Abstract
In this study, a combination of growth factors was used to induce bone marrow mesenchymal stem cells differentiation into neuron-like cells, in a broader attempt to observe the role of thrombospondin 1 in synapse formation. Results showed that there was no significant difference in the differentiation rate of neuron-like cells between bone marrow mesenchymal stem cells with thrombospondin induction and those without. However, the cell shape was more complex and the neurites were dendritic, with unipolar, bipolar or multipolar morphologies, after induction with thrombospondin 1. The induced cells were similar in morphology to normal neurites. Immunohistochemical staining showed that the number of positive cells for postsynaptic density protein 95 and synaptophysin 1 protein was significantly increased after induction with thrombospondin 1. These findings indicate that thrombospondin 1 promotes synapse formation in neuron-like cells that are differentiated from bone marrow mesenchymal stem cells.
Keywords: neural regeneration, stem cells, bone marrow mesenchymal stem cells, neuron-like cells, synapse, thrombospondin 1, neurite, postsynaptic density protein 95, synaptophysin 1, neuron-specific enolase, glial fibrillary acidic protein, grants-supported paper, neuroregeneration
Research Highlights
-
(1)
A combination of growth factors was used as inducers for the successful and high differentiation of bone marrow-derived mesenchymal stem cells into neuron-like cells.
-
(2)
Thrombospondin 1 induced the formation of a larger synaptic network that exhibited dendritic, unipolar, dipolar or multipolar shapes. The morphology of induced cells was similar to normal neurites.
-
(3)
After thrombospondin 1 induced bone marrow mesenchymal stem cells to differentiate into neuron-like cells, the positive cells for postsynaptic density protein 95 and synaptophysin 1 were significantly increased. This indicates that synaptic functions were produced.
INTRODUCTION
Growing evidence indicates that new treatments, such as cell transplantation and gene therapy[1,2], can restore neurological function in adult animals with nervous system impairment. Bone marrow mesenchymal stem cells are widely studied for cell transplantation and gene therapy because they are easy to isolate, culture and grow, and they have low immunogenicity and stable genetic characteristics. Moreover, bone marrow mesenchymal stem cells have lower risk for tumor formation than embryonic stem cells and have the potential for long-term multi-lineage differentiation. Cell transplantation has been regarded as an effective means for treating spinal cord injury in animal and clinical studies[3,4,5]; however, the mechanism remains unclear.
There are two main mechanisms by which bone marrow mesenchymal stem cells may help repair spinal cord injury. The first theory is that implanted bone marrow mesenchymal stem cells secrete trophic factors, such as brain-derived neurotrophic factor or vascular endothelial growth factor, which may promote neuronal survival and growth, and attenuate the inflammatory response. In vitro experimentsshowed that chondroitin sulfate proteoglycan[6,7,8], Nogo-A and MAG significantly inhibit the growth of axons. Wright et al[9] added these factors into culture medium of dorsal root ganglion explants and compared their effects to culture medium from human bone marrow mesenchymal stem cells. Results showed that the inhibitory effect of these factors on the axonal outgrowth was weakened by conditioned medium from human bone marrow mesenchymal stem cells. Thus, this suggests that bone marrow mesenchymal stem cells can attenuate the inhibitory effects of extracellular chondroitin sulfate proteoglycan, Nogo-A and MAG on axonal outgrowth, through the secretion of various neurotrophic factors. Nakajima et al[10] showed that bone marrow mesenchymal stem cells changed the immunophenotype of macrophages from M1 to M2 following acute spinal cord injury. In addition, bone marrow mesenchymal stem cells inhibited the formation of scar tissue during subacute or chronic periods, which promoted axonal outgrowth. The second mechanism by which bone marrow mesenchymal stem cells may help repair spinal cord injury is the transplanted bone marrow mesenchymal stem cells can differentiate into neurons in vivo and trigger the formation of synapses.
In vitro study showed that bone marrow mesenchymal stem cells can be induced to differentiate into neurons under a variety of conditions; however, each induction method achieves various differentiation rates. Therefore it is important to find a specific differentiation mechanism and an efficient induction method for improving the differentiation rate after induction. These measures will help cell transplantation treatment of nervous system impairment. Moreover, with an appropriate amount of immunosuppressive agents, the gene-modified human bone marrow mesenchymal stem cells survive for at least 3 weeks in mouse spinal cord. Therefore bone marrow mesenchymal stem cells can serve as transgenic vectors and provide a good carrier for gene modification treatment of spinal cord injury[11]. In addition, bone marrow mesenchymal stem cells transplantation in the treatment of ischemic brain injury has made great progress.
In neurobiology, the regulation of synapse formation is a process involving multiple factors, such as glial cells, neurotrophic factors, synaptophysin, synaptic cell adhesion molecules and synaptic differentiation markers[12,13,14,15,16,17]. The effective regulation of synaptic formation and functional recovery are significant in the treatment of nervous system diseases. Recent studies have shown that thrombospondin, which is secreted by astrocytes, is involved in the regulation of synaptic formation, post-injury remodeling, the proliferation and differentiation of neural progenitor cells, and the neural mechanisms of certain drugs through its interactions with its receptor α2δ-1 and neuroligin 1[18,19,20,21,22]. There are five members in the thrombospondin family, and each member has its own independent coding gene. Thrombospondin 1 and thrombospondin 2 are trimer proteins with similar structures and functional domains, while thrombospondin 4 is a pentamer protein that has different structural domains to thrombospondin 1/thrombospondin 2. Thrombospondin 1 is the main component of platelet alpha-granules, where it acts as a platelet activating factor and cell adhesion molecule that is released from platelet particles following activation. Thrombospondin 1 contributes to platelet aggregation and inflammation, as well as the formation of synapses. Pfrieger et al[23,24] reported that astrocyte proliferation closely matched synapse formation, and that signals secreted by glial cells can regulate neuronal differentiation and promote synapse formation. Accordingly, growing evidence is emerging on the mechanism underlying glial cell promotion of synapse formation. Recently, Christopherson et al[25] found that retinal ganglion cells cultured in vitro using either thrombospondin 1 or thrombospondin 2 could greatly increase the number of synapses, and this increase was not observed with the co-culture of astrocytes and retinal ganglion cells. In the thrombospondin 1 and thrombospondin 2 knockout mice, the number of excitatory synapses was significantly reduced, indicating that thrombospondin 1 and thrombospondin 2 also maintain synaptic stability. Subsequently, there has been increasing attention on the role of thrombospondin in promoting synapse formation, as well as preliminary insights into the mechanism and related pathophysiological studies. Eroglu et al[18] found a repeated sequence in thrombospondin that is responsible for its binding to von Willebrand factor, and proposed that its epidermal growth factor-like domain might directly bind the α2δ-1 receptor to induce synapse formation, as α2δ-1 knockdown with small interfering RNA inhibits the effects of thrombospondin on synapse formation. In the studies of Xu et al[26], SP1 and neurexin showed similar effects on synapse formation in hippocampal neuronal cultures. Their results further demonstrated that the neurexin protein receptor, neuroligin1, is involved in the mechanism of thrombospondin 1 increased synapse formation.
