Abstract
Minocylcine, a tetracycline derivate, has been shown to cross the blood-brain barrier and enter the central nervous system. In this study, cerebral ischemia-reperfusion injury models were established using the suture method, and minocycline was immediately injected intraperitoneally after cerebral ischemia-reperfusion (22.5 mg/kg, initially 45 mg/kg) at a 12-hour interval. Results showed that after minocycline treatment, the volume of cerebral infarction was significantly reduced, the number of surviving cell in the hippocampal CA1 region increased, the number of apoptotic cells decreased, the expression of caspase-3 and poly(adenosine diphosphate-ribose) polymerase-1 protein was down-regulated, and the escape latency in the water maze test was significantly shortened compared with the ischemia-reperfusion group. Our experimental findings indicate that minocycline can protect against neuronal injury induced by focal ischemia-reperfusion, which may be mediated by the inhibition of caspase-3 and poly(adenosine diphosphate-ribose) polymerase-1 protein expression.
Keywords: neural regeneration, brain injury, minocycline, cerebral ischemia-reperfusion, hippocampus, poly (adenosine diphosphate-ribose) polymerase-1, caspase-3, apoptosis, grants-supported paper, neuroregeneration
Research Highlights
-
(1)
The effect of minocycline treatment on neuronal apoptosis and learning and memory was monitored following ischemic reperfusion injury.
-
(2)
Inhibition of caspase-3 and poly(adenosine diphosphate-ribose) polymerase-1 protein expression by minocycline protected neurons from focal ischemia-reperfusion injury.
INTRODUCTION
Cerebral ischemia triggers a cascade of pathophysiological events including excitotoxicity, ionic imbalance, oxidative stress and apoptotic-like cell death mechanisms[1,2,3,4,5,6,7,8]. Caspase-dependent neuronal apoptosis and caspase-independent neuronal apoptosis have been documented as two classic signaling pathways that occur during cerebral ischemia-reperfusion. Caspase-3 and poly (adenosine diphosphate-ribose) polymerase-1 are two key downstream implementation factors of the caspase-dependent neuronal apoptosis signaling pathway. Moreover, caspase-3 and poly(adenosine diphosphate-ribose) polymerase-1 have been identified as key mediators of apoptosis in animal models of cerebral ischemia-reperfusion[9]. Apoptotic neurons in the penumbra area can be saved if intervention occurs at a certain time after cerebral ischemia-reperfusion. Therefore, blocking apoptosis to reverse the damage in neurons is the focus of many treatments for ischemic cerebrovascular disease.
Minocycline is a tetracycline derivative and has been used in clinical situations for the treatment of pityrosporum folliculitis and pyoderma[10]. Minocycline can readily cross the blood-brain barrier and easily penetrates the central nervous system to produce a protective effect in various models of acute neurological injury[11]. Moreover, minocycline has been shown to promote neuronal survival through suppression of apoptotic cell death via Bcl-2/cytochrome c against ischemia in kidney cells and spinal cord injury through heat stress[12,13,14]. However, no evidence exists involving the protective effects of minocycline on neuronal injuries induced by focal ischemia-reperfusion. This study aimed to observe the effect of minocycline in rats following focal ischemia-reperfusion injury by assaying the change in cerebral infarction volume, cell apoptosis in the hippocampal CA1 region, and the expression of caspase-3 and poly(adenosine diphosphate-ribose) polymerase-1 protein.
RESULTS
Quantitative analysis of experimental animals
Healthy male Sprague-Dawley rats (n = 108) were randomly assigned into a sham-operation group (n = 36), ischemia-reperfusion group (n = 36), and a minocycline treatment group (n = 36). The latter two groups were subjected to ischemia-reperfusion, and the minocycline group was treated with minocycline via intraperitoneal injection. All rats were used in the final analysis.
Minocycline enhanced learning and memory abilities in rats following cerebral ischemia-reperfusion
In the water maze test, the escape latencies were significantly prolonged in rats in the ischemia-reperfusion group compared with the sham-operation group (P < 0.05). In addition, escape latencies were significantly shorter in the minocycline treatment group than in the ischemia-reperfusion group (P < 0.05; Table 1).
Table 1.
Effects of minocycline on learning and memory in rats following cerebral ischemia-reperfusion
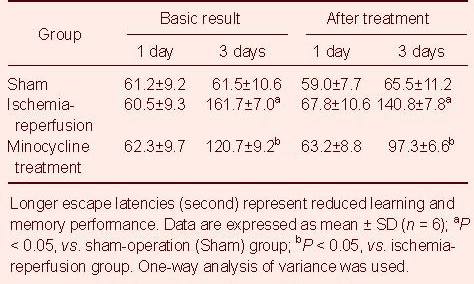
Minocycline decreased the volume of cerebral infarction in rats following cerebral ischemia-reperfusion
Tetrazolium chloride staining results showed that uniform staining in bilateral cortical hemispheres was visible in the sham-operation group, indicating no infarction. In the ischemia-reperfusion group and minocycline treatment group, the infarction areas were large and pale. Compared with the ischemia-reperfusion group, infarction volume was significantly reduced in the minocycline treatment group (P < 0.05; Figure 1).
