent stem cells for the treatment of neurological disorders.
Recent studies have demonstrated that human umbilical mesenchymal stem cells are able to break germ layer commitment and differentiate into cells exhibiting oligodendrocyte properties[16]. Using an established animal model of spinal cord injury, the present study investigated the potential therapeutic effects of human umbilical mesenchymal stem cell-derived oligodendrocyte precursor cells in vivo.
RESULTS
Quantitative analysis of experimental animals
Forty-seven adult Sprague-Dawley rats were divided into a sham-operated group (n = 5; rats were subjected to laminectomy but not contusion); a human umbilical mesenchymal stem cell-oligodendrocyte precursor cell- treated group (n = 21; oligodendrocyte precursor cell transplantation after contusion); and a saline-treated group (n = 21; saline injection after contusion). All 47 rats were included in the final analysis.
Isolation and culture of human umbilical mesenchymal stem cells and differentiation into oligodendrocyte precursor cells
Human umbilical mesenchymal stem cells at passage 1 displayed large and full cell bodies with abundant cytoplasm, and the cells were round or oval-shaped. Human umbilical mesenchymal stem cells at passage 5 were fibroblast-like and grew in a whirlpool configuration (Figure 1A).
Figure 1.
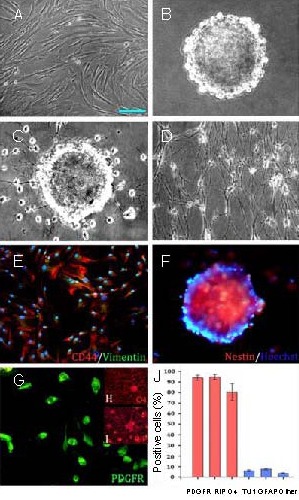
Morphology and phenotype of human umbilical mesenchymal stem cells (HUMSCs) after induced differentiation (scale bar shown in A, A–D, F–I: 50 μm, E: 100 μm).
(A) Morphology of HUMSCs at passage 5. HUMSCs at passage 5 developed into elongated or spindle-shaped cells and became relatively homogeneous in appearance. (B) HUMSCs were induced into neurospheres. (C) Neurospheres were plated in oligodendrocyte precursor cell (OPC) differentiation medium and 4 days later, cells with bipolar and tripolar morphology migrated out of the spheres.
(D) The OPC-like cells terminally differentiated into oligodendrocyte-like cells. (E) HUMSCs co-express CD44 and vimentin. (F) HUMSC-derived neurospheres were recognized by the nestin antibody. (G) OPC-like cells induced from neurospheres displayed platelet-derived growth factor receptor (PDGFR) immunoreactivity. (H) OPC-like cells readily differentiated into oligodendrocytes, which expressed oligodendrocyte 4 (O4) and (I) replication protein A-interacting protein (RIP).
(J) Statistical analysis of the HUMSC surface markers at passage 5. Data are expressed as mean ± SD. The experiment was repeated five times. GFAP: Glial fibrillary acidic protein.
After 1 or 2 days of continuous culture in induction medium (neurobasal medium supplemented with 20 ng/mL epidermal growth factor, 20 ng/mL basic fibroblast growth factor and N2), human umbilical mesenchymal stem cells began to aggregate. Many small floating spheres of cells appeared within 3–4 days after induction. These spheres proliferated rapidly and the diameter of a single sphere was over 100 μm at 10–14 days post-induction (Figure 1B). After human umbilical mesenchymal stem cell-derived neurospheres were plated in oligodendrocyte precursor cell differentiation medium for 4 days, some cells with bipolar or tripolar morphologies migrated out of the spheres (Figure 1C). Seven days later, the small oligodendrocyte precursor cell-like cells connected with each other to form a network (Figure 1D).
Phenotypic characteristics of human umbilical mesenchymal stem cells and transdifferentiation into oligodendrocyte precursor cells
The phenotypic characteristics of human umbilical mesenchymal stem cells were demonstrated in our previous studies[11,16,17,18]. The majority of human umbilical mesenchymal stem cells at passage 5 showed strong CD44 staining (94.33 ± 8.21%) and some cells co-expressed the mesodermal marker vimentin (Figure 1E). The neurospheres derived from human umbilical mesenchymal stem cells were recognized by a nestin antibody (a total of 97.65 ± 5.45% cells showed nestin immunoreactivity) (Figure 1F). A majority of the oligodendrocyte precursor cell-like cells induced from neurospheres (93.97 ± 1.17%) displayed the immature oligodendrocyte precursor cell marker platelet-derived growth factor receptor (Figure 1G). The oligodendrocyte precursor cell-like cells terminally differentiated into replication protein A-interacting protein-positive (a mature oligodendrocyte marker) (94.75 ± 1.27%) and oligodendrocyte 4-positive (another mature oligodendrocyte marker) (80.51 ± 4.03%) cells (Figures 1H–I). Few of the induced cells expressed surface markers of neuronal and glial cells. The statistical analysis of the results is shown in Figure 1J. Oligodendrocytes induced from human umbilical mesenchymal stem cell-oligodendrocyte precursor cells are morphologically and phenotypically identical to oligodendrocyte precursor cells.