Lu et al[20] found that thrombospondin 1 promotes the proliferation and differentiation of neural progenitor cells, and maintains stability through the regulation of multiple pathways. In addition, thrombospondin is involved in the pathophysiological processes of epilepsy, stroke, Down's syndrome and other nervous system diseases. Thrombospondin receptor α2δ-1 is the target of the antiepileptic drugs gabapentin and pregabalin, and morphine activity is also concomitant with changes in thrombospondin 1 levels in the central nervous system[27,28,29]. Therefore, thrombospondin plays important roles both in central nervous system development and function.
In this study, we investigated the role of thrombospondin 1 in the differentiation of bone marrow mesenchymal stem cells into neural cells by analyzing changes in morphology and synapse formation after induction. We found that postsynaptic differentiation protein 95 and synapsin-1, which are markers of mature synapses, were increased after differentiation. This provides theoretical evidence for determining an efficient induction method for producing healthy neurons and exploring the mechanism associated with the formation of functional synapses.
RESULTS
Isolation, culture and amplification of bone marrow mesenchymal stem cells
Whole bone marrow was cultured for 2–3 days. Following this period, adherent cells gradually became visible on the bottom of the culture flask, which were small in size, with round or polygonal morphologies. Primary cells grew slowly and began to actively proliferate after 5–6 days in culture, and this was accompanied by an increase in cell size. Cells displayed a typical fibroblast-like morphology. A large number of cells grew in a radial manner in neat rows, and the density increased significantly. At 10–15 days, radial or spiral fibroblast colonies were visible in a variety of sizes. When the cells were 80–90% confluent, subpassaging was performed. As the cell generation increased, the morphology of the bone marrow mesenchymal stem cells became more uniform, with a fibroblast-like shape. Some cells became wide and flat, with round or oval nuclei, intracellular vacuoles or granules appeared, and the refraction was reduced (Figure 1).
Figure 1.
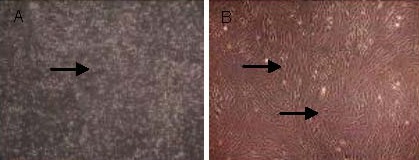
Morphology of primary bone marrow mesenchymal stem cells (A) and bone marrow mesenchymal stem cells at the third passage (B; inverted phase contrast microscope).
(A) Primary cells (arrow) were suspended particles and some cells were adherent (× 40).
(B) Cells at the third passage (arrows) of fusiform shape began to adhere and rapidly proliferate (× 100). Cells were closely attached to one another, gradually became confluent, and arranged in order along the long axis of cells. Their morphology was single and even, showing typical polarity and a vortex shape after fusion.
Identification of bone marrow mesenchymal stem cells
The expressions of different surface antigens of passage 3 bone marrow mesenchymal stem cells were detected by flow cytometry. Results showed that 99.1% were CD90-positive, while only 2.4% were CD34-positive and 3% were CD45-positive Figure 2.
Figure 2.
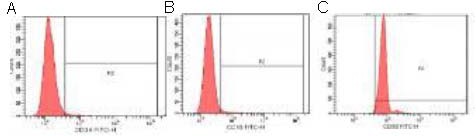
Identification of bone marrow mesenchymal stem cells by flow cytometry.
Among the positive cell samples, the percentage of fluorescein isothiocyanate (FITC)-CD34 (A), CD45 (B) and CD90 (C) positive cells were 2.4%, 3% and 99.1%, respectively, indicating that the cultured cells were bone marrow mesenchymal stem cells.
Osteogenic and adipogenic differentiation
After bone marrow mesenchymal stem cells were cultured with osteogenic induction medium, their morphology gradually transformed into large polygonal or irregular shapes. After 21 days of induction, a large number of calcium nodules formed in the cytoplasms, and stained orange by alizarin red staining. In addition, after bone marrow mesenchymal stem cells were cultured with adipogenic induction medium, their morphology changed to an irregular polygonal shape. After 14 days of induction, their cytoplasms were filled with a large number of red lipid droplets as displayed by oil red O staining (Figure 3).
Figure 3.
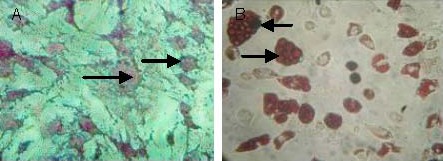
Morphological changes in bone marrow mesenchymal stem cells after osteogenic and adipogenic induction (inverted phase contrast microscope, × 200).
(A) At day 21 after the osteogenic induction, a large number of calcium nodules formed in the cytoplasm (alizarin red staining, arrows).
(B) At day 14 after the adipogenic induction, salmon-colored lipid droplets of varying sizes were visible in the cytoplasm (oil red O staining, arrows).