Figure 1.
Effect of minocycline (MT) on cerebral infarction volume in the hippocampal CA1 region of rats after cerebral ischemia-reperfusion (I/R) (tetrazolium chloride staining).
(A) Tetrazolium chloride staining results showed uniform staining in the sham-operation (Sham) group, and a pale infracted locus in the I/R group and MT group. Compared with the I/R group, infarction volume was significantly reduced in the MT group.
(B) Infarction volume analysis of cerebral I/R. Data are expressed as mean ± SD (n = 6), and were analyzed using one-way analysis of variance. aP < 0.05, vs. sham group; bP < 0.05, vs. I/R group. 1 and 3 days mean the time of MT treatment.
Minocycline attenuated neuronal injury in the hippocampal CA1 region in rats following cerebral ischemia-reperfusion
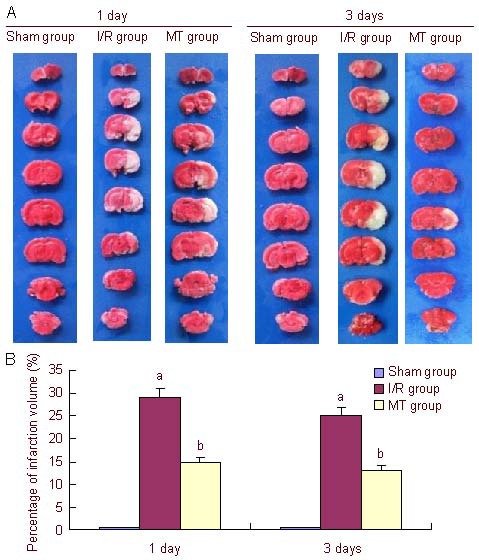
Hematoxylin-eosin staining showed that in the sham-operation group, the normal neurons in the hippocampal CA1 region arranged in neat rows, the cell body was full, and the outline of the nucleus was clear; in the ischemia- reperfusion group, neurons in the hippocampal CA1 region began to undergo necrosis and the number of neurons was significantly reduced and cell bodies were swollen. In addition, the number of pyramidal cells was decreased and lacked unity and coherence. In the minocycline treatment group, the number of intact neurons in the hippocampal CA1 region was increased significantly, and the morphology of neurons slightly recovered (Figure 2).
Figure 2.
Effect of minocycline on morphological changes in the hippocampal CA1 region of rats after cerebral ischemia-reperfusion (I/R) (hematoxylin-eosin staining, × 200).
In the sham-operation group, normal neurons arranged in neat rows, the cell bodies were full, and the outline of the nucleus was clear.
In the I/R group, neuronal necrosis in the hippocampal CA1 region and significantly less neurons were observed, the cell bodies were swollen and the number of pyramidal cells was decreased and lacked unity and coherence.
In the minocycline group, structural integrity of neurons increased significantly, and neurons slightly recovered in number.
Minocycline reduced cell apoptosis in the hippocampal CA1 region in rats following cerebral ischemia-reperfusion
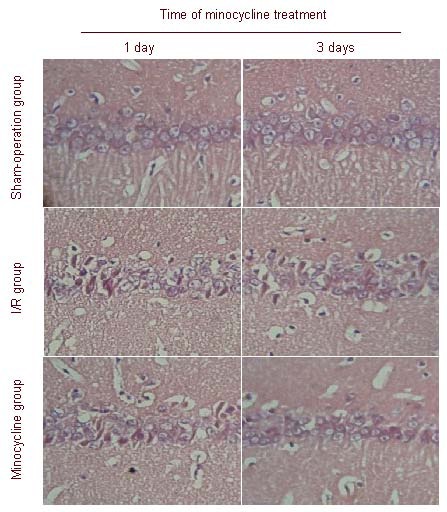
Terminal deoxynucleotidyl transferase biotin-dUTP nick end labeling (TUNEL) staining showed that in the sham-operation group, only a few apoptotic cells in the hippocampal CA1 region were observed, which suggested physiological death of neurons. In the ischemia-reperfusion group, the number of apoptotic cells increased after ischemia-reperfusion; in the minocycline treatment group, the number of apoptotic neurons was significantly decreased after treatment. The apoptotic index was greatest in the ischemia-reperfusion group, followed by the minocycline treatment group and sham-operation group (P < 0.05; Figure 3).