Oligodendrocyte precursor cell transplantation improved functional recovery after rat spinal cord injury
As shown in Figure 2, all animals scored 21 points on the Basso, Beattie and Bresnahan scale before spinal cord injury. One day after spinal cord injury, all animals received a score of 0, and, 3 days after injury, a score under 2. The rats in the sham-operated group recovered to the normal level 1 week after injury. During the week after injury, the locomotor performance substantially improved and the human umbilical mesenchymal stem cell-oligodendrocyte precursor cell-treated group started to show an early increase in recovery compared with the rats who received saline treatment. The Basso, Beattie and Bresnahan scores in the human umbilical mesenchymal stem cell-oligodendrocyte precursor cell-treated group were consistently higher than in the saline-treated group starting from the 5th week and continuing until the 11th week after injury (P < 0.05).
Figure 2.
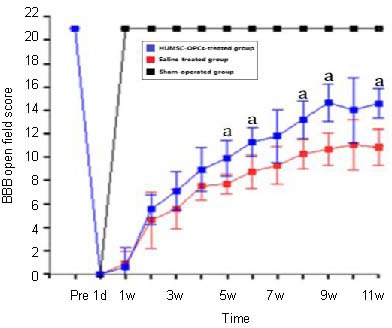
Locomotor evaluation of hindlimbs using Basso, Beattie and Bresnahan (BBB) scores.
The locomotor function of the rats in the sham-operated group recovered to the normal level 1 week after injury and remained at normal level to the end of observation. The animals (n = 5) in the sham-operated group were sacrificed after 11 weeks. The human umbilical mesenchymal stem cell-oligodendrocyte precursor cells (HUMSC-OPCs)-treated group achieved a significant improvement in locomotor performance compared with the saline-treated group.
Data are given as mean ± SD. There are 21 rats in each group. After 4 weeks, eight rats in the HUMSC-OPCs-treated group and the saline-treated group were sacrificed for immunoreactive staining after functional evaluation. From the beginning of the 5th week until the end of the experiment, there were five rats in the sham-operated group and 13 rats each in the two other groups. a P < 0.05, vs. saline-treated group (one-way analysis of variance with repeated measures, followed by the post-hoc Newman-Keuls test).
A higher BBB score indicates improved neurological function. pre: Before model establishment; d: day; w: week.
The human umbilical mesenchymal stem cell-oligodendrocyte precursor cell-treated group achieved a significant improvement in locomotor performance to a final score of 14.59 ± 0.62, compared with the saline-treated group (10.84 ± 0.78) 11 weeks after transplantation (P < 0.05). Basso, Beattie and Bresnahan score analysis showed that transplantation of human umbilical mesenchymal stem cell-oligodendrocyte precursor cells was effective in improving hindlimb function.
Survival, differentiation and effects of oligodendrocyte precursor cells on spinal axon regeneration
Platelet-derived growth factor receptor immunoreactivity (Figure 3A1) showed that some grafted cells changed into immature oligodendrocyte precursor cells. Furthermore, HuNu (a marker for human nuclei) and replication protein A-interacting protein (Figure 3A2) or oligodendrocyte 4 (Figure 3A3) double-labeling indicated that grafted cells changed into mature oligodendrocytes. Only a few cells were, however, observed in the spinal cord of cell-treated animals 8 weeks post-injury. This suggested that most of the transplanted cells did not survive until this time (Figure 3A4).
Figure 3.
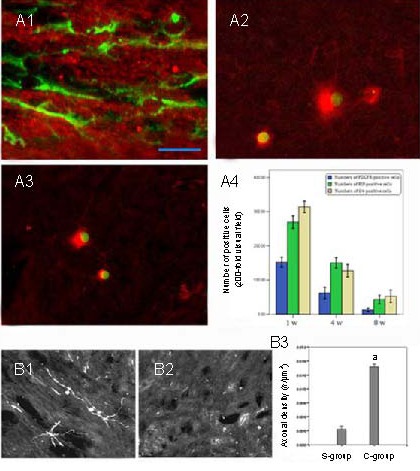
Effects of oligodendrocyte precursor cells (OPCs) on spinal axon regeneration (scale bar shown in A1, A1: 12.5 μm, A2, A3, B1, B2: 50 μm).
(A1) Platelet-derived growth factor receptor (PDGFR)-positive labeling adjacent to the epicenter of the spinal cord in human umbilical mesenchymal stem cell (HUMSC)-OPC-treated animals 4 weeks after injury (green indicates PDGFR-positive labeling for immature OPCs). HuNu and replication protein A-interacting protein (RIP) (A2) or oligodendrocyte 4 (O4) (A3) double-labeling indicated that grafted cells changed into mature oligodendrocytes 4 weeks after injury. Green in A2 and A3 indicates HuNu-positive labeling for human nuclei; red indicates RIP-positive labeling in A2 and O4-positive labeling in A3 for mature oligodendrocytes (immunofluorescence double-labeling staining, fluorescence microscope). (A4) Quantification of immunolabeled cell numbers in the injured epicenter.
