Abstract
The hippocampus is a brain region responsible for learning and memory functions. The purpose of this study was to investigate the effects of low-intensity exercise and bright light exposure on neurogenesis and brain-derived neurotrophic factor expression in adult rat hippocampus. Male Sprague-Dawley rats were randomly assigned to control, exercise, light, or exercise + light groups (n = 9 per group). The rats in the exercise group were subjected to treadmill exercise (5 days per week, 30 minutes per day, over a 4-week period), the light group rats were irradiated (5 days per week, 30 minutes per day, 10 000 lx, over a 4-week period), the exercise + light group rats were subjected to treadmill exercise in combination with bright light exposure, and the control group rats remained sedentary over a 4-week period. Compared with the control group, there was a significant increase in neurogenesis in the hippocampal dentate gyrus of rats in the exercise, light, and exercise + light groups. Moreover, the expression level of brain-derived neurotrophic factor in the rat hippocampal dentate gyrus was significantly higher in the exercise group and light group than that in the control group. Interestingly, there was no significant difference in brain-derived neurotrophic factor expression between the control group and exercise + light group. These results indicate that low-intensity treadmill exercise (first 5 minutes at a speed of 2 m/min, second 5 minutes at a speed of 5 m/min, and the last 20 minutes at a speed of 8 m/min) or bright-light exposure therapy induces positive biochemical changes in the brain. In view of these findings, we propose that moderate exercise or exposure to sunlight during childhood can be beneficial for neural development.
Keywords: neural regeneration, neurogenesis, neurorehabilitation, exercise, bright light, brain-derived neurotrophic factor, hippocampus, childhood, grants-supported paper, neuroregeneration
Research Highlights
-
(1)
This study was designed to reveal whether neurogenesis occurs in the hippocampus throughout the lifespan.
-
(2)
The results indicate that low-intensity exercise or exposure to bright light increases neurogenesis in the hippocampal dentate gyrus of adult rats and the combined treatment does not have an additive effect.
-
(3)
Low-intensity exercise or exposure to bright light increases hippocampal brain-derived neurotrophic factor expression and the combined treatment does not have an additive effect.
-
(4)
The results suggest that children and elderly individuals should be continuously encouraged to perform appropriate outdoor exercise during the daytime to promote neural development and improve learning and memory abilities.
INTRODUCTION
The mammalian brain produces neuronal precursor cells throughout development[1,2,3]. Neurogenesis occurs in the hippocampus during both periods of neuron generation and after active growth, and the hippocampus is critically involved in learning and the formation of memories[4,5].
Neurogenesis occurs in the subgranular zone of hippocampal dentate gyrus where the hippocampus induces cellular responses to external stimulation, and repeated responses plays an important role in learning and narrative and spatial memory[6,7,8,9]. Newly generated neurons provide the foundation for structural plasticity in the hippocampus by forming new synapses in the existing hippocampal neuronal circuit through generation of axons and neurites[10]. Thus, the generation and reduction of neurons is closely related to hippocampal functions, and it is causally associated with exercise physiology. Indeed, effects of exercise on neurogenesis have been studied extensively[11,12,13,14,15,16].
Exercise is known to efficiently enhance neurogenesis through induction of neural stem cell proliferation in the hippocampus[17,18]. In a previous study, van Praag et al[18] showed that voluntary wheel running in experimental mice promotes cellular proliferation and survival. Consistent with this finding, Trejo et al[17] reported that forced treadmill running stimulates cell proliferation in the hippocampal dentate gyrus of rats. Another study revealed that appropriate aerobic exercise increases human recognition and memory[19], whereas Blumenthal et al[20] reported that exercise induces physiological stability. These studies collectively indicate that exercise improves hippocampal functions via induction of neurogenesis, and neurogenesis in the hippocampal dentate gyrus is strongly related to learning and memory.
At present, children and adolescents often lack sufficient physical activity, and exposure to sunlight is limited by reduced outdoor activities. In addition, some students tend to prefer indoor activities to outdoor activities because they fear skin aging or dark skin colorations induced by exposure to sunlight. Sunlight deficiency triggers numerous diseases, including depression, insomnia, and vitamin D deficiency syndrome[21,22,23,24,25]. Light stimulates the retina, and is delivered to and recognized by the pineal gland through the retino-hypothalamic tract, which in turn controls the secretion of melatonin and serotonin. In this process, light enters the brain through the eyes and corrects abnormal symptoms by controlling the biochemical actions and physiology of the brain[26], subsequently influencing emotional and behavioral functions through effects on neurotransmitters that participate in biochemical activity. Since light exposure can influence brain functions, many studies have been conducted on bright light therapy as an intervention for excited behavior and somnipathy in aged people suffering from dementia[27,28,29].