Figure 3.
Effect of minocycline on neuronal apoptosis in the hippocampal CA1 region of rats after cerebral ischemia-reperfusion (I/R) (TUNEL staining, × 200).
(A) Cell apoptosis in the hippocampal CA1 region. Cell apoptosis was not observed in the sham-operation group; the number of apoptotic cells in the minocycline group was significantly less than in the I/R group. Arrows point to apoptotic cells.
(B) Apoptotic index analysis in cerebral I/R rats after treatment for 1 and 3 days. The apoptotic index was calculated using the equation: TUNEL-positive cells/total number of cells × 100%. Data are expressed as mean ± SD (n = 6), and were analyzed using one-way analysis of variance. aP < 0.05, vs. sham-operation group; bP < 0.05, vs. I/R group.
TUNEL: Terminal deoxynucleotidyl transferase biotin-dUTP nick end labeling.
Minocycline decreased the number of caspase-3-positive cells in the hippocampal CA1 region in rats following cerebral ischemia-reperfusion
Immunohistochemical staining showed that caspase-3-positive cells were not observed in the sham-operation group. In the ischemia-reperfusion group, the number of caspase-3-positive cells increased after ischemia-reperfusion; however, this number significantly decreased after minocycline treatment. The number of caspase-3-positive cells was greatest in the ischemia-reperfusion group, followed by the minocycline treatment group and sham-operation group (Figure 4).
Figure 4.
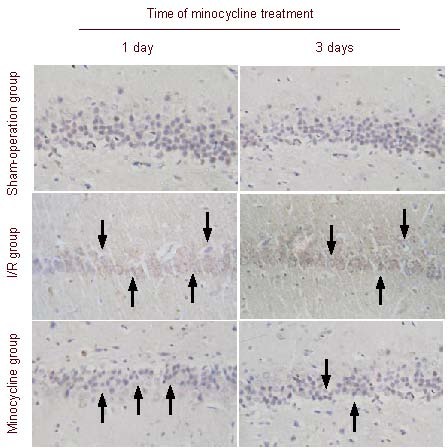
Effect of minocycline on caspase-3 expression in the hippocampal CA1 region of rats after cerebral ischemia-reperfusion (I/R) (immunohistochemical staining, × 200).
Caspase-3-positive cells were not observed in the sham-operation group; the number of caspase-3-positive cells in the minocycline group was significantly less than in the I/R group. Arrows point to caspase-3-positive cells.
Minocycline decreased the number of poly (adenosine diphosphate-ribose) polymerase-1-positive cells in the hippocampal CA1 region in rats following cerebral ischemia-reperfusion
Immunohistochemical staining showed that no poly (adenosine diphosphate-ribose) polymerase-1-positive cells were observed in the sham-operation group. In the ischemia-reperfusion group, the number of poly (adenosine diphosphate-ribose) polymerase-1-positive cells increased after ischemia-reperfusion; however, numbers significantly decreased after minocycline treatment. The number of poly(adenosine diphosphate-ribose) polymerase-1-positive cells was greatest in the ischemia-reperfusion group, followed by the minocycline treatment group and sham-operation group (Figure 5).
Figure 5.
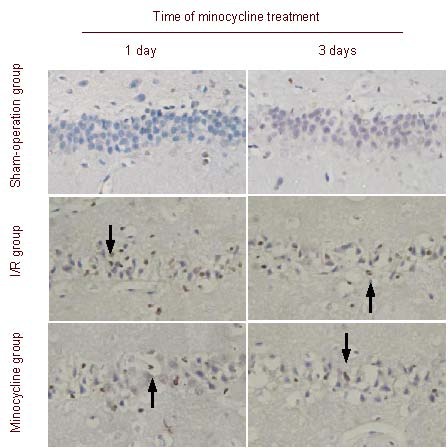
Effect of minocycline on poly(adenosine diphosphate-ribose) polymerase-1 expression in the hippocampal CA1 region of rats after cerebral ischemia-reperfusion (I/R) (immunohistochemical staining, × 200).
Poly(adenosine diphosphate-ribose) polymerase-1-positive cells were not observed in the sham-operation group; changes in the number of poly(adenosine diphosphate-ribose) polymerase-1-positive cells in the I/R group; the number of poly(adenosine diphosphate-ribose) polymerase-1-positive cells in the minocycline group were significantly less than in the I/R group. Arrows point to poly(adenosine diphosphate-ribose) polymerase-1-postive cells.
In the sham-operation group, activated caspase-3 and poly(adenosine diphosphate-ribose) polymerase-1 levels were reduced, as determined by western blot assay; In the ischemia-reperfusion group, activated caspase-3 and poly(adenosine diphosphate-ribose) polymerase-1 levels were significantly increased when compared with the sham-operation group (P < 0.05), but levels decreased following minocycline treatment (P < 0.05; Figure 6).