Morphology and identification of bone marrow mesenchymal stem cells-differentiated neuron-like cells
After bone marrow mesenchymal stem cells were induced with neural inducers for 3 days, cells induced with epidermal growth factor and basic fibroblast growth factor and cells induced with epidermal growth factor, basic fibroblast growth factor and thrombospondin 1 were flat, with inward contractions, small cell bodies, rounded or oval shapes, and long processes. The cell processes became slender and dendritic as the induction period increased. At day 4, typical morphological characteristics of neuron-like cells were visible. At day 5, the majority of cells showed a neuronal morphology, with a three-dimensional sense and strong refraction, and were unipolar, bipolar or multipolar, while very few cones were visible. Compared with the epidermal growth factor + basic fibroblast growth factor group, the number of neurites significantly increased and cell morphology was more complex in the epidermal growth factor + basic fibroblast growth factor + thrombospondin 1 group, with more synaptic connections between cells. In the blank control group (no inducers in the culture medium), the cell morphology remained unchanged. The number of cells did not increase with culture period. At day 6, the cytoplasm gradually shrank, and the cells detached from the culture plate and died (Figure 4).
Figure 4.
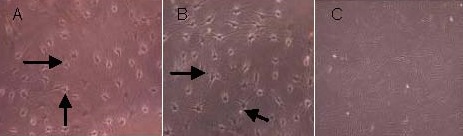
Morphology of bone marrow mesenchymal stem cells differentiated into neuron-like cells (inverted phase contrast microscope).
After 5 days of induction, both the epidermal growth factor + basic fibroblast growth factor group (A, × 200) and epidermal growth factor + basic fibroblast growth factor + thrombospondin 1 group (B, × 200) showed neuron-like morphologies (arrows), with a three-dimensional sense and strong refraction, and unipolar, bipolar or multipolar shapes. Cell processes were visible; the morphology of neurites in the epidermal growth factor + basic fibroblast growth factor + thrombospondin 1 group was more similar to normal neurites than the epidermal growth factor + basic fibroblast growth factor group, showing more complex structures and longer processes. In the blank control group (C, × 100), cell morphology maintained the original morphology of bone marrow mesenchymal stem cells, with no formation of processes.
After bone marrow mesenchymal stem cells were induced to differentiate into neuron-like cells, they were stained with the streptavidin biotin-peroxidase complex method. Positive cells had orange-yellow or brown cytoplasm and dark blue nuclei. This indicates that the induced cells expressed neuron-specific enolase or glial fibrillary acidic protein. Negative cells had no staining in the cytoplasm, and were only identified by nuclear staining.
In the epidermal growth factor + basic fibroblast growth factor group, neuron-specific enolase-positive cells accounted for 64% of total cells (Figures 5, 7), and glial fibrillary acidic protein-positive cells were 10% (Figures 6, 7). In the epidermal growth factor + basic fibroblast growth factor + thrombospondin 1 group, neuron-specific enolase-positive cells accounted for 66% of the total cells (Figures 5, 7), and glial fibrillary acidic protein-positive cells were 11% (Figures 6, 7). In the blank control group, neuron-specific enolase and glial fibrillary acidic protein were both negative. There was no significant difference in the staining for these neuronal specific markers between the epidermal growth factor + basic fibroblast growth factor and epidermal growth factor + basic fibroblast growth factor + thrombospondin 1 groups (Figure 7).
Figure 5.

Morphology of neuron-specific enolase-positive cells (arrows) after induction of bone marrow mesenchymal stem cells (immunohistochemical staining, × 200, streptavidin biotin-peroxidase complex method).
In the epidermal growth factor + basic fibroblast growth factor group (A) and epidermal growth factor + basic fibroblast growth factor + thrombospondin 1 group (B), neuron-specific enolase-positive nuclei were stained dark blue, and the cytoplasm was stained as brown-yellow. The control group (C) showed no pigmentation.
Figure 7.
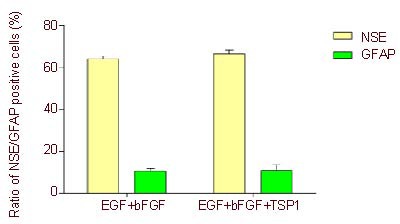
Changes in neuron-specific enolase (NSE)-and glial fibrillary acidic protein (GFAP)-positive cells after induction (fluorescent microscope, × 200).
Five visual fields were randomly selected from each group for the detection of expression of NSE and GFAP under an inverted phase contrast microscope (Olympus). Data are expressed as mean ± SD (n = 5), and two samples t-test was used. There was no significant difference in NSE- and GFAP-positive expression rates between epidermal growth factor (EGF) + basic fibroblast growth factor (bFGF) and EGF + bFGF + thrombospondin 1 (TSP1) groups.
Figure 6.

Morphology of glial fibrillary acidic protein-positive cells (arrows) after induction of bone marrow mesenchymal stem cells (immunohistochemical staining, × 200, streptavidin biotin-peroxidase complex method).
In the epidermal growth factor + basic fibroblast growth factor group (A) and epidermal growth factor + basic fibroblast growth factor + thrombospondin 1 group (B), glial fibrillary acidic protein-positive nuclei were stained dark blue, and the cytoplasm was stained as brown-yellow. The control group (C) showed no pigmentation.
Morphology of cell synapses after bone marrow mesenchymal stem cells induction
Under an inverted phase contrast microscope, we observed that the morphology of synapses in the epidermal growth factor + basic fibroblast growth factor + thrombospondin 1 group was more complicated than the epidermal growth factor + basic fibroblast growth factor group, presenting dendritic, unipolar, bipolar or multipolar shapes. The synapse morphology in the epidermal growth factor + basic fibroblast growth factor group was relatively simple. In addition, the neurites in the epidermal growth factor + basic fibroblast growth factor + thrombospondin 1 group continuously extended during the culture period, while cells in the blank control group were unchanged in morphology, with a long-fusiform shape (Figure 4). Compared with the epidermal growth factor + basic fibroblast growth factor group, the number and length of synapses in the epidermal growth factor + basic fibroblast growth factor + thrombospondin 1 group were significantly increased. These results indicate that thrombospondin 1 increased the number of synapses formed and promoted the neurite extension (P < 0.05; Figure 8).
Figure 8.
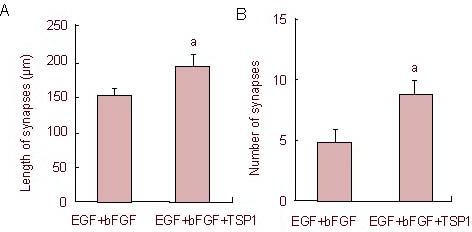
Effect of thrombospondin 1 on synaptic length (A) and number (B) after induction of bone marrow mesenchymal stem cells.