Brain-derived neurotrophic factor, the most abundant neurotrophic factor present in diverse areas of the brain, is critical for the growth and development of neurons as well as for neuroplasticity[30]. It improves neuronal survival by increasing resistance to nerve damage. Notably, brain-derived neurotrophic factor manifested in the hippocampus is known to control the generation and survival of neurons[31]. It would therefore be of interest to establish whether brain-derived neurotrophic factor is regulated by exercise or light exposure and plays a role in hippocampal neurogenesis.
The theory that exercise or light exposure improves brain function is well accepted. However, the majority of research to date has analyzed the effects of exercise and light therapy separately, and few studies have attempted to determine the effects of a combination of exercise and light exposure. However, some previous studies have shown positive effects of a combination of exercise and light exposure[32,33,34,35]. Therefore, we believe that exercise under sunlight may improve brain functions in childhood. Accordingly, in this study, we examined the effects of low-intensity treadmill exercise and bright light exposure over a 4-week period on neurogenesis and brain-derived neurotrophic factor expression in the brains of male Sprague-Dawley rats.
RESULTS
Quantitative analysis of experimental animals
Thirty-six rats were randomly divided into control, exercise, light, and exercise + light groups (n = 9 per group). The rats in the exercise group were subjected to treadmill exercise (5 days per week, 30 minutes per day, over a 4-week period), the light group rats were subjected to bright light exposure (5 days per week, 30 minutes per day, 10 000 lx, over a 4-week period), the exercise + light group rats were subjected to treadmill exercise in combination with bright light exposure, and the control group rats remained sedentary over a 4-week period. Four rats per group were sacrificed for immunohistochemistry and five rats per group were sacrificed for immunoblotting.
Neurogenesis in the hippocampal dentate gyrus
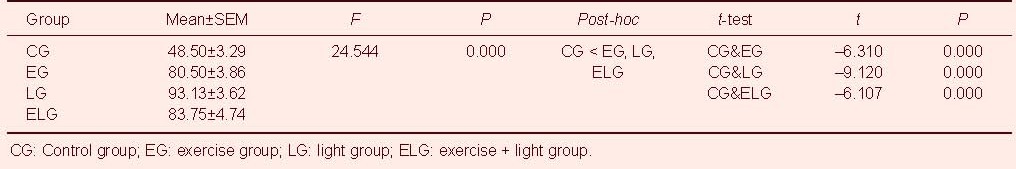
Immunohistochemistry was performed to detect 5-bromo-2’-deoxyuridine (BrdU)-positive cells in the hippocampal dentate gyrus. Typical BrdU-positive cells in each group are shown in Figure 1A. One-way analysis of variance and t-tests showed that neurogenesis in the hippocampal dentate gyrus was significantly higher in the exercise, light, and exercise + light groups compared with the control group (P < 0.001; Table 1). However, one-way analysis of variance showed no significant differences among the exercise, light, and exercise + light groups (Figure 1B).
Figure 1.
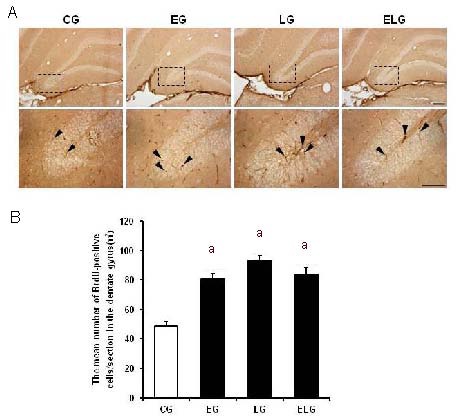
Effects of low-intensity treadmill exercise or bright light exposure on neurogenesis in the hippocampal dentate gyrus (immunohistochemistry).
(A) Photomicrographs of 5-bromo-2’-deoxyuridine (BrdU)-positive cells in the hippocampal dentate gyrus (3,3’-diaminobenzidine stained with anti-BrdU). Arrowheads show BrdU-positive cells in the hippocampal dentate gyrus. Scale bars: 200 μm for upper panel photomicrographs, and 100 μm for the lower panel photomicrographs. Upper panel photomicrographs were taken at 10 ×, and lower panel photomicrographs at 40 ×.
(B) Analysis of BrdU-positive cells in the hippocampal dentate gyrus(/mm2). Four weeks of low-intensity treadmill exercise and bright light exposure induced hippocampal neurogenesis. The data are presented as mean ± SEM with four rats in each group. aP < 0.001, vs. control group (CG)(one-way analysis of variance followed by t-tests). However, one-way analysis of variance showed no significant differences among the exercise group (EG), light group (LG), and exercise + light group (ELG). Multiple comparisons were performed with Scheffe's method.
Table 1.