Figure 6.
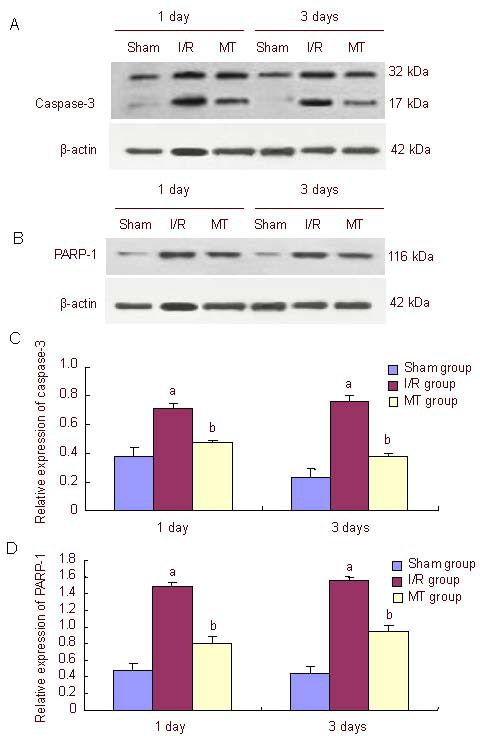
Effect of minocycline (MT) on caspase-3 and PARP-1 protein expression in the hippocampal region of rats after cerebral ischemia-reperfusion (I/R) (western blot assay).
(A, B) Caspase-3 and PARP-1 protein expression was detected by western blot assay. In the sham-operation (Sham) group, activated caspase-3 and PARP-1 levels were low. In the I/R group, pro-caspase-3 was activated (17 kDa fragment detected); PARP-1 levels were higher than in the sham group and MT group. In the MT group, activated caspase-3 levels were lower than in the I/R group.
(C, D) Quantification of caspase-3 and PARP-1 protein expression in the hippocampal region. Data in the graph represent the densitometric ratios of caspase-3/β-actin and PARP-1/β-actin among the sham, I/R and MT groups, respectively. Data are expressed as mean ± SD (n = 3). aP < 0.05, vs. sham group; bP < 0.05, vs. I/R group.
PARP-1: Poly (adenosine diphosphate-ribose) polymerase-1. 1 and 3 days mean the time of MT treatment.
DISCUSSION
In the present study, the rat model of cerebral ischemia-reperfusion injury was established using the suture method. Results showed that rats subjected to ischemia-reperfusion injury displayed right ptosis, miosis, enophthalmos, attenuation of left forelimb pain and a rear-end sign after ischemia-reperfusion, which indicated successful establishment of the model. The concentration of minocycline in the brain can reach 50% of the peak concentration in the blood. When the concentration of minocycline is 100 mg/kg, approximately 50% can reach the brain following ischemic damage. A concentration of 20 mg/kg can effectively reduce brain damage, and minocycline treatment of other brain diseases usually adopts an effective concentration of 22.5 mg/kg. The half-life of minocycline is 16–18 hours.
Therefore, the present experiment used an initial intraperitoneal injection dose of 45 mg/kg, and subsequent administration of 22.5 mg/kg every 12 hours to achieve a stable effective concentration of 22.5 mg/kg in the blood[15], in a broader attempt to explore the protective effects of minocycline on neuronal injuries induced by focal ischemia-reperfusion.
The water maze test can detect the learning and memory abilities of rats[16,17]. Baseline detection in this experiment allows the rats to become familiar with the environment and observe the differences in learning and memory abilities in each group. We suggest that these baseline determinations did not overly influence the subsequent test sessions because the natural amnesia time of the rat has been described to be 4 days[18], and we conducted this experiment at 6 and 9 days. Therefore, the memory obtained by test trainings prior to the experiment had no impact on the final results. In the sham-operation group, learning and memory in rats showed no significant difference before and after ischemia-reperfusion. In the ischemia- reperfusion group, learning and memory impairment was apparent and minocycline treatment could significantly improve the impairment. This result indicated that minocycline can improve learning and memory abilities of rats after ischemia-reperfusion.
Measuring the cerebral infarction volume in rat brain tissues is an important objective indicator to evaluate the extent of damage. Infarct volume measurements are commonly used to provide a foundation in the treatment of cerebral ischemia[19]. Tetrazolium chloride staining displays the location and volume of the cerebral infarct after ischemia-reperfusion. Using this method, we found that the infarct volume was significantly reduced after minocycline treatment.