Longitudinal sections of the injured spinal cord stained for biotinylated dextran amines 11 weeks after injury in representative rats of the HUMSC-OPC-treated group (B1) and the saline group (B2). Biotinylated dextran amine tracing shows the regrowth of cerebrospinal fibers. As shown in B1, the branches of some cerebrospinal fibers were obviously labeled with biotinylated dextran amines in the epicenter of the lesion in the HUMSC-OPC-treated group. However, in B2, almost no labeled fibers were observed in the epicenter of the lesion in the saline-treated group. A significantly higher density of corticospinal tracts was found in the lesion epicenter of HUMSC-OPC-treated rats than of saline-treated rats (B3). A t-test was used to perform the quantification analysis. Data are expressed as mean ± SD; n = 4, a P < 0.01, vs. saline-treated group. S-group: Saline-treated group; C-group: HUMSC-OPC-treated group.
Within the rostral stump of the injury site, corticospinal tracts were mainly localized deep in the dorsal columns and fewer tracts in the ventral funiculi, using biotinylated dextran amine tracing. There was no difference in the density of corticospinal tracts in the rostral stumps between the human umbilical mesenchymal stem cell-oligodendrocyte precursor cell-treated group and the saline-treated group. However, a significantly higher (Figure 3B3) density of corticospinal tracts was found in the lesion epicenter and caudal stump of human umbilical mesenchymal stem cell-oligodendrocyte precursor cell-treated rats (Figure 3B1) than in those of saline-treated rats (Figure 3B2).
Evaluation of remyelination after oligodendrocyte precursor cell transplantation
To investigate the effect of human umbilical mesenchymal stem cell-oligodendrocyte precursor cells on myelin preservation, the extent of residual myelination, stained with Luxol fast blue, was examined at the injury epicenter 11 weeks after contusive spinal cord injury. Residual myelination was also examined 1, 2, 3, and 4 mm rostral and caudal to the injury epicenter. As shown in Figure 4A, the volume of residual myelin was significantly increased in the human umbilical mesenchymal stem cell-oligodendrocyte precursor cell-treated group at the epicenter as well as at 1 and 2 mm rostral and caudal to the lesion epicenter compared with that in the saline-treated group (P < 0.01).
Figure 4.
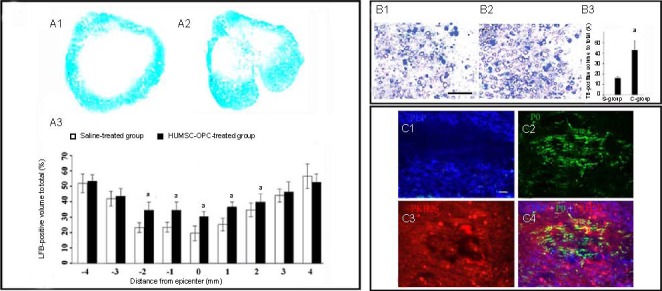
Oligodendrocyte precursor cells (OPCs) promoted remyelination of injured spinal cord (scale bar in B: 25 μm (B1); in C: 12.5 μm (C1).
(A) Residual myelination in injured spinal cords 11 weeks after contusive spinal cord injury using Luxol fast blue (LFB) staining. (A1) LFB staining of residual myelination in the saline-treated group (× 40); (A2) LFB staining of residual myelination in the HUMSC-OPC-treated group (× 40); (A3) Quantification of residual myelination. For the quantification analysis, an independent sample t-test was performed. Data are expressed as mean ± SD, n = 5; a P < 0.01, vs. saline-treated group.
(B) Spared myelin in injured spinal cords 11 weeks after contusive spinal cord injury. (B1) Toluidine blue (TB) staining of residual myelination in the saline-treated group (× 40). (B2) TB staining of residual myelination in the HUMSC-OPC-treated group (× 40); (B3) Quantification of spared myelination. For the quantification analysis, an independent sample t-test was performed. Data are expressed as mean ± SD, n = 5; a P < 0.01, vs. saline-treated group.
(C) Double immunofluorescence staining for proteolipid protein (PLP; C1) and P0 (C2) and concurrent observation of PKH26-labeled (C3) transplanted cells in regenerated tissues. Proteolipid protein-positive staining (blue), representing a central nervous system-type myelin sheath, was more centrally located in the lesion, and mostly co-localized with the PKH26-labeled grafted cells (red). However, P0-positive staining (green) was localized mainly at the lesion periphery (C4).
To verify the Luxol fast blue results, a subset of sections from the injury epicenter of the above groups was stained with toluidine blue. As shown in Figure 4B, the spared myelin was more abundant in the human umbilical mesenchymal stem cell-oligodendrocyte precursor cell-treated group than in the saline-treated group (P < 0.01).
To further determine whether the injured spinal cord injury transplanted with human umbilical mesenchymal stem cell-oligodendrocyte precursor cells regenerated a central nervous system-type myelin sheath rather than a peripheral nervous system-type sheath in the repaired spinal cord injury site, double immunofluorescence staining was performed using an anti-myelin P0 antibody to detect peripheral nervous system peripheral nervous system-type sheaths and an anti-myelin proteolipid protein antibody to identify central nervous system-type sheaths (Figure 4C).
Proteolipid protein-expressing cells were concentrated mainly in the lesion epicenter, accurately coinciding with the region of PKH26-labeled cells (Red Fluorescent Cell Linker Mini Kit-26 for labeling grafted cells in vitro). P0-expressing cells were distinctly separated from transplanted PKH26-labeled and P0-expressing cells in the lesion center, and were mainly localized in the periphery.