Immunohistochemical results of neurogenesis in the hippocampal dentate gyrus using analysis of variance and t-tests
Brain-derived neurotrophic factor expression in the hippocampus
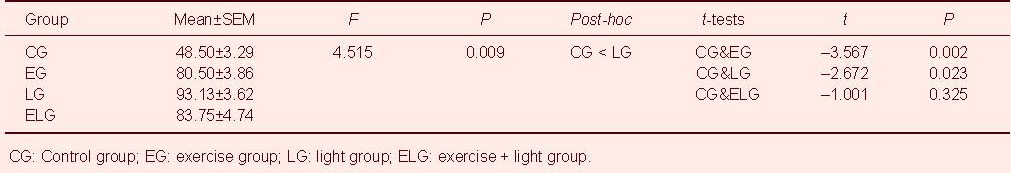
Brain-derived neurotrophic factor protein expression in the hippocampus of rats from each group was assessed using western blot analysis (Figure 2A). Table 2 shows the results of one-way analysis of variance and t-tests. One-way analysis of variance showed significant differences only between the control and light groups (P < 0.01). However, t-tests showed that brain-derived neurotrophic factor expression in the hippocampus was significantly higher in the exercise group and light group compared with the control group (P < 0.01, P < 0.05). Interestingly, there were no significant differences in brain-derived neurotrophic factor expression between the control and exercise + light groups (Figure 2B).
Figure 2.
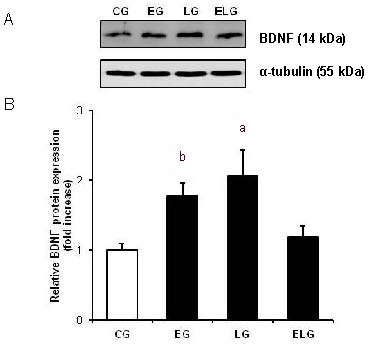
Effects of low-intensity treadmill exercise and/or bright light on brain-derived neurotrophic factor (BDNF) protein levels in the hippocampus.
(A) BDNF expression was assessed using western blot analysis. Western blot analysis was performed using sodium dodecyl sulfate-polyacrylamide gel electrophoresis.
(B) All data are presented as mean ± SEM with five rats in each group. aP < 0.05, bP < 0.01, vs. control group (CG; one-way analysis of variance followed by t-tests). BDNF expression was slightly, but not significantly, increased in the exercise + light group (ELG) compared with the control group (CG). EG: Exercise group.
Table 2.
Western blot results of brain-derived neurotrophic factor expression in the hippocampus using one-way analysis of variance and t-tests
DISCUSSION
The effects of exercise on the generation and pruning of neurons in the brain are well accepted[4,36,37,38,39,40,41]. In addition, exercise has been shown to upregulate brain-derived neurotrophic factor protein and mRNA expression in hippocampal tissue[13,42,43,44,45,46]. It has been also reported that neurogenesis in the hippocampal dentate gyrus is affected by diverse factors, including exercise, learning, and enriched environments[18,47,48,49]. In mice administered BrdU, van Praag et al[18] reported a survival rate of newly generated neurons in animals subjected to 4 weeks of running exercise twice that of animals in a non-exercise group. Also, it has been reported that the survival rate of proliferated cells in animals allowed to exercise is higher than that in a control group[50]. In the current study, compared with the control group, the number of BrdU-positive cells and neurogenesis were higher in the exercise, light, and exercise + light groups compared with the control group. The number of BrdU-positive cells was 1.5 times higher in the exercise, light, and exercise + light groups, which is consistent with a previous study[51]. In combination with findings that neurogenesis can be significantly increased by exercise in both healthy adults and the aged[51], our results clearly demonstrate that exercise is an important factor in neurogenesis. However, although exercise and/or light exposure clearly induce neurogenesis in the hippocampal dentate gyrus, combined treatment does not appear to have an additive effect. Although exercise is a potent stimulator of hippocampal neurogenesis[16,52], that is only true with voluntary exercise. Also, bright light may be less effective in nocturnal than diurnal animals. Therefore, additive effects of combined treatment may depend on the species or precise procedures used.
Griesbach and colleagues[53] reported that voluntary wheel exercise after brain damage alleviates impaired recognition abilities through upregulation of hippocampal brain-derived neurotrophic factor protein expression. Many other studies have shown that exercise improves brain functions such as learning, memory, and recognition abilities and increases neurogenesis and expression of neuron growth factor[54,55,56,57]. We found that brain-derived neurotrophic factor expression was significantly increased by exercise and light, as observed on a western blot analysis. However, there was no significant effect of a combination of exercise + light. Bright light exposure therapy has been shown to effectively reduce depression and anxiety in humans. In fact, infrared light may be as effective as anti-depressant or anti-anxiety drugs[58]. It has been suggested that infrared light is involved in hippocampal neurogenesis. However, we did not find any additive effects of combined treatment with exercise and bright light on neurogenesis and brain-derived neurotrophic factor expression. Follow-up studies are thus necessary to determine whether the effects of high-intensity light exposure offset those of exercise in rats, which are nocturnal animals. Rats in the light group were allowed to rest during the light exposure whereas those in the exercise + light group were forced to exercise. Therefore, the use of forced exercise may have attenuated the effects of the light exposure, and diurnal exercise under natural light may have been more effective.