Five visual fields were randomly selected from each group for observations of the average synaptic number and synaptic length (mean ± SD, n = 5) under an inverted phase contrast microscope after induction. aP < 0.05, vs. epidermal growth factor (EGF) + basic fibroblast growth factor (bFGF) group (two samples t-test).
The induced neuron-like cells were stained with rabbit anti-rat postsynaptic density protein 95 and rabbit anti-rat synaptophysin 1, followed by the secondary antibody TRITC-labeled goat anti-rabbit. Results showed that neuron-like cells in both the epidermal growth factor + basic fibroblast growth factor and epidermal growth factor + basic fibroblast growth factor + thrombospondin 1 groups were stained with evenly distributed particles by confocal microscopy. As shown in Figures 9–11, the fluorescence staining intensity of postsynaptic density protein 95 and synaptophysin 1 in the epidermal growth factor + basic fibroblast growth factor + thrombospondin 1 group was significantly higher than that in the epidermal growth factor + basic fibroblast growth factor group. This indicates that thrombospondin 1 promotes the increases in the expressions of postsynaptic density protein 95 and synaptophysin 1 (Figures 10, 12; P < 0.05).
Figure 9.
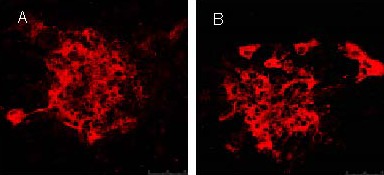
Expression of postsynaptic density protein 95 after induction of bone marrow mesenchymal stem cells (fluorescent microscope, × 200).
Under confocal laser scanning microscope, the expression of postsynaptic density protein 95 in the epidermal growth factor + basic fibroblast growth factor group (A) and epidermal growth factor + basic fibroblast growth factor + thrombospondin 1 group (B) was observed. Results showed that expression (TRITC-labeled) in the epidermal growth factor + basic fibroblast growth factor + thrombospondin 1 group was higher than in the epidermal growth factor + basic fibroblast growth factor group. TRITC: Tetramethyl rhodamin isothiocyanate.
Figure 11.
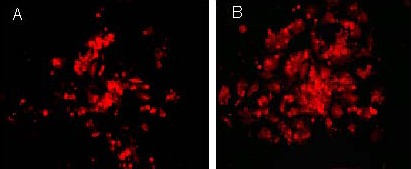
Expression of synaptophysin 1 after induction of bone marrow mesenchymal stem cells (fluorescent microscope, × 200).
Under a confocal laser scanning microscope, the expression of synaptophysin 1 in the epidermal growth factor + basic fibroblast growth factor group (A) and the epidermal growth factor + basic fibroblast growth factor + thrombospondin 1 group (B) was observed. Results showed that expression (TRITC-labeled) in the epidermal growth factor + basic fibroblast growth factor + thrombospondin 1 group was higher than in the epidermal growth factor + basic fibroblast growth factor group. TRITC: Tetramethyl rhodamin isothiocyanate.
Figure 10.
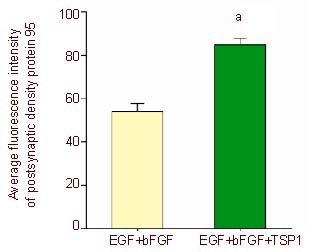
Expression of postsynaptic density protein 95 after induction of bone marrow mesenchymal stem cells.
Five visual fields in each group were randomly selected to detect the fluorescence density using Image Pro Plus 6.0 software, and the average absorbance values of postsynaptic density protein 95 after induction were calculated. aP < 0.05, vs. epidermal growth factor (EGF) + basic fibroblast growth factor (bFGF) group (two samples t-test). Data are represented as mean ± SD, n = 5.
Figure 12.
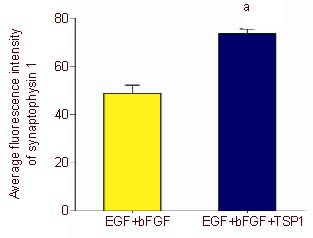
Expression of synaptophysin 1 after induction of bone marrow mesenchymal stem cells.
Five visual fields in each group were randomly selected to detect the fluorescence density using Image Pro Plus 6.0 software, and the average absorbance values of synaptophysin 1 after induction were calculated. aP < 0.05, vs. epidermal growth factor (EGF) + basic fibroblast growth factor (bFGF) group (two samples t-test). Data are represented as mean ± SD, n = 5.
DISCUSSION
As a target cell, bone marrow mesenchymal stem cells play an important role in cell transplantation and gene therapy treatments for spinal cord injury. Bone marrow mesenchymal stem cells are mainly derived from bone marrow, where the content of bone marrow mesenchymal stem cells is relatively low. Therefore, the in vitro isolation and culture of bone marrow mesenchymal stem cells of a high purity is extremely important. The common method for the in vitro isolation of bone marrow mesenchymal stem cells is an adherent screening method or density gradient centrifugation. Using the adherent screening method, the extracted bone marrow mesenchymal stem cells adhere quickly, are highly active and strongly proliferative, and primary cells require only 7–10 days for fusion. The density gradient centrifugation method also obtains a high purity of cells.
Previous studies mainly adopt one method to extract target cells, while in this study we applied both methods in the extraction and purification procedure, and obtained active target cells of a high purity. Currently, there is no specific molecular marker for bone marrow mesenchymal stem cells. Bone marrow mesenchymal stem cells are mainly identified through cell morphology, cell surface differentiation antigens and the potential for multi-lineage differentiation. It was previously reported that CD44, CD90 and CD106 are highly expressed on the surface of bone marrow mesenchymal stem cells, whereas CD34, CD45 and other hematopoietic lineage markers are scarcely expressed[30]. In this study, CD90, CD34 and CD45 expressions were detected with flow cytometry to exclude bone marrow hematopoietic stem cells. Bone marrow mesenchymal stem cells can be differentiated into three kinds of cell lineages: adipose, osteoblast, and cartilage[31,32,33]. The identification of bone marrow mesenchymal stem cells depends on the characteristics of stem cells and their differentiation abilities. Target cells were isolated from rat bone marrow using the adherent screening and gradient centrifugation methods. The obtained cells were wide and flat, with a fibroblast-like shape, and were arranged in radial or spiral colonies. After induction medium was added, cells can differentiate into osteoblasts and adipocytes, indicating a potential for multi-lineage differentiation. Flow cytometry results showed a high expression of CD90 and low expression of CD34 and CD45, which is consistent with the characteristics of bone marrow mesenchymal stem cells surface markers. Together, these results indicate that the cultured cells were bone marrow mesenchymal stem cells.