DISCUSSION
This study showed that a combination of trophic factors was able to induce the differentiation of human umbilical mesenchymal stem cells into cells with morphological characteristics of the oligodendrocyte phenotype and oligodendrocyte cell surface markers in cell culture medium. Consistent with this finding, Kennea et al[19] have demonstrated that primary human fetal mesenchymal stem cells could also differentiate into cells with an oligodendrocyte phenotype both in vitro and in vivo by exposing human fetal mesenchymal stem cells to conditioned medium with inducing factors or by introducing pro-oligodendrocyte genes. Our previous study showed that human umbilical cord blood-derived stromal cells can also differentiate into cells with an oligodendrocyte phenotype[20].
Thus, mesenchymal stem cells from different sources can be induced into oligodendrocyte-like cells by inducing factors, and human umbilical mesenchymal stem cell-oligodendrocyte precursor cells might provide a choice for the treatment of spinal cord injury.
Human umbilical mesenchymal stem cell-oligodendrocyte precursor cells were able to support axonal regeneration and remyelination after being grafted into the injured spinal cord. Oligodendrocyte differentiation of grafted cells is vital for the remyelination of the injured spinal cord[21,22]. Multiple cell types, including fetal and adult neural stem cells or precursors, have been grafted into the injured spinal cord. However, neural stem cells mainly differentiated into astrocytes and only a few oligodendrocytes after transplantation into the injured spinal cord[1,23].
Embryonic-derived oligodendrocyte precursor cells co-transplanted with mesenchymal stem cells differentiated into oligodendrocytes and mesenchymal stem cells, which reduced microglial activation and astrocytosis in the brain of transplanted animals[24]. However, Lv and colleagues[25,26] recently reported that almost no embryonic oligodendrocyte precursor cells (isolated from rat spinal cord) differentiated into oligodendrocytes after in situ transplantation. In contrast, a much higher percentage (42%) of grafted human umbilical mesenchymal stem cell- oligodendrocyte precursor cells differentiated into mature oligodendrocytes, as shown in this study. Several factors are responsible for these differences. Above all, the scaffold used in this study may improve the local microenvironment, which may promote the survival and differentiation of human umbilical mesenchymal stem cell-oligodendrocyte precursor cells. Compared with a previous study[25], a better functional recovery was achieved in this study. It is thus important to use fibrin glue, which significantly promotes the survival of grafted cells via chemotactic and mitogenic stimuli[27]. Improving the local microenvironment of the injured spinal cord is of great significance when grafting oligodendrocyte precursor cells. Secondly, sources of oligodendrocyte precursor cells and transplantation methods may contribute to the differences observed between our study and previous work. Different animal models and cell labeling methods may also affect the results. Further systematic investigations are needed to compare the therapeutic effects of oligodendrocyte precursor cells isolated from different sources.
In this study, human umbilical mesenchymal stem cell-oligodendrocyte precursor cells promoted axon regeneration after spinal cord injury. However, we observed only a small quantity and a short length of regenerated axons, considering the rapidity of the recovery in the saline-treated group. Therefore, it was unlikely that axon regeneration itself influenced the outcome in the injured spinal cord[11]. Interestingly, there was a significant increase in spared myelinated axons in the human umbilical mesenchymal stem cell-oligodendrocyte precursor cell-treated group compared with the vehicle-treated group. This histological improvement correlated well with the increase in behavioral recovery. These results are consistent with previous studies[28]. A regenerative environment created by grafted human umbilical mesenchymal stem cell-oligodendrocyte precursor cells is a possible explanation for the functional recovery in this study[29]. Our previous study showed that human umbilical mesenchymal stem cell-oligodendrocyte precursor cells are capable of producing numerous neurotrophins including hepatocyte growth factor, transforming growth factor-beta 1 and brain-derived neurotrophic factor[16]. Oligodendrocyte precursor cells even promoted neurite outgrowth of rat sensory neurons in vitro[30]. Additionally, co-culture of human embryonic stem cell-derived oligodendrocyte precursor cells with cortical neurons enhanced neurite outgrowth from the cortical neurons, indicating that the factors secreted by the human umbilical mesenchymal stem cell-oligodendrocyte precursor cells can affect surrounding cells[30,31]. These results suggested that human umbilical mesenchymal stem cell-oligodendrocyte precursor cells may contribute to providing trophic support.
Our findings suggest that, in vivo as well as in vitro, human umbilical mesenchymal stem cell- oligodendrocyte precursor cells retain their oligodendrocyte precursor cell-like characteristics even after transplantation, thereby supporting axonal remyelination. Although more precise characterization of human umbilical mesenchymal stem cell- oligodendrocyte precursor cells is needed, the present results show that human umbilical mesenchymal stem cell-oligodendrocyte precursor cells are morphologically, phenotypically and functionally identical to original oligodendrocyte precursor cells. Additionally, human umbilical mesenchymal stem cell-oligodendrocyte precursor cells can be induced without the introduction of exogenous genes, by the simple administration of trophic factors[16,30]. This is beneficial for the safety of this cell-based therapy. It encourages us to put human umbilical mesenchymal stem cell-oligodendrocyte precursor cell transplantation forward as a candidate for clinical applications. To achieve more effective functional recovery after spinal cord injury, future research is needed to establish optimal conditions for the survival and differentiation of human umbilical mesenchymal stem cell-oligodendrocyte precursor cells.