In summary, low-intensity exercise and bright light separately affect neurogenesis in the hippocampus, but their combination has no additive effects on neuron generation. Moreover, both exercise and bright light induce positive biochemical changes in the brain. Accordingly, we suggest that children should be continuously encouraged to perform appropriate exercise and exposed to natural light by avoiding enclosed and dimly lit spaces, with the expectation that this will improve their learning and memory abilities.
MATERIALS AND METHODS
Design
A randomized, controlled animal study.
Time and setting
This experiment was performed at the Open Animal Laboratory, Gyeongsang National University, Republic of Korea from June to October in 2011.
Materials
Male Sprague-Dawley rats, weighing 160 ± 10 g, aged 5 weeks, were obtained from a commercial breeder (KOATECH, Gyeonggi, Republic of Korea). Experimental procedures were performed in accordance with the animal care guidelines of National Institutes of Health (NIH). Animals were housed under controlled temperature (22 ± 2°C) conditions with an alternating 12-hour light/dark cycle, and provided food and water ad libitum.
Methods
BrdU injections
BrdU injections were performed as described previously with some modifications[59,60,61]. After 1 week of environment adaptation, BrdU (Sigma, St. Louis, MO, USA) was intraperitoneally administered to all rats (50 mg/kg) once a day over 5 consecutive days each week for 4 weeks. The BrdU was administered 60 minutes before the treadmill exercise, light exposure, or their combination.
Treadmill exercise regimen
Animals from the exercise and exercise + light groups were subjected to treadmill (PARK TECH, Daegu, Republic of Korea) exercise for 30 minutes once a day over 5 consecutive days each week for 4 weeks. The exercise load consisted of a running speed of 2 m/min for the first 5 minutes, 5 m/min for the next 5 minutes, and 8 m/min for the last 20 minutes at a 0° inclination. The exercise was performed in the morning (between 10:00 a.m. and 12:00 a.m.).
Bright light exposure
Light therapy was commonly administered at an intensity of 10 000 lx[62]. An artificial lamp with 10 000 lx of ray illumination was obtained from a commercial company (Danbee, Gyeonggi, Republic of Korea), and placed at a distance of 80 cm from animals in these experiments. In the light cycle, bright light exposure was performed during a 30-minute period matched to the period of exercise. The combined treadmill exercise and light exposure were performed over the same duration.
Tissue preparation
Experimental animals were sacrificed. For immunohistochemical analysis, animals were sacrificed 24 hours after the end of the 4-week treatments. Rats were anesthetized with Zoletil 50 (10 mg/kg, intraperitoneal; Virbac, S.A., France), transcardially perfused with 0.1 M PBS, and fixed with 4% neutralized buffered paraformaldehyde. Brains were removed, postfixed in the same fixative for 48 hours, and transferred to 20% sucrose solution for cryoprotection. Coronal sections of 30 μm thickness were prepared using a freezing microtome (Leica, Nussloch, Germany).
To extract protein, frozen hippocampal samples of rats were transferred to sterile 1.5 mL microcentrifuge tubes containing 550 μL of lysis buffer (protease inhibitor cocktail: T-buffer = 1:100). Homogenized tissues were incubated for 10 minutes on ice and sonicated. Next, samples were centrifuged at 12 000 r/min for 30 minutes at 4°C, and supernatant fractions were collected. Protein concentrations were quantified using the bicinchoninic acid protein assay (Bio-Rad, Rockford, IL, USA), and samples were stored at –80°C until use.
BrdU immunohistochemistry
For detection of neurogenesis in the dentate gyrus, 5-bromo-2’-deoxyuridine-specific immunohistochemistry was performed[50]. In brief, free floating 30 μm-thick tissue sections were pretreated with 0.3% H2O2 for 10 minutes. Sections were blocked in 0.1 M PBS containing 1.5% normal horse serum and 0.1% Triton X-100 for 30 minutes, and incubated with rat monoclonal primary antibody against 5-bromo-2’-deoxyuridine (1:100; Abcam, Cambridge, England) overnight at 4°C, followed by three washes with 0.1 M PBS, and subsequent incubation for 1 hour at room temperature with a biotinylated anti-rat IgG antibody (1:200; Vector Laboratories, Burlingame, CA, USA). After three more washes with 0.1 M PBS, sections were incubated in an avidin-biotin-peroxidase complex solution (ABC kit, Vector Laboratories, CA, USA) for 1 hour, and developed with 0.03% diaminobenzidine tetrahydrochloride (DAB; Sigma) containing 0.003% H2O2 for 3 minutes. Next, sections were mounted on gelatin-coated slides, heat-dried, dehydrated through a graded alcohol series, cleared in xylene, and coverslipped with Permount (Sigma). Sections were subsequently visualized under a photomicroscope (Olympus, Wendenstrasse, Hamburg, Germany), and digital images were captured and documented. BrdU immunoreactive cells were counted by stereologic methods using a photomicroscope. BrdU-positive cells in the granule cell layer and hilus of every 8th and 12th sections (2 sections/animal) were counted through the entire dentate gyrus according to a previously published method[7]. Photomicrographs were assessed via densitometry using the Image-Pro plus program (Media Cybernetics, Silver Spring, MD, USA) to determine staining densities. Total cells within the log range of intensity were counted to allow for quantification and group comparisons.