Hematoxylin-eosin staining is the most widely used technique in histology, embryology, pathology teaching and research experiments. In the sham-operation group, the normal neurons were arranged in neat rows, the cell bodies were round, the outline of the nucleus was clear; in the ischemia-reperfusion group, hematoxylin-eosin staining detected neuronal necrosis in the hippocampal CA1 region and a significantly reduced number of neurons, and the cell bodies were swollen. In addition, the number of pyramidal cells was decreased and lacked unity and coherence; in the minocycline treatment group, structural integrity of neurons increased significantly and the number of neurons slightly recovered. These results are evidence that minocycline can effectively protect neurons in the hippocampal CA1 region.
TUNEL is commonly used for the detection of apoptotic cells. The number of apoptotic cells in the minocycline treatment group was significantly lower than in the ischemia-reperfusion group. Therefore, we can speculate that minocycline can reduce neuronal apoptosis after ischemia-reperfusion and has a neuroprotective effect.
By immunohistochemistry and western blot analysis, we found that caspase-3 and poly(adenosine diphosphate-ribose) polymerase-1 protein expression positively correlated with neural apoptosis following cerebral ischemia-reperfusion, which was consistent with previous studies[20,21]. In the minocycline treatment group, caspase-3 and poly(adenosine diphosphate-ribose) polymerase-1 expression was significantly less than the ischemia-reperfusion group at 1 and 3 days, which suggested that minocycline significantly reduced caspase-3 and poly(adenosine diphosphate-ribose) polymerase-1 expression following cerebral ischemia-reperfusion, thereby decreasing neural apoptosis. Immunohistochemistry and western blot analysis were used to qualitatively and semi-quantitatively determine caspase-3 and poly(adenosine diphosphate-ribose) polymerase-1 expression, respectively, following cerebral ischemia-reperfusion. However, quantitative protein analysis should be performed in future studies.
In summary, minocycline reduced caspase-3 and poly(adenosine diphosphate-ribose) polymerase-1 expression following cerebral ischemia-reperfusion. However, the mechanism of action remains unclear.
MATERIALS AND METHODS
Design
A randomized, controlled, animal experiment.
Time and setting
The present study was performed at the laboratory of Physiology Department, Zhuhai Campus of Zunyi Medical College, Zhuhai, Guangdong Province, China between September 2010 and April 2012.
Materials
Animals
Male Sprague-Dawley rats (n = 108), of specific pathogen free grade, aged 10–12 weeks and weighing 250 ± 10 g were provided by the Medical Animal Experimental Center of Guangdong Province, China (certificate No. SCXK2008-0002). All rats were maintained at 21 ± 2°C in 30–70% humidity under a 12-hour light-dark cycle. All experimental procedures were in accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals, published by the Ministry of Science and Technology of China[22].
Drugs
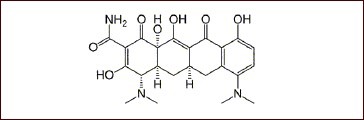
Minocycline was obtained from Sigma, St. Louis, MO, USA. Synonyms: [4S-(4α, 4a α5,5a α,12a α)]-4,7-bis (dimethylamino)-14,4a,5,5a,6,11,12a-octahydro-3,10,12,12a-tetrahydroxy-1, 11-dioxo-2-naphthacenecarboxamie; 7-dimethylamino-6-demethyl-6-deoxytetracycline.
Molecular formula: C23H27N3O7·HCl; molecular weight: 493.9; structure formula:
Minocycline was preserved in 1 M PBS (pH 7.4) containing 15 mM sodium azide.
Methods
Preparation of cerebral ischemia-reperfusion model
According to methods described in a previous study[23], rats were anesthetized with an intraperitoneal injection of 10% (w/v) chloral hydrate (350 mg/kg; Boster, Wuhan, China). A 2-cm incision was made in the middle of the neck line, separating the right carotid artery, as well as the internal and external carotid communicating arteries, which were tied to the line. Through a small incision to the external carotid artery, a previously selected plug thread was inserted from the right external carotid artery into the right internal carotid artery to a depth of 18.0 ± 0.5 mm (beginning with the common carotid artery bifurcation), and the thread was maintained for 2 hours and subsequently removed to restore blood flow to the common carotid and internal carotid arteries (Figure 7).
Figure 7.
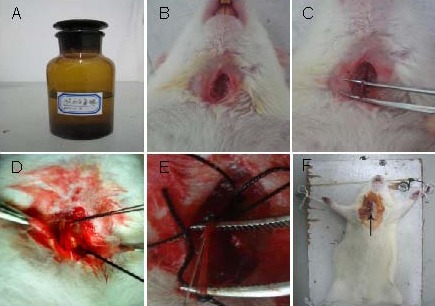
Establishing cerebral ischemia-reperfusion model in the rat.
(A) Intraperitoneal injection of 10% (w/v) chloral hydrate.