MATERIALS AND METHODS
Design
A randomized, controlled animal experiment.
Time and setting
Experiments were performed at the Institute of Neurosurgery of the Military General Hospital of Beijing PLA between March 2010 and December 2011.
Materials
Animals
Forty-seven adult male Sprague-Dawley rats, weighing 250–300 g and aged 10–12 weeks old, were purchased from the Experimental Animal Center, the Academy of Military Medical Sciences in China (license No. SCXK (Military) 2011013). All animal procedures were performed under protocols conforming to National Institutes of Health Guidelines.
Human umbilical cord
With the written consent of the umbilical cord donors, fresh human umbilical cords were obtained after birth from the Department of Obstetrics in the Military General Hospital of Beijing, China and collected in Hanks’ Balanced Salt Solution (Gibco, 14185-052, Grand Island, NY, USA) at 4°C. This was performed according to the Declaration of Helsinki.
Methods
Culture of human umbilical mesenchymal stem cells
The isolation and culture of human umbilical mesenchymal stem cells were carried out according to previously described methods[17]. As described previously[11], following disinfection in 75% ethanol for 30 seconds, the umbilical cord vessels were removed while they were still in Hanks Balanced Salt Solution. The mesenchymal tissue (in Wharton's jelly) was then diced into cubes of about 0.5–1 cm3 and centrifuged at 1 200 r/min for 5 minutes, d = 10 cm. After removal of the supernatant fraction, the precipitate (mesenchymal tissue) was washed with serum-free Dulbecco's modified Eagle's medium/F12 (Gibco) and centrifuged at 1 000 r/min for 5 minutes. The precipitate was then enzymatically dissociated for 30 minutes at 37 °C using 0.075% collagenase type II (Sigma, St. Louis, MO, USA), followed by digestion with 0.125% trypsin/ethylenediamine tetraacetic acid (Gibco) at 37°C for 30 minutes. The suspension was neutralized with 10% (v/v) fetal bovine serum (Gibco) and cells were counted under a microscope (Olympus, Tokyo, Japan) with the aid of a hemocytometer (CELL-VU®; Millennium Sciences, Mulgrave, Victoria, Australia). The identification of human umbilical mesenchymal stem cells by flow cytometric analysis and immunofluorescence staining was described in our previous studies[11,16,17,18].
Transdifferentiation of human umbilical mesenchymal stem cells into neurospheres and oligodendrocyte precursor cells
Human umbilical mesenchymal stem cells at passage 5 were digested with 0.25% trypsin (Gibco) and then plated onto culture flasks at a concentration of 2 × 105/cm2 in neurobasal medium (Invitrogen, Carlsbad, CA, USA) supplemented with 20 ng/mL epidermal growth factor (Peprotech, London, UK), 20 ng/mL basic fibroblast growth factor (Peprotech) and N2 (1:100, Gibco) at 37°C in 5% CO2. We added fresh neurobasal medium every 3 to 4 days and changed the medium once a week. Four or five days after initial differentiation, free-floating neurospheres formed. Neurospheres were passaged every 7–10 days by trituration using a fire-polished Pasteur pipette and re-plating in fresh medium.
For oligodendrocyte precursor cell commitment, neurospheres were plated onto 6-well plates containing neurobasal medium supplemented with human sonic hedgehog (Sigma) (100 ng/mL), basic fibroblast growth factor (10 ng/mL; Sigma, St. Louis, MO, USA), insulin-like growth factor (50 ng/mL, Peprotech) and platelet-derived growth factor-BB (10 ng/mL, Peprotech) for 4–5 days.
Immunocytochemistry
The procedure for immunocytochemistry was described previously[17]. The primary antibodies used were polyclonal mouse anti-nestin (1:400), mouse anti-CD44 (1:400), rabbit anti-vimentin (1:200), rabbit anti-platelet-derived growth factor receptor (1:400), mouse anti-oligodendrocyte 4 and mouse anti-replication protein A-interacting protein (1:200) (all from Chemicon, Billerica, MA, USA). The sections were incubated in the primary antibody solution for 48 hours at 4°C. Following three washes in 0.1 M PBS for 5 minutes each, sections were incubated with secondary antibodies (Alexa Fluor 594 goat anti-mouse IgG (red color, 1:200), Alexa Fluor 488 goat anti-rabbit IgG (green color, 1:200) (all from Molecular Probes, Eugene, OR, USA) for 1 hour at room temperature. The nuclei were counterstained with Hoechst 33342 (Invitrogen). Samples were examined using a fluorescence microscope (Leica, Vertrieb, Germany) and an IM50 imaging system (Thorlabs, Newton, NJ, USA). The positively stained cells were counted in 20 random fields (× 200).
Establishment of spinal cord injury model and intervention
Contusive spinal cord injury was performed using a New York University Impactor. Briefly, the animals were anesthetized with sodium pentobarbital (30 mg/kg) and received a laminectomy at the T9 level. After the spinous processes of T7 and T11 were clamped to stabilize the spine, the exposed dorsal surface of the cord was subjected to a weight drop injury using a 10 g rod (2.5 mm in diameter) dropped at a height of 12.5 mm. Sham-operated rats were subjected to laminectomy but not contusion. Manual bladder emptying was performed three times daily until reflex bladder emptying was established.