Western blot analysis of brain-derived neurotrophic factor protein
For brain-derived neurotrophic factor protein analysis, hippocampal lysates (30 μg/lane) were separated using 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, followed by electrophoretic transfer onto a polyvinylidene difluoride membrane (Millipore, Bedford, MA, USA). Polyclonal rabbit brain-derived neurotrophic factor (1:1 000; Santa Cruz Biotechnology, Santa Cruz, CA, USA) and goat anti-rabbit secondary antibody (1:10 000; Thermo Scientific, Rockford, IL, USA) were used for detection of brain-derived neurotrophic factor protein expression on the polyvinylidene difluoride membrane (Millipore). The brain-derived neurotrophic factor protein expression level was assessed with an enhanced chemiluminescence solution (Pierce, Rockford, IL, USA) and the LAS-4000 system (Fujifilm, Japan). The intensities of brain-derived neurotrophic factor bands were normalized to those of α-tubulin (Sigma).
Statistical analysis
The differences between control and experimental groups were determined using the unpaired Student's t-tests. One-way analysis of variance was used to analyze all group differences. Values are expressed as mean ± SEM, with P < 0.05 accepted as significant.
Footnotes
Funding: This study was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology, No. 2012-0000301.
Conflicts of interest: None declared.
Ethical approval: This study was approved by the Animal Care and Use Committee of Gyeongsang National University in Republic of Korea.
(Reviewed by Gu Y, Ercan F, Xi YW, Murnane K)
(Edited by Li CH, Song LP)
REFERENCES
- [1].Altman J, Bayer SA. Migration and distribution of two populations of hippocampal granule cell precursors during the perinatal and postnatal periods. J Comp Neurol. 1990;301(3):365–381. doi: 10.1002/cne.903010304. [DOI] [PubMed] [Google Scholar]
- [2].Hirabayashi Y, Gotoh Y. Stage-dependent fate determination of neural precursor cells in mouse forebrain. Neurosci Res. 2005;51(4):331–336. doi: 10.1016/j.neures.2005.01.004. [DOI] [PubMed] [Google Scholar]
- [3].Temple S. The development of neural stem cells. Nature. 2001;414(6859):112–117. doi: 10.1038/35102174. [DOI] [PubMed] [Google Scholar]
- [4].Eriksson PS, Perfilieva E, Bjork-Eriksson T, et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4(11):1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- [5].Lie DC, Song H, Colamarino SA, et al. Neurogenesis in the adult brain: new strategies for central nervous system diseases. Annu Rev Pharmacol Toxicol. 2004;44:399–421. doi: 10.1146/annurev.pharmtox.44.101802.121631. [DOI] [PubMed] [Google Scholar]
- [6].Biegler R, McGregor A, Krebs JR, et al. A larger hippocampus is associated with longer-lasting spatial memory. Proc Natl Acad Sci U S A. 2001;98(12):6941–6944. doi: 10.1073/pnas.121034798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Cameron HA, McKay RD. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J Comp Neurol. 2001;435(4):406–417. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- [8].Gage FH. Mammalian neural stem cells. Science. 2000;287(5457):1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- [9].Greenough WT, Cohen NJ, Juraska JM. New neurons in old brains: learning to survive? Nat Neurosci. 1999;2(3):203–205. doi: 10.1038/6300. [DOI] [PubMed] [Google Scholar]
- [10].Ramirez-Amaya V, Marrone DF, et al. Integration of new neurons into functional neural networks. J Neurosci. 2006;26(47):12237–12241. doi: 10.1523/JNEUROSCI.2195-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Asakura R, Matsuwaki T, Shim JH, et al. Involvement of progranulin in the enhancement of hippocampal neurogenesis by voluntary exercise. Neuroreport. 2011;22(17):881–886. doi: 10.1097/WNR.0b013e32834bf4ca. [DOI] [PubMed] [Google Scholar]
- [12].Chae CH, Lee HC, Jung SL, et al. Swimming exercise increases the level of nerve growth factor and stimulates neurogenesis in adult rat hippocampus. Neuroscience. 2012;212:30–37. doi: 10.1016/j.neuroscience.2012.03.030. [DOI] [PubMed] [Google Scholar]