(B) A 2-cm incision was made in the middle of the neck line.
(C) Separating the right carotid artery.
(D) Ligation of the internal carotid artery and common carotid artery.
(E) A selected plug thread was inserted through the incision.
(F) Suturing the incision (arrow).
Minocycline administration
Minocycline (45 mg/kg) was immediately injected intraperitoneally after the cerebral ischemia-reperfusion model was established, and then intraperitoneally injected (22.5 mg/kg) every 12 hours afterwards[15] until death. Normal saline (22.5 mg/kg) was injected intraperitoneally in the sham-operation and ischemia-reperfusion groups.
Detection of learning and memory abilities by water maze test
The water maze test was conducted after 1 and 2 days of treatment and the results were recorded. A DB001-type water maze device (Zhishu Duobao Beijing Biotech Co., Ltd., China) is shown in Figure 8. The water maze consisted of a circular pool with a 150 cm diameter and a 60 cm height, divided into four quadrants (N, S, W, E). An escape platform (5 cm) was placed between the N and E quadrants, 0.5 cm lower than the horizontal plane. Water in the pool was dyed dark with carbon ink, and the water temperature was maintained at 24 ± 2°C. According to previously described methods[18], the rats were put in the pool from a fixed position (with their backs opposite the escape platform), and the time taken for the rats to find the platform was recorded as their escape latency. Each rat was trained five times with a 15-second rest period between each trial. If a rat did not find the platform within 120 seconds, the time was recorded as 120 seconds (baseline value), and the rat was allowed to take a rest on the platform for 15 seconds before continuing to the next trial[24]. If a rat did not find the platform during any of the five trials, the rat was excluded from the study and replaced. In the water maze test, longer escape latencies represent reduced learning and memory performance[25].
Figure 8.

Picture (A) and schematic diagram (B) of the water maze device.
The W, E, N, S represent west, east, north, and south quadrants, respectively, in Figure B.
Cerebral infraction volume as determined by tetrazolium chloride staining
After the water maze test, the rats were decapitated and the brains were removed and sectioned into 2-mm-thick consecutive coronal slices, with a total of eight slices per rat. The sections were incubated in 2% tetrazolium chloride phosphate buffer solution at 37°C for 10 minutes, followed by 4% (w/v) paraformaldehyde fixation. Normal brain tissue stained red and infracted tissue was pale[26]. Using digital imaging, the percent cerebral infarct volume was calculated using Auto-CAD analysis software (Autodesk Co., Ltd., San Francisco, CA, USA).
Hippocampal morphology as observed by hematoxylin-eosin staining
After treatment for 1 and 3 days, paraffinized hippocampal sections were dewaxed with xylene and hydrated with gradient alcohol, washed in distilled water[27], then stained in Harris hematoxylin solution for 8 minutes, washed with running tap water for 5 minutes, counterstained in eosin-phloxine solution for 30 seconds to 1 minute, dehydrated, cleared with xylene, and mounted with neutral gum. The pathological changes in the hippocampal CA1 region were observed under light microscope (Olympus, Tokyo, Japan).
Cell apoptosis in the hippocampal CA1 region as assayed by TUNEL staining
Paraffinized sections were dewaxed with xylene and hydrated in gradient alcohol, rinsed in 0.01 M PBS (pH 7.4) for 5 minutes, treated with 3% (v/v) H2O2 for 10 minutes at room temperature, rinsed in 0.01 M PBS (pH 7.4), microwaved in 0.01 M citrate buffer solution (pH 6.0), blocked in goat serum at room temperature for 30 minutes, rinsed twice in 0.01 M PBS for 5 minutes, incubated in TUNEL reaction (Boster, Wuhan, Hubei Province, China) solution in the dark at 37°C for 1 hour, and rinsed three times in 0.01 M PBS (pH 7.4) for 5 minutes. A multi-fusion microscope (Olympus, Tokyo, Japan) was used to detect and document positive immunohistochemistry expression. Image-pro plus 6.0 medical imaging system (Media Cybernetics, Silver Spring, MD, USA) was used to quantify immunohistochemistry expression. Cells were selected in three visual fields (200 × magnification) to count the immunopositive cells, the apoptotic index was calculated with the equation as: TUNEL-positive cells/total number of cells × 100%.
Caspase-3 and poly(adenosine diphosphate-ribose) polymerase-1 expression in the hippocampal CA1 region as detected by immunohistochemistry
Paraffinized sections were dewaxed in xylene, hydrated in gradient alcohol, rinsed in distilled water, treated with 3% (v/v) hydrogen peroxide and blocked with goat serum at room temperature. Slices were then rinsed in PBS-Tween 20, and incubated with primary rabbit anti-caspase-3 polyclonal antibody (1:120; Sigma) or rabbit anti-rat poly(adenosine diphosphate-ribose) polymerase-1 monoclonal antibody (1:100; Sigma) in primary antibody dilution buffer overnight at 4°C; rinsed with PBS-Tween 20, and incubated with goat anti-rabbit antibody (1:200; Boster) for 30 minutes at room temperature and streptavidin-horseradish peroxidase for 30 minutes at room temperature, and colored with diazoaminobenzene. Immunohistochemistry products were observed under the light microscope.