To trace the transplanted cells, cells were harvested, labeled with PKH26 (Sigma) according to the manufacturer's protocol, and diluted to 1 × 105/μL in saline. One week after impact injury, the sham-operated group received 5 μL saline in fibrin glue (Greenplast, Greencross PBM, Korea) injected within the epicenter of injury. In experimental groups, 5 μL of saline or PBS containing 1 × 106 human umbilical mesenchymal stem cell-oligodendrocyte precursor cells in fibrin glue were injected into the epicenter of injury. The saline or PBS containing human umbilical mesenchymal stem cell-oligodendrocyte precursor cells in fibrin glue was injected into the epicenter of the syrinx at the T9 level over 5 minutes with a 10 μL Hamilton syringe and the aid of a spinal stereotaxic frame.
Functional evaluation
Locomotor function of the hindlimbs was evaluated according to the Basso, Beattie, and Bresnahan locomotor rating scale[32]. The initial locomotor scores were equalized between groups. Hindlimb locomotion was scored from 0 to 21 points. A score of 0 was given if there was no spontaneous movement, and a score of 21 indicated normal locomotion[17]. Two independent examiners determined motor ability for 4 minutes.
Biotinylated dextran amine tracing study
Eight weeks post-transplantation, a biotinylated dextran amine tracing study was performed. Corticospinal tracing using biotinylated dextran amines was performed according to Erceg et al[28]. Briefly, after the rat was anesthetized with sodium pentobarbital (30 mg/kg), 10% biotinylated dextran amine solution (Invitrogen) in 0.01 M PBS was slowly injected into the sensorimotor cortex using a Hamilton syringe inserted into burr-holes. Biotinylated dextran amines were injected stereotaxically at a depth of 1.5 mm at three sites distributed over the sensorimotor cortex[33], 1 μL per injection. Two weeks later (11 weeks after injury), four animals from each group were euthanized by anesthesia overdose and transcardially perfused with PBS followed by 4% paraformaldehyde in 0.1 M PBS. T11–L1 spinal cord segments were removed and postfixed in the same solution at 4°C overnight, followed by cryoprotection in 30% sucrose overnight. Tissue was frozen at the optimal cutting temperature and 30 μm longitudinal sections of the spinal segments were cut with a cryostat. The T11–L1 free-floating longitudinal sections were incubated with avidin-horseradish peroxidase overnight at 4°C.
Histological staining
Immunohistochemistry staining: The rats from the human umbilical mesenchymal stem cell-oligodendrocyte precursor cell-treated and saline-treated groups were sacrificed and perfused transcardially with ice-cold PBS and 4% paraformaldehyde at 4 (n = 8 for human umbilical mesenchymal stem cell-oligodendrocyte precursor cell-treated and saline-treated groups) and 11 weeks (n = 13 for human umbilical mesenchymal stem cell-oligodendrocyte precursor cells-treated and saline-treated groups, n = 5 for sham-operated group) after injury. The 20 mm transverse segments of the spinal cord injury region were dissected and stored in the same fixative overnight. The tissues were frozen and cryosectioned as 10 μm thick longitudinal sections by cryomicrotome (Microm/HM500V, Walldorf, Germany). Individual sections were incubated with human nuclear protein (HuNu, mAb 1281, Chemicon; 1:200), rabbit anti-P0 (Chemicon; 1:500) and mouse polyclonal anti-proteolipid protein antibodies (AbCam, Cambridge, UK; 1:1 000) for 2 hours at 37°C. They were then incubated with secondary antibody (Alexa Fluor 594 goat anti-mouse IgG (red color; 1:200), Alexa Fluor 488 goat anti-rabbit IgG (green color; 1:200) for 2 hours at 37°C, washed, and mounted on glass slides with fluorescent mounting medium (Vectorshield, Vector Laboratories, Burlingame, CA, USA). The positively stained cells were counted in 20 random fields (× 200).
Luxol fast blue staining: Two sets of slides (each set containing serial sections spaced 200 μm apart) were stained with Luxol fast blue to identify myelinated white matter. The lesion epicenter was defined as the section containing the least amount of spared white matter. The total and cross-sectional area of the spinal cord and the lesion boundary were measured with an Olympus BX60 microscope attached to a Neurolucida system (Microbrightfield Inc., Colchester, VT, USA). The total volume of the lesion area (which included areas of cavitation) was calculated by summing their individual subvolumes. Individual subvolumes of the lesion area were calculated by multiplying the cross-sectional area (A) × D, where D represented the distance between sections (200 μm). The percentage total volume of the injured area was calculated by dividing the total volume of the lesion area by the total spinal cord volume[1]. Luxol fast blue- stained sections at the lesion epicenter and 1, 2, 3, and 4 mm rostral and caudal to the epicenter were analyzed. The myelinated white matter was quantified by Image pro-plus 5.1 (Media Cybernetics, Inc, Atlanta, GA, USA).