- [13].Ferreira AF, Real CC, Rodrigues AC, et al. Short-term, moderate exercise is capable of inducing structural, BDNF-independent hippocampal plasticity. Brain Res. 2011;1425:111–122. doi: 10.1016/j.brainres.2011.10.004. [DOI] [PubMed] [Google Scholar]
- [14].Koltai E, Zhao Z, Lacza Z, et al. Combined exercise and insulin-like growth factor-1 supplementation induces neurogenesis in old rats, but do not attenuate age-associated DNA damage. Rejuvenation Res. 2011;14(6):585–596. doi: 10.1089/rej.2011.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Marlatt MW, Potter MC, Lucassen PJ, et al. Running throughout middle-age improves memory function, hippocampal neurogenesis, and BDNF levels in female C57BL/6J mice. Dev Neurobiol. 2012;72(6):943–952. doi: 10.1002/dneu.22009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Onksen JL, Briand LA, Galante RJ, et al. Running- induced anxiety is dependent on increases in hippocampal neurogenesis. Genes Brain Behav. 2012;11(5):529–538. doi: 10.1111/j.1601-183X.2012.00788.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Trejo JL, Carro E, Torres-Aleman I. Circulating insulin-like growth factor I mediates exercise-induced increases in the number of new neurons in the adult hippocampus. J Neurosci. 2001;21(5):1628–1634. doi: 10.1523/JNEUROSCI.21-05-01628.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2(3):266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- [19].Hill RD, Storandt M, Malley M. The impact of long-term exercise training on psychological function in older adults. J Gerontol. 1993;48(1):P12–17. doi: 10.1093/geronj/48.1.p12. [DOI] [PubMed] [Google Scholar]
- [20].Blumenthal JA, Emery CF, Madden DJ, et al. Long-term effects of exercise on psychological functioning in older men and women. J Gerontol. 1991;46(6):P352–361. doi: 10.1093/geronj/46.6.p352. [DOI] [PubMed] [Google Scholar]
- [21].Dean E. Time to see the light. Nurs Stand. 2012;26(31):20–21. doi: 10.7748/ns.26.31.20.s22. [DOI] [PubMed] [Google Scholar]
- [22].Laaksi I. Vitamin D and respiratory infection in adults. Proc Nutr Soc. 2012;71(1):90–97. doi: 10.1017/S0029665111003351. [DOI] [PubMed] [Google Scholar]
- [23].Nowson CA, McGrath JJ, Ebeling PR, et al. Vitamin D and health in adults in Australia and New Zealand: a position statement. Med J Aust. 2012;196(11):686–687. doi: 10.5694/mja11.10301. [DOI] [PubMed] [Google Scholar]
- [24].Tariq MM, Streeten EA, Smith HA, et al. Vitamin D: a potential role in reducing suicide risk? Int J Adolesc Med Health. 2011;23(3):157–165. doi: 10.1515/ijamh.2011.038. [DOI] [PubMed] [Google Scholar]
- [25].Zeitzer JM, Friedman L, Yesavage JA. Effectiveness of evening phototherapy for insomnia is reduced by bright daytime light exposure. Sleep Med. 2011;12(8):805–807. doi: 10.1016/j.sleep.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Rosenthal NE. Issues for DSM-V: seasonal affective disorder and seasonality. Am J Psychiatry. 2009;166(8):852–853. doi: 10.1176/appi.ajp.2009.09020188. [DOI] [PubMed] [Google Scholar]
- [27].Lovell BB, Ancoli-Israel S, Gevirtz R. Effect of bright light treatment on agitated behavior in institutionalized elderly subjects. Psychiatry Res. 1995;57(1):7–12. doi: 10.1016/0165-1781(95)02550-g. [DOI] [PubMed] [Google Scholar]
- [28].Lyketsos CG, Lindell Veiel L, Baker A, et al. A randomized, controlled trial of bright light therapy for agitated behaviors in dementia patients residing in long-term care. Int J Geriatr Psychiatry. 1999;14(7):520–525. [PubMed] [Google Scholar]
- [29].van Someren EJ, Kessler A, Mirmiran M, et al. Indirect bright light improves circadian rest-activity rhythm disturbances in demented patients. Biol Psychiatry. 1997;41(9):955–963. doi: 10.1016/S0006-3223(97)89928-3. [DOI] [PubMed] [Google Scholar]
- [30].Mizuno M, Yamada K, Olariu A, et al. Involvement of brain-derived neurotrophic factor in spatial memory formation and maintenance in a radial arm maze test in rats. J Neurosci. 2000;20(18):7116–7121. doi: 10.1523/JNEUROSCI.20-18-07116.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Rossi C, Angelucci A, Costantin L, et al. Brain-derived neurotrophic factor (BDNF) is required for the enhancement of hippocampal neurogenesis following environmental enrichment. Eur J Neurosci. 2006;24(7):1850–1856. doi: 10.1111/j.1460-9568.2006.05059.x. [DOI] [PubMed] [Google Scholar]