Caspase-3 and poly(adenosine diphosphate-ribose) polymerase-1 expression in the hippocampus as detected by western blot analysis
Hippocampal tissues from each group were homogenated at 4°C and centrifuged at 12 000 r/min for 15 minutes. The supernatants were harvested, and protein concentrations were measured using BCA methods[28] and electrophoresed on sodium dodecylsulfate polyacrylamide gels. The proteins were transferred to a polyvinylidene fluoride membrane and blocked with 5% (w/v) defatted milk powder overnight at 4°C. The membranes were incubated with rabbit anti-caspase-3 polyclonal antibody (1:2 000; Sigma) or rabbit anti-rat poly(adenosine diphosphate-ribose) polymerase-1 monoclonal antibody (1: 2 000; Sigma) and rabbit anti-rat β-actin monoclonal antibody (1:200; Sigma). Membrane were washed with Tris-buffered saline Tween, then incubated with horseradish peroxidase-labeled goat anti-rabbit polyclonal (1:5 000; Boster) antibody at room temperature for 1 hour, followed by enhanced chemiluminescence reagent and exposure. The target protein bands were measured using Quantity one system (Bio-Rad, Chicago, IL, USA). Results were expressed as the densitometric ratios of caspase-3/β-actin and poly(adenosine diphosphate-ribose) polymerase-1/β-actin.
Statistical analysis
All data were expressed as mean ± SD. Statistical analysis was performed using one-way analysis of variance with SPSS 16.0 software (SPSS, Chicago, IL, USA). A P < 0.05 value was considered statistically significant.
Footnotes
Funding: This study was supported by the National Natural Science Foundation of China, No. 81160157; and the Key Program of the Science and Technology Department of Guizhou Province, No. SY20093075; Nomarch Foundation for Excellent Talents in Science, Technology and Education Field of Guizhou Province, No. 201209.
Conflicts of interest: None declared.
Ethical approval: The study was approved by the Animal Ethics Committee of Zunyi Medical College in China.
(Reviewed by Diwakarla S, Stow A, Su SW, Liu JG)
(Edited by Yu J, Yang Y, Li CH, Song LP)
REFERENCES
- [1].Fiskum G, Murphy AN, Beal MF. Mitochondria in neurodegeneration: acute ischemia and chronic neurodegenerative diseases. J Cereb Blood Flow Metab. 1999;19(4):351–369. doi: 10.1097/00004647-199904000-00001. [DOI] [PubMed] [Google Scholar]
- [2].Zipfel GJ, Lee JM, Choi DW. Reducing calcium overload in the ischemic brain. N Engl J Med. 1999;341(20):1543–1544. doi: 10.1056/NEJM199911113412011. [DOI] [PubMed] [Google Scholar]
- [3].Paschen W. Role of calcium in neuronal cell injury: which subcellular compartment is involved? Brain Res Bull. 2000;53(4):409–413. doi: 10.1016/s0361-9230(00)00369-5. [DOI] [PubMed] [Google Scholar]
- [4].Yuan J, Yankner BA. Apoptosis in the nervous system. Nature. 2000;407(6805):802–809. doi: 10.1038/35037739. [DOI] [PubMed] [Google Scholar]
- [5].Chan PH. Reactive oxygen radicals in signaling and damage in the ischemic brain. J Cereb Blood Flow Metab. 2001;21(1):2–14. doi: 10.1097/00004647-200101000-00002. [DOI] [PubMed] [Google Scholar]
- [6].Horn J, Limburg M. Calcium antagonists for ischemic stroke: a systematic review. Stroke. 2001;32(2):570–576. doi: 10.1161/01.str.32.2.570. [DOI] [PubMed] [Google Scholar]
- [7].Yu SP, Choi DW. Ions, cell volume, and apoptosis. Proc Natl Acad Sci U S A. 2000;97(17):9360–9362. doi: 10.1073/pnas.97.17.9360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci. 2003;4(5):399–415. doi: 10.1038/nrn1106. [DOI] [PubMed] [Google Scholar]
- [9].Broughton BR, Reutens DC, Sobey CG. Apoptotic mechanisms after cerebral ischemia. Stroke. 2009;40(5):e331–339. doi: 10.1161/STROKEAHA.108.531632. [DOI] [PubMed] [Google Scholar]
- [10].Yong VW, Wells J, Giuliani F, et al. The promise of minocycline in neurology. Lancet Neurol. 2004;3(12):744–751. doi: 10.1016/S1474-4422(04)00937-8. [DOI] [PubMed] [Google Scholar]