Toluidine blue staining: In brief, spinal cord segments were fixed overnight in a solution containing 2% glutaraldehyde and 5% sucrose in 0.1 M sodium cacodylate buffer, pH 7.4, followed by 1% osmium tetroxide in the same buffer for 1 hour. The tissue was embedded in Spurr's epoxy resin and cured at 70°C. Transverse semi-thin sections (1 μm) were stained with a mixture of 1% toluidine blue (Sigma) and 1% sodium borate. For statistical analysis, the number of spared myelinated fibers was calculated in four random 10 × 40-fold microscope views (about 67 500 μm2 per view) in the middle of the lesion border and the pial border.
Statistical analysis
All data are expressed as mean ± SD. Data for these measurements, assuming equal variance, were analyzed using one-way analysis of variance with repeated measures, followed by the post-hoc Newman-Keuls test and independent sample t-test with SPSS 11.0 software (SPSS, Chicago, IL, USA). A value of P < 0.05 was considered statistically significant.
Footnotes
Funding: This research was supported by the National Natural Science Foundation of China, No. 81100916, 30400464, 81271316; and the Postdoctoral Science Foundation of China, No. 201104901907.
Conflicts of interest: None declared.
Ethical approval: For the human umbilical cord use, ethical approval was obtained from the Military General Hospital of Beijing PLA, China.
(Reviewed by McAlpine F, Wysong S, Zhu WJ, Zhao H)
(Edited by Wang LM, Qiu Y, Li CH, Song LP)
REFERENCES
- [1].Cao Q, Xu XM, Devries WH, et al. Functional recovery in traumatic spinal cord injury after transplantation of multineurotrophin-expressing glial-restricted precursor cells. J Neurosci. 2005;25(30):6947–6957. doi: 10.1523/JNEUROSCI.1065-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Liu S, Qu Y, Stewart TJ, et al. Embryonic stem cells differentiate into oligodendrocytes and myelinate in culture and after spinal cord transplantation. Proc Natl Acad Sci U S A. 2000;97(11):6126–6131. doi: 10.1073/pnas.97.11.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Mekhail M, Almazan G, Tabrizian M. Oligodendrocyte- protection and remyelination post-spinal cord injuries: a review. Prog Neurobiol. 2012;96(3):322–339. doi: 10.1016/j.pneurobio.2012.01.008. [DOI] [PubMed] [Google Scholar]
- [4].Karimi-Abdolrezaee S, Schut D, Wang J, et al. Chondroitinase and growth factors enhance activation and oligodendrocyte differentiation of endogenous neural precursor cells after spinal cord injury. PLoS One. 2012;7(5):e37589. doi: 10.1371/journal.pone.0037589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Cho YK, Kim G, Park S, et al. Erythropoietin promotes oligodendrogenesis and myelin repair following lysolecithin-induced injury in spinal cord slice culture. Biochem Biophys Res Commun. 2012;417(2):753–759. doi: 10.1016/j.bbrc.2011.12.029. [DOI] [PubMed] [Google Scholar]
- [6].Yasuda A, Tsuji O, Shibata S, et al. Significance of remyelination by neural stem/progenitor cells transplanted into the injured spinal cord. Stem Cells. 2011;29(12):1983–1994. doi: 10.1002/stem.767. [DOI] [PubMed] [Google Scholar]
- [7].Keirstead HS, Nistor G, Bernal G, et al. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury. J Neurosci. 2005;25(19):4694–4705. doi: 10.1523/JNEUROSCI.0311-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bambakidis NC, Miller RH. Transplantation of oligodendrocyte precursors and sonic hedgehog results in improved function and white matter sparing in the spinal cords of adult rats after contusion. Spine J. 2004;4(1):16–26. doi: 10.1016/j.spinee.2003.07.004. [DOI] [PubMed] [Google Scholar]
- [9].Lukovic D, Moreno Manzano V, Stojkovic M, et al. Concise review: human pluripotent stem cells in the treatment of spinal cord injury. Stem Cells. 2012;30(9):1787–1792. doi: 10.1002/stem.1159. [DOI] [PubMed] [Google Scholar]
- [10].Ogawa SI, Tokumoto Y, Miyake J, et al. Induction of oligodendrocyte differentiation from adult human fibroblast-derived induced pluripotent stem cells. In Vitro Cell Dev Biol Anim. 2011;47(7):464–469. doi: 10.1007/s11626-011-9435-2. [DOI] [PubMed] [Google Scholar]
- [11].Zhang L, Zhang HT, Hong SQ, et al. Cografted Wharton's jelly cells-derived neurospheres and BDNF promote functional recovery after rat spinal cord transaction. Neurochem Res. 2009;34(11):2030–2039. doi: 10.1007/s11064-009-9992-x. [DOI] [PubMed] [Google Scholar]
- [12].Kern S, Eichler H, Stoeve J, et al. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24(5):1294–1301. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- [13].Shang AJ, Hong SQ, Xu Q, et al. NT-3-secreting human umbilical cord mesenchymal stromal cell transplantation for the treatment of acute spinal cord injury in rats. Brain Res. 2011;1391:102–113. doi: 10.1016/j.brainres.2011.03.019. [DOI] [PubMed] [Google Scholar]