There are three methods for bone marrow mesenchymal stem cells differentiation into neurons in vitro: (1) induction with antioxidants, such as β-mercaptoethanol or dimethylsulfoxide/butylated hydroxyanisole[34]; (2) induction with dibutyryl-cyclic AMP and isobutyl methylxanthine to enhance intracellular cyclic AMP content, thereby inducing bone marrow mesenchymal stem cells to differentiate into neuron-like cells[35]; and (3) induction with cell growth factors. In this experiment, bone marrow mesenchymal stem cells were differentiated into bone marrow-derived neuron-like cells using growth factors as the inducers, where thrombospondin 1 played an important role in the formation of synapses during the differentiation period. Therefore, our study provides an efficient method for producing safe target cells for transplantation in the treatment of spinal cord injury. Our results indicate that although thrombospondin 1 cannot increase the differentiation rate of bone marrow mesenchymal stem cells, it significantly upregulated the expression of synaptophysin 1 and postsynaptic density protein 95, and increased the number of synapses after differentiation. This indicates that thrombospondin 1 has an important role in establishing functional synapses and in the transmission of neural signals. It has been confirmed in in vitro experiments that chemical reagents can induce bone marrow mesenchymal stem cells to differentiate into neural cells with a high induction rate; however, the induced cells only survive for a short period and cannot meet the needs of further research. Furthermore, the side effects after cell implantation remain unclear. Woodbury et al[34] used β-mercaptoethanol and butylated hydroxyanisole to induce bone marrow mesenchymal stem cells differentiation into neuron-like cells in a short time period, with the differentiation rate up to 80%. However, it takes a few days to induce neuronal cells with chemical inducers, and these neuron-like cells have no physiological characteristics of nerve cells. Although the induction of bone marrow mesenchymal stem cells with cell-derived factors is a longer process and obtains low efficiency, its induction effect is mild and can effectively simulate the growth environment of neural cells in vivo. This result is consistent with the normal development process of human neurons and the induced neurons exhibit a high safety profile, thus ensuring their safe application in cell transplantation therapy. In this study, after a cell growth factor was used as an inducer, the flat cells inwardly contracted, and the cell bodies became small and were round- or oval-shaped. The surrounding processes were longer in both the basic fibroblast growth factor + glial cell line-derived neurotrophic factor and basic fibroblast growth factor + glial cell line-derived neurotrophic factor + thrombospondin 1 groups. These neurites became slender and dendritic as the induction advanced. At day 5, most cells showed changes in neuronal morphology, with a three-dimensional sense, strong refraction, and unipolar, bipolar or multipolar morphologies, while very few cells were cone-shaped. In addition, the neuron-specific enolase-positive cells accounted for 65% of the total cells, while the glial fibrillary acidic protein-positive cells were only 10% in the basic fibroblast growth factor + glial cell line-derived neurotrophic factor and basic fibroblast growth factor + glial cell line-derived neurotrophic factor + thrombospondin 1 groups. This evidence suggests that the induced cells mainly expressed the surface antigen of mature neurons, and the surface antigen expression of glial cells was rarely seen. After cells were induced with the cytokine, the cell morphology and cell surface antigen expression were similar to mature neurons. Statistical analyses revealed that there was no significant difference in positive staining for the neuron-specific markers between the two groups. Thus, thrombospondin 1 has no impact on the rate of bone marrow mesenchymal stem cells differentiation.
Lu et al[20] highlighted that thrombospondin 1 regulates the proliferation and stability of neural progenitor cells through multiple signal pathways. In addition, they showed thrombospondin 1 induced the differentiation of neural progenitors into neurons. Christopherson et al[25] revealed that astrocyte-secreted thrombospondin 1 could enhance the formation of synapses and increase the number of synapses by several folds. We speculate that, although thrombospondin 1 can promote synaptic formation in bone marrow mesenchymal stem cells-differentiated neuron-like cells and promote the differentiation of neural progenitor cells into neurons, it does not promote bone marrow mesenchymal stem cells differentiation into neurons.
In the process of bone marrow mesenchymal stem cells differentiation into neuron-like cells, although thrombospondin 1 did not affect the differentiation rate, it significantly increased synaptic density, length, and formation in the induced neurons. The synaptic density and length in the basic fibroblast growth factor + glial cell line-derived neurotrophic factor + thrombospondin 1 group were significantly higher than the basic fibroblast growth factor + glial cell line-derived neurotrophic factor group. The expressions of postsynaptic density protein 95 and synaptophysin 1, which are markers of synaptic formation, were significantly increased in the basic fibroblast growth factor + glial cell line-derived neurotrophic factor + thrombospondin 1 group compared with the basic fibroblast growth factor + glial cell line-derived neurotrophic factor group. Christopherson et al[25] tried to culture retinal ganglion cells with thrombospondin 1 and thrombospondin 2 secreted by astrocytes in vitro and obtained similar results. This indicates that the thrombospondin 1-induced neuron-like cells are similar to mature neurons in morphological structure, and that thrombospondin 1 promotes synaptic differentiation in neuron-like cells differentiated from bone marrow mesenchymal stem cells. The mechanism associated with thrombospondin 1 promotion of synapse formation may be mediated by its binding to the receptor α2δ, and activation of downstream presynaptic and postsynaptic cascades, resulting in neural cytoskeletal rearrangement[36]. In addition, thrombospondin 1 has cellular de-adhesion properties[37], which may induce excessive growth of the neuronal matrix and promote synaptic formation. In conclusion, the mechanism of thrombospondin 1 promotion of synapse formation may be achieved by the interactions of a series of growth factors, extracellular matrix proteins, cell adhesion molecules and receptor proteins.