- [11].Fraser CL, Biousse V, Newman NJ. Minocycline-induced fulminant intracranial hypertension. Arch Neurol. 2012;69(8):1067–1070. doi: 10.1001/archneurol.2012.144. [DOI] [PubMed] [Google Scholar]
- [12].Wang J, Wei Q, Wang CY, et al. Minocycline up-regulates Bcl-2 and protects against cell death in mitochondria. J Biol Chem. 2004;279(19):19948–19954. doi: 10.1074/jbc.M313629200. [DOI] [PubMed] [Google Scholar]
- [13].Matsuki S, Iuchi Y, Ikeda Y, et al. Suppression of cytochrome c release and apoptosis in testes with heat stress by minocycline. Biochem Biophys Res Commun. 2003;312(3):843–849. doi: 10.1016/j.bbrc.2003.10.191. [DOI] [PubMed] [Google Scholar]
- [14].Teng YD, Choi H, Onario RC, et al. Minocycline inhibits contusion-triggered mitochondrial cytochrome c release and mitigates functional deficits after spinal cord injury. Proc Natl Acad Sci U S A. 2004;101(9):3071–3076. doi: 10.1073/pnas.0306239101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Granville DJ, Jiang H, An MT, et al. Bcl-2 overexpression blocks caspase activation and downstream apoptotic events instigated by photodynamic therapy. Br J Cancer. 1999;79(1):95–100. doi: 10.1038/sj.bjc.6690017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006;1(2):848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].D’Hooge R, De Deyn PP. Applications of the Morris water maze in the study of learning and memory. Brain Res Brain Res Rev. 2001;36(1):60–90. doi: 10.1016/s0165-0173(01)00067-4. [DOI] [PubMed] [Google Scholar]
- [18].Wang D, Dai TJ, Ma T, et al. Relationship between amnestic effect of ketamine propofol or sodium oxybate and NMDA receptor. Zhongguo Yaoli Xue Tongbao. 2010;26(7):890–893. [Google Scholar]
- [19].Emery E, Aldana P, Bunge MB, et al. Apoptosis after traumatic human spinal cord injury. J Neurosurg. 1998;89(6):911–920. doi: 10.3171/jns.1998.89.6.0911. [DOI] [PubMed] [Google Scholar]
- [20].Springer JE, Azbill RD, Knapp PE. Activation of the caspase-3 apoptotic cascade in traumatic spinal cord injury. Nat Med. 1999;5(8):943–946. doi: 10.1038/11387. [DOI] [PubMed] [Google Scholar]
- [21].Rao R, Sperr D, Ennis K, et al. Postnatal age influences hypoglycemia-induced poly(ADP-ribose) polymerase-1 activation in the brain regions of rats. Pediatr Res. 2009;66(6):642–647. doi: 10.1203/PDR.0b013e3181bbce69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animais 2006-9-30 [Google Scholar]
- [23].Forsyth R, Thuy Vu, Salorio C, et al. Review: efficient rehabilitation trial designs using disease progress modeling: a pediatric traumatic brain injury example. Neurorehabil Neural Repair. 2010;24(3):225–234. doi: 10.1177/1545968309354534. [DOI] [PubMed] [Google Scholar]
- [24].Xu M, Lu ZJ, Zhang FJ. Effect of sevoflurane on spatial learning and memory in mice. Shanghai Jiaotong Daxue Xuebao: Yixue Ban. 2011;31(2):182–186. [Google Scholar]
- [25].Morris RGM. Spatial localization does not require the presence of local cues. Learn Motiv. 1981;12(2):239–260. [Google Scholar]
- [26].Lou M, Chen Y, Ding M, et al. Involvement of the mitochondrial ATP-sensitive potassium channel in the neuroprotective effect of hyperbaric oxygenation after cerebral ischemia. Brain Res Bull. 2006;69(2):109–116. doi: 10.1016/j.brainresbull.2005.11.009. [DOI] [PubMed] [Google Scholar]
- [27].Matsukawa N, Yasuhara T, Hara K, et al. Therapeutic targets and limits of minocycline neuroprotection in experimental ischemic stroke. BMC Neurosci. 2009;10:126. doi: 10.1186/1471-2202-10-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hill HD, Straka JG. Protein determination using bicinchoninic acid in the presence of sulfhydryl reagents. Anal Biochem. 1988;170(1):203–208. doi: 10.1016/0003-2697(88)90109-1. [DOI] [PubMed] [Google Scholar]