- [32].Demura T, Demura S, Aoki H, et al. Effect of linear polarized near-infrared light irradiation and light exercise on muscle performance. J Physiol Anthropol. 2011;30(3):91–96. doi: 10.2114/jpa2.30.91. [DOI] [PubMed] [Google Scholar]
- [33].Dunai A, Novak M, Chung SA, et al. Moderate exercise and bright light treatment in overweight and obese individuals. Obesity (Silver Spring) 2007;15(7):1749–1757. doi: 10.1038/oby.2007.208. [DOI] [PubMed] [Google Scholar]
- [34].Kantermann T, Forstner S, Halle M, et al. The stimulating effect of bright light on physical performance depends on internal time. PLoS One. 2012;7(7):e40655. doi: 10.1371/journal.pone.0040655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].McCurry SM, Pike KC, Vitiello MV, et al. Increasing walking and bright light exposure to improve sleep in community-dwelling persons with Alzheimer's disease: results of a randomized, controlled trial. J Am Geriatr Soc. 2011;59(8):1393–1402. doi: 10.1111/j.1532-5415.2011.03519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Chae CH, Lee HC, Jung SL, et al. Swimming exercise increases the level of nerve growth factor and stimulates neurogenesis in adult rat hippocampus. Neuroscience. 2012;212:30–37. doi: 10.1016/j.neuroscience.2012.03.030. [DOI] [PubMed] [Google Scholar]
- [37].Kramer AF, Erickson KI. Capitalizing on cortical plasticity: influence of physical activity on cognition and brain function. Trends Cogn Sci. 2007;11(8):342–348. doi: 10.1016/j.tics.2007.06.009. [DOI] [PubMed] [Google Scholar]
- [38].Lafenetre P, Leske O, Ma-Hogemeie Z, et al. Exercise can rescue recognition memory impairment in a model with reduced adult hippocampal neurogenesis. Front Behav Neurosci. 2010;3(34):1–9. doi: 10.3389/neuro.08.034.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Larsen JO, Skalicky M, Viidik A. Does long-term physical exercise counteract age-related Purkinje cell loss? A stereological study of rat cerebellum. J Comp Neurol. 2000;428(2):213–222. [PubMed] [Google Scholar]
- [40].Mattson MP. Neuroprotective signaling and the aging brain: take away my food and let me run. Brain Res. 2000;886(1-2):47–53. doi: 10.1016/s0006-8993(00)02790-6. [DOI] [PubMed] [Google Scholar]
- [41].Sim YJ, Kim H, Kim JY, et al. Long-term treadmill exercise overcomes ischemia-induced apoptotic neuronal cell death in gerbils. Physiol Behav. 2005;84(5):733–738. doi: 10.1016/j.physbeh.2005.02.019. [DOI] [PubMed] [Google Scholar]
- [42].Aguiar AS, Jr, Castro AA, Moreira EL, et al. Short bouts of mild-intensity physical exercise improve spatial learning and memory in aging rats: involvement of hippocampal plasticity via AKT, CREB and BDNF signaling. Mech Ageing Dev. 2011;132(11-12):560–567. doi: 10.1016/j.mad.2011.09.005. [DOI] [PubMed] [Google Scholar]
- [43].Baj G, D’Alessandro V, Musazzi L, et al. Physical exercise and antidepressants enhance BDNF targeting in hippocampal CA3 dendrites: further evidence of a spatial code for BDNF splice variants. Neuropsychopharmacology. 2012;37(7):1600–1611. doi: 10.1038/npp.2012.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Groves-Chapman JL, Murray PS, Stevens KL, et al. Changes in mRNA levels for brain-derived neurotrophic factor after wheel running in rats selectively bred for high- and low-aerobic capacity. Brain Res. 2011;1425:90–97. doi: 10.1016/j.brainres.2011.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Lin TW, Chen SJ, Huang TY, et al. Different types of exercise induce differential effects on neuronal adaptations and memory performance. Neurobiol Learn Mem. 2012;97(1):140–147. doi: 10.1016/j.nlm.2011.10.006. [DOI] [PubMed] [Google Scholar]
- [46].Miladi-Gorji H, Rashidy-Pour A, Fathollahi Y, et al. Voluntary exercise ameliorates cognitive deficits in morphine dependent rats: the role of hippocampal brain-derived neurotrophic factor. Neurobiol Learn Mem. 2011;96(3):479–491. doi: 10.1016/j.nlm.2011.08.001. [DOI] [PubMed] [Google Scholar]