We conducted a quantitative analysis on the levels of postsynaptic density protein 95 and synaptophysin 1 to detect the influence of thrombospondin 1 on synapse formation after bone marrow mesenchymal stem cells were differentiated into neurons. Experimental findings showed that thrombospondin 1 promoted synaptic formation; however, this effect was not as evident as it was in retinal ganglion cells. The synaptic density in thrombospondin 1-induced retinal ganglion cells was increased several-fold compared with primarily cultured neurons[25]. After the induction of thrombospondin 1, the density of postsynaptic density protein 95 and synaptophysin 1 was less than twice as high as that of the control group. Differences in these experimental results may be due to variations in the timing of the neuronal cultures with thrombospondin 1. At different developmental stages of neurons, thrombospondin 1 has varying degrees of promoting effects[25]. Thus, at different time points of induction with thrombospondin 1, its role in synapse formation may be directly affected. In addition, as thrombospondin 1 acts by different mechanisms for promoting synapse formation in different types of neurons, this may also explain the variance in experimental findings.
After bone marrow mesenchymal stem cells are induced to differentiate into neural cells in vitro, it is important to observe structural changes and to identify cell functions. In addition, certain stimuli should be examined to see if they induce the release of neurotransmitters, or generate action potentials that cross synapses and transfer excitatory or inhibitory signals to neighboring cells. In this study, we confirmed the high differentiation of bone marrow-derived neuron-like cells, and the influence of thrombospondin 1 on synaptic formation at the morphological and protein expression levels. However, there is no electrophysiological evidence to support the theory that these synapses had normal physiological functions. A previous study found a transient membrane depolarization (mainly a transient change in K+ current) in the induced neuron-like cells by electrophysiological examination[38], but the depolarization process was not stable and disappeared as cells died. The identification of the differentiated neuron-like cells should not be confined to analyses of cell morphology and the expression of marker proteins, but focus also on the physiological functions of neuron-like cells and the formed synapses. For example, whole cell patch-clamp detection using voltage clamp and current clamp methods[39,40] should be performed to record Na+ and K+ currents and action potentials in cells to confirm the existence of functional synapses between the neurons. Synaptic vesicles have been labeled using a fluorescent probe, without any impact on vesicle activity[41]. Accordingly, neural endocytosis and exocytosis can be examined by measuring fluorescence intensity, thus allowing tracking of transport vesicles in an activity-dependent manner. This evidence can confirm the neurotransmitter releasing capacities of nerve cells, with glutamic acid being the main neurotransmitter in excitatory synapses[42,43,44]. There are two types of neurotransmitter receptor: metabotropic and ionotropic. The ionotropic receptor has three members: the AMPA receptor, NMDA receptor and KA receptor. If a postsynaptic membrane expresses only the NMDA receptor, and not the AMPA receptor, the synapses lack normal physiological function, and are known as silent synapses[45,46].
In this study, thrombospondin 1 induction significantly upregulated the expression of postsynaptic density protein 95 and synaptophysin 1. However, Malinow et al[45] found that the density of the glutamate receptor 1 subunit cluster of the AMPA receptor did not change significantly, even when postsynaptic density protein 95 density was significantly increased by thrombospondin 1. This indicates that thrombospondin 1-induced synapses are silent synapses because of the absence or low expression of the AMPA receptor, and thus will have no normal physiological function. Further studies are needed to examine synaptic transmission and to confirm that these neuron-like cells have silent synapses.
In summary, we simulated the synaptic growth environment in vivo and obtained highly differentiated bone marrow-derived neuron-like cells using a combination of growth factors. Moreover, thrombospondin 1 showed an obvious effect on synapse formation after induction of bone marrow mesenchymal stem cells differentiation into neurons, where synaptic morphology, number and length were significantly improved. The number of cells positive for postsynaptic density protein 95 and synaptophysin 1 was also greatly increased[47,48]. Experimental findings indicate a possible mechanism for thrombospondin 1 in synapse formation, and provide an effective induction method and theoretical evidence for the use of bone marrow mesenchymal stem cells transplantation in the treatment of spinal cord injury.
MATERIALS AND METHODS
Design
A randomized, controlled, in vitro experiment.
Time and setting
Experiments were completed in the Central Laboratory in Affiliated Hospital to Guangdong Medical College, China from October 2011 to July 2012.
Materials
Healthy, clean Sprague-Dawley rats, aged 4 weeks, weighing 125 ± 20 g, specific-pathogen free, were purchased from the Experimental Animal Center of Guangdong Medical College, China, under the license No. A2010067SCXK (Yue) 2008-0008.
All experimental disposals were in line with the Guidance Suggestions for the Care and Use of Laboratory Animals, formulated by the Ministry of Science and Technology of China[49].
Methods
Acquisition and amplification of bone marrow mesenchymal stem cells
Rats were killed by cervical dislocation under anesthesia. Femoral and tibial bones were isolated, and the ends were removed in medium. Tissues were then incubated with low-glucose Dulbecco's modified Eagle's medium (DMEM; Hyclone, Logan, UT, USA) containing 10% fetal bovine serum, 100 U/mL penicillin and 100 U/mL streptomycin. The bone marrow cavity was washed and prepared into a uniform cell suspension. The cell suspension was centrifuged at 1 000 r/min for 10 minutes. The supernatant was discarded and cells were inoculated into 25-mm2 cell culture flasks (4.5 mL) with 5% CO2 at 37°C for 3 days. The culture medium was completely changed, and the non-adherent cells were removed. Then, culture medium was replenished every 2 or 3 days. When the primary cells grew 80–90%, cells were subcultured at a 1:2 ratio. This study used bone marrow mesenchymal stem cells at passage 3, in a good growth state[34].
Identification of bone marrow mesenchymal stem cells
Fluorescein isothiocyanate (FITC)-labeled CD34, CD45, CD90 antibody identification: 100 μL of cell suspension, at a density of 1 × 105 cells/mL, was added to four centrifuge tubes, following which 20 μL of FITC-labeled rabbit anti-rat monoclonal antibodies to CD45, CD34, CD90, or the negative control, rabbit anti-rat 20% IG1-FITC (Jackson Immunoresearch, West Grove, PA, USA), were added separately to each of the tubes. Each tube was incubated at room temperature in the dark for 20 minutes. Then, 1.5 mL PBS was added to each tube and centrifuged at 1 800 r/min for 5 minutes. The supernatant was removed and 500 μL PBS was added to each tube to prepare the cell suspension for analysis by flow cytometry[50,51,52].