- [14].Yang CC, Shih YH, Ko MH, et al. Transplantation of human umbilical mesenchymal stem cells from Wharton's jelly after complete transection of the rat spinal cord. PLoS One. 2008;3(10):e3336. doi: 10.1371/journal.pone.0003336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Margossian T, Reppel L, Makdissy N, et al. Mesenchymal stem cells derived from Wharton's jelly: comparative phenotype analysis between tissue and in vitro expansion. Biomed Mater Eng. 2012;22(4):243–254. doi: 10.3233/BME-2012-0714. [DOI] [PubMed] [Google Scholar]
- [16].Zhang HT, Fan J, Cai YQ, et al. Human Wharton's jelly cells can be induced to differentiate into growth factor-secreting oligodendrocyte progenitor-like cells. Differentiation. 2010;79(1):15–20. doi: 10.1016/j.diff.2009.09.002. [DOI] [PubMed] [Google Scholar]
- [17].Zhang HT, Chen H, Zhao H, et al. Neural stem cells differentiation ability of human umbilical cord mesenchymal stromal cells is not altered by cryopreservation. Neurosci Lett. 2011;487(1):118–122. doi: 10.1016/j.neulet.2010.10.008. [DOI] [PubMed] [Google Scholar]
- [18].Hong SQ, Zhang HT, You J, et al. Comparison of transdifferentiated and untransdifferentiated human umbilical mesenchymal stem cells in rats after traumatic brain injury. Neurochem Res. 2011;36(12):2391–2400. doi: 10.1007/s11064-011-0567-2. [DOI] [PubMed] [Google Scholar]
- [19].Kennea NL, Waddington SN, Chan J, et al. Differentiation of human fetal mesenchymal stem cells into cells with an oligodendrocyte phenotype. Cell Cycle. 2009;8(7):1069–1079. doi: 10.4161/cc.8.7.8121. [DOI] [PubMed] [Google Scholar]
- [20].Luo YC, Zhang HT, Cheng HY, et al. Differentiation of cryopreserved human umbilical cord blood-derived stromal cells into cells with an oligodendrocyte phenotype. In Vitro Cell Dev Biol Anim. 2010;46(7):585–589. doi: 10.1007/s11626-010-9314-2. [DOI] [PubMed] [Google Scholar]
- [21].Siebert JR, Stelzner DJ, Osterhout DJ. Chondroitinase treatment following spinal contusion injury increases migration of oligodendrocyte progenitor cells. Exp Neurol. 2011;231(1):19–29. doi: 10.1016/j.expneurol.2011.05.002. [DOI] [PubMed] [Google Scholar]
- [22].Plemel JR, Chojnacki A, Sparling JS, et al. Platelet- derived growth factor-responsive neural precursors give rise to myelinating oligodendrocytes after transplantation into the spinal cords of contused rats and dysmyelinated mice. Glia. 2011;59(12):1891–1910. doi: 10.1002/glia.21232. [DOI] [PubMed] [Google Scholar]
- [23].Shihabuddin LS, Horner PJ, Ray J, et al. Adult spinal cord stem cells generate neurons after transplantation in the adult dentate gyrus. J Neurosci. 2000;20(23):8727–8735. doi: 10.1523/JNEUROSCI.20-23-08727.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hicks AU, Lappalainen RS, Narkilahti S, et al. Transplantation of human embryonic stem cell-derived neural precursor cells and enriched environment after cortical stroke in rats: cell survival and functional recovery. Eur J Neurosci. 2009;29(3):562–574. doi: 10.1111/j.1460-9568.2008.06599.x. [DOI] [PubMed] [Google Scholar]
- [25].Lü HZ, Wang YX, Zhou JS, et al. Cyclosporin A increases recovery after spinal cord injury but does not improve myelination by oligodendrocyte progenitor cell transplantation. BMC Neurosci. 2010;11:127. doi: 10.1186/1471-2202-11-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Lü HZ, Wang YX, Zou J, et al. Differentiation of neural precursor cell-derived oligodendrocyte progenitor cells following transplantation into normal and injured spinal cords. Differentiation. 2010;80(4-5):228–240. doi: 10.1016/j.diff.2010.09.179. [DOI] [PubMed] [Google Scholar]
- [27].Lee OK. Fibrin glue as a vehicle for mesenchymal stem cell delivery in bone regeneration. J Chin Med Assoc. 2008;71(2):59–61. doi: 10.1016/S1726-4901(08)70075-3. [DOI] [PubMed] [Google Scholar]
- [28].Erceg S, Ronaghi M, Oria M, et al. Transplanted oligodendrocytes and motoneuron progenitors generated from human embryonic stem cells promote locomotor recovery after spinal cord transection. Stem Cells. 2010;28(9):1541–1549. doi: 10.1002/stem.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Sharp J, Frame J, Siegenthaler M, et al. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants improve recovery after cervical spinal cord injury. Stem Cells. 2010;28(1):152–163. doi: 10.1002/stem.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Zhang YW, Denham J, Thies RS. Oligodendrocyte progenitor cells derived from human embryonic stem cells express neurotrophic factors. Stem Cells Dev. 2006;15(6):943–952. doi: 10.1089/scd.2006.15.943. [DOI] [PubMed] [Google Scholar]
- [31].Watson RA, Yeung TM. What is the potential of oligodendrocyte progenitor cells to successfully treat human spinal cord injury? BMC Neurol. 2011;11:113. doi: 10.1186/1471-2377-11-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12(1):1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- [33].Paxions G, Watson C. 6th ed. San Diego: Academic Press; 2005. The Rat Brain in Stereotaxic Coordinates. [Google Scholar]