- [47].Kesslak JP, So V, Choi J, et al. Learning upregulates brain-derived neurotrophic factor messenger ribonucleic acid: a mechanism to facilitate encoding and circuit maintenance? Behav Neurosci. 1998;112(4):1012–1019. doi: 10.1037//0735-7044.112.4.1012. [DOI] [PubMed] [Google Scholar]
- [48].Liu J, Solway K, Messing RO, et al. Increased neurogenesis in the dentate gyrus after transient global ischemia in gerbils. J Neurosci. 1998;18(19):7768–7778. doi: 10.1523/JNEUROSCI.18-19-07768.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Ueda S, Sakakibara S, Yoshimoto K. Effect of long-lasting serotonin depletion on environmental enrichment-induced neurogenesis in adult rat hippocampus and spatial learning. Neuroscience. 2005;135(2):395–402. doi: 10.1016/j.neuroscience.2005.06.065. [DOI] [PubMed] [Google Scholar]
- [50].Wu CW, Chang YT, Yu L, et al. Exercise enhances the proliferation of neural stem cells and neurite growth and survival of neuronal progenitor cells in dentate gyrus of middle-aged mice. J Appl Physiol. 2008;105(5):1585–1594. doi: 10.1152/japplphysiol.90775.2008. [DOI] [PubMed] [Google Scholar]
- [51].van Praag H, Shubert T, Zhao C, et al. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25(38):8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Garrett L, Lie DC, Hrabe de Angelis M, et al. Voluntary wheel running in mice increases the rate of neurogenesis without affecting anxiety-related behaviour in single tests. BMC Neurosci. 2012;13(1):61. doi: 10.1186/1471-2202-13-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Griesbach GS, Gomez-Pinilla F, Hovda DA. The upregulation of plasticity-related proteins following TBI is disrupted with acute voluntary exercise. Brain Res. 2004;1016(2):154–162. doi: 10.1016/j.brainres.2004.04.079. [DOI] [PubMed] [Google Scholar]
- [54].Farmer J, Zhao X, van Praag H, et al. Effects of voluntary exercise on synaptic plasticity and gene expression in the dentate gyrus of adult male Sprague-Dawley rats in vivo. Neuroscience. 2004;124(1):71–79. doi: 10.1016/j.neuroscience.2003.09.029. [DOI] [PubMed] [Google Scholar]
- [55].Kim H, Lee SH, Kim SS, et al. The influence of maternal treadmill running during pregnancy on short-term memory and hippocampal cell survival in rat pups. Int J Dev Neurosci. 2007;25(4):243–249. doi: 10.1016/j.ijdevneu.2007.03.003. [DOI] [PubMed] [Google Scholar]
- [56].Lee HH, Kim H, Lee MH, et al. Treadmill exercise decreases intrastriatal hemorrhage-induced neuronal cell death via suppression on caspase-3 expression in rats. Neurosci Lett. 2003;352(1):33–36. doi: 10.1016/j.neulet.2003.08.039. [DOI] [PubMed] [Google Scholar]
- [57].Radak Z, Kaneko T, Tahara S, et al. Regular exercise improves cognitive function and decreases oxidative damage in rat brain. Neurochem Int. 2001;38(1):17–23. doi: 10.1016/s0197-0186(00)00063-2. [DOI] [PubMed] [Google Scholar]
- [58].Tanaka Y, Akiyoshi J, Kawahara Y, et al. Infrared radiation has potential antidepressant and anxiolytic effects in animal model of depression and anxiety. Brain Stimul. 2011;4(2):71–76. doi: 10.1016/j.brs.2010.04.001. [DOI] [PubMed] [Google Scholar]
- [59].Baek SS, Jun TW, Kim KJ, et al. Effects of postnatal treadmill exercise on apoptotic neuronal cell death and cell proliferation of maternal-separated rat pups. Brain Dev. 2012;34(1):45–56. doi: 10.1016/j.braindev.2011.01.011. [DOI] [PubMed] [Google Scholar]
- [60].Chae CH, Jung SL, An SH, et al. Treadmill exercise improves cognitive function and facilitates nerve growth factor signaling by activating mitogen-activated protein kinase/extracellular signal-regulated kinase1/2 in the streptozotocin-induced diabetic rat hippocampus. Neuroscience. 2009;164(4):1665–1673. doi: 10.1016/j.neuroscience.2009.09.075. [DOI] [PubMed] [Google Scholar]
- [61].Sim YJ, Kim SS, Kim JY, et al. Treadmill exercise improves short-term memory by suppressing ischemia-induced apoptosis of neuronal cells in gerbils. Neurosci Lett. 2004;372(3):256–261. doi: 10.1016/j.neulet.2004.09.060. [DOI] [PubMed] [Google Scholar]
- [62].Gagne AM, Gagne P, Hebert M. Impact of light therapy on rod and cone functions in healthy subjects. Psychiatry Res. 2007;151(3):259–263. doi: 10.1016/j.psychres.2006.09.004. [DOI] [PubMed] [Google Scholar]