Multi-lineage differentiation potentials of bone marrow mesenchymal stem cells[53]: (1) Osteogenic induction: bone marrow mesenchymal stem cells at passage 3 were incubated in 6-well culture plates at 1 × 108 cells/L. When the cells reached 80% confluence, the medium was replaced with osteogenic induction medium, containing 0.1 M dexamethasone, 50 mg/L vitamin C, 10 mM β-glycerophosphate sodium (Sigma, St. Louis, MO, USA) at 37°C with 5% CO2. The induction medium was replenished every 3 days. At day 21 of differentiation, the osteogenic induction medium was aspirated and the cells were washed with PBS. Then, the cells were fixed with 95% ethanol for 10 minutes and stained with alizarin red. The orange-red nodule size, number and distribution of staining were observed under a CKX41 inverted phase contrast microscope (Olympus, Tokyo, Japan). The nodule distribution represents the distribution of calcium nodules in osteoblasts. (2) Adipogenic induction: bone marrow mesenchymal stem cells at passage 3 were incubated in 6-well culture plates at a density of 1 × 108 cells/L. Cell slices were prepared and cultured for 24 hours. Cells were then incubated with adipogenic inducing medium, composed of DMEM/Nutrient Mixture F-12, containing 0.25 μM dexamethasone, 50 μM indomethacin, 0.5 mM IBMX, 10 mg/L bovine insulin, and 10% fetal bovine serum (Sigma) at 37°C with 5% CO2. The induction medium was replenished every 3 days. At day 14 after differentiation, the medium was removed and the cells were washed with PBS and fixed with 10% neutral formalin for 1 hour at room temperature. Oil red O staining was performed to detect fat deposition. The distribution of red lipid droplets was observed under CKX41 inverted phase contrast microscope (Olympus), to determine lipid droplet distribution and fat secretion.
Neural induction and identification of induced neurons
Epidermal growth factor + basic fibroblast growth factor group: Passage 3 bone marrow mesenchymal stem cells at a density of 2 × 107 cells/L were cultured in 6-well culture plates[54,55,56,57]. Pre-induction was performed for 24 hours using DMEM containing 10% fetal bovine serum, 20 μg/L epidermal growth factor (Sigma) and 20 μg/L basic fibroblast growth factor (Sigma). Then, the culture medium was completely changed and cells were rinsed with PBS twice. Cells were incubated for 5 hours to 6 days using serum-free DMEM and then with Neurobasal Medium/B27 medium. Both culture mediums contained 20 μg/L basic fibroblast growth factor and 20 μg/L epidermal growth factor. The morphology and the number of cells were observed daily.
Epidermal growth factor + basic fibroblast growth factor + thrombospondin 1 group: Passage 3 bone marrow mesenchymal stem cells at a density of 2 × 107 cells/L were cultured in 6-well culture plates. Pre-induction was performed for 24 hours using DMEM containing 10% fetal bovine serum, 20 μg/L epidermal growth factor and 20 μg/L basic fibroblast growth factor. Then, the culture medium was completely changed and cells were rinsed with PBS twice. Cells were incubated for 5 hours to 6 days using serum-free DMEM and then with Neurobasal Medium/B27 medium (Gibco, New York, NY, USA). Both culture mediums contained 20 μg/L basic fibroblast growth factor and 20 μg/L epidermal growth factor. Cell morphology was observed daily. At days 3–4 of induction, cells began to contract and peripheral neurites were visible, following which cells were incubated with thrombospondin 15 μg/L[38].
Blank control group: Bone marrow mesenchymal stem cells were conventionally cultured without basic fibroblast growth factor, epidermal growth factor or thrombospondin 1.
Identification of neuron-like cells: Cells were fixed with 4% paraformaldehyde and rinsed with 0.01 M PBS. Then, cells were incubated with goat anti-rat neuron-specific enolase and glial fibrillary acidic protein polyclonal antibody (Qiyun Biotechnology Co., Ltd., Guangzhou, Guangdong Province, China; 1:100) at 4°C overnight. Next, cells were incubated with an antibody (Qiyun Biotechnology Co., Ltd.; 1:100) and streptavidin-biotin-peroxidase complex at room temperature for 30 minutes. Stains were developed with 3,3′-diaminobenzidine for 30 minutes. The percentage of cells positive for neuron-specific enolase and glial fibrillary acidic protein in the total population was calculated in each visual field. Positive results were as follows: the cytoplasm was stained orange-yellow or brown, with the nuclei as deep blue. Five non-overlapping views were randomly selected in each group under the light microscope (Olympus). The positive cell rate was calculated according to the following formula: positive rate = number of positive cells/number of total cells × 100%.
Determination of synaptic morphology
The 6-well culture plate was stained using the streptavidin-biotin-peroxidase complex method. The neurites were measured under an inverted phase contrast microscope using a micrometer. Five visual fields were selected from each hole at 200 × magnification. The neurite number and length were calculated and analyzed statistically.
Determination of postsynaptic density protein 95 and synaptophysin 1
Cells cultured for 12 days were used for immunocytochemical staining. In brief, cell slices were incubated on ice for 30 minutes and treated with 0.1% Triton X-100 for 10 minutes. Cells were then rinsed with 0.1 M PBS three times, each for 5 minutes, and blocked with the serum for 20 minutes at room temperature. Cells were finally incubated with rabbit anti-mouse postsynaptic density protein 95 polyclonal antibody (Qiyun Biotechnology Co., Ltd.; 1:100) and synaptophysin 1 polyclonal antibody (Qiyun Biotechnology Co., Ltd.) at 4°C overnight. Subsequently, cells were rinsed with 0.1 M PBS three times for 5 minutes each. Blank controls were incubated with PBS and tetramethyl rhodamin isothiocyanate (TRITC; Jackson Immunoresearch, West Grove, PA, USA)-labeled goat anti-rabbit antibody. Then cells were rinsed with PBS three times, for 5 minutes each. Cell slices were stained with monoclonal propidium iodide (1:500; Sigma) and mounted with neutral resin. Cell morphology and staining were observed under fluorescence microscopy (Leica, Solms, Germany).
Image analysis
The fluorescence intensity of postsynaptic density protein 95 and synaptophysin 1 was analyzed using Image Pro Plus Version 6.0 image analysis software (Media Cybernetics Company, Bethesda, MD, USA).
Statistical analysis
Measurement data were expressed as mean ± SD and statistical analysis was performed using SPSS 18.0 statistical software (SPSS, Chicago, IL, USA). The mean values between groups were compared with two samples t-test, with α = 0.05.
Acknowledgments
We would like to thank Guangdong Medical College and the Affiliated Hospital of Guangdong Medical College in China for technical support; and the staff of the Library of Guangdong Medical College in China for their help.
Footnotes
Funding: This study was supported by the Natural Science Foundation of Guangdong Province, No. S2011010004096, and the Medical Scientific Research Foundation of Guangdong Province, No. A2010431; A2009477.
Conflicts of interest: None declared.
Ethical approval: The study gained full ethical approval from the Animal Ethics Committee of Guangdong Medical College in China.
(Reviewed by Wallace M, Haase R, Zhang GR, Zhang JM)
(Edited by Wang J, Yang Y, Li CH, Song LP)
REFERENCES
- [1].De Feo D, Merlini A, Laterza C, et al. Neural stem cell transplantation in central nervous system disorders: from cell replacement to neuroprotection. Curr Opin Neurol. 2012;25(3):322–333. doi: 10.1097/WCO.0b013e328352ec45. [DOI] [PubMed] [Google Scholar]
- [2].Pereira Lopes FR, Martin PK, Frattini F, et al. Double gene therapy with G-CSF and VEGF acts synergistically to improve nerve regeneration and functional outcome after sciatic nerve injury in mice. Neuroscience. 2012 doi: 10.1016/j.neuroscience.2012.10.025. [DOI] [PubMed] [Google Scholar]
- [3].Nishida H, Nakayama M, Tanaka H, et al. Safety of autologous bone marrow stromal cell transplantation in dogs with acute spinal cord injury. Vet Surg. 2012;41(4):437–442. doi: 10.1111/j.1532-950X.2011.00959.x. [DOI] [PubMed] [Google Scholar]
- [4].Nakamura M, Tsuji O, Nori S, et al. Cell transplantation for spinal cord injury focusing on iPSCs. Expert Opin Biol Ther. 2012;12(7):811–821. doi: 10.1517/14712598.2012.681774. [DOI] [PubMed] [Google Scholar]
- [5].Hawryluk GW, Mothe A, Wang J, et al. An in vivo characterization of trophic factor production following neural precursor cell or bone marrow stromal cell transplantation for spinal cord injury. Stem Cells Dev. 2012;21(12):2222–2238. doi: 10.1089/scd.2011.0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Walker BA, Ji SJ, Jaffrey SR. Intra-axonal translation of rhoa promotes axon growth inhibition by CSPG. J Neurosci. 2012;32(41):14442–14447. doi: 10.1523/JNEUROSCI.0176-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Pernet V, Schwab ME. The role of Nogo-A in axonal plasticity, regrowth and repair. Cell Tissue Res. 2012;349(1):97–104. doi: 10.1007/s00441-012-1432-6. [DOI] [PubMed] [Google Scholar]
- [8].Nagai J, Goshima Y, Ohshima T. CRMP4 mediates MAG-induced inhibition of axonal outgrowth and protection against Vincristine-induced axonal degeneration. Neurosci Lett. 2012;519(1):56–61. doi: 10.1016/j.neulet.2012.05.020. [DOI] [PubMed] [Google Scholar]
- [9].Wright KT, El Masri W, Osman A, et al. Bone marrow stromal cells stimulate neurite outgrowth over neural proteoglycans (CSPG), myelinassociated glycoprotein and Nogo-A. Biochem Biophys Res Commun. 2007;354(2):559–566. doi: 10.1016/j.bbrc.2007.01.013. [DOI] [PubMed] [Google Scholar]
- [10].Nakajima H, Uchida K, Guerrero AR, et al. Transplantation of mesenchymal stem cells promotes an alternative pathway of macrophage activation and functional recovery after spinal cord injury. J Neurotrauma. 2012;29(8):1614–1625. doi: 10.1089/neu.2011.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ronsyn MW, Daans J, Spaepen G, et al. Plasmid-based genetic modification of human bone marrow-derived stromal cells: analysis of cell survival and transgene expression after transplantation in rat spinal cord. BMC Biotechnol. 2007;7:90. doi: 10.1186/1472-6750-7-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Suqiura Y, Lin W. Neuron-glia interactions: the roles of Schwann cells in neuromuscular synapse formation and function. Biosci Rep. 2011;31(5):295–302. doi: 10.1042/BSR20100107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Rousse I, St-Amour A, Darabid H, et al. Synapse-glia interactions are governed by synaptic and intrinsic glial properties. Neuroscience. 2010;167(3):621–632. doi: 10.1016/j.neuroscience.2010.02.036. [DOI] [PubMed] [Google Scholar]
- [14].Wang Z, Wang B, Yang L, et al. Presynaptic and postsynaptic interaction of the amyloid precursor protein promotes peripheral and central synaptogenesis. J Neurosci. 2009;29(35):10788–10801. doi: 10.1523/JNEUROSCI.2132-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Fu Z, Vicini S. Neuroligin-2 accelerates GABAergic synapse maturation in cerebellar granule cells. Mol Cell Neurosci. 2009;42(1):45–55. doi: 10.1016/j.mcn.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Perlini LE, Botti F, Fornasiero EF, et al. Effects of phosphorylation and neuronal activity on the control of synapse formation by synapsin 1. J Cell Sci. 2011;124(Pt 21):3643–3653. doi: 10.1242/jcs.086223. [DOI] [PubMed] [Google Scholar]
- [17].Díaz E. SynDIG1 regulation of synaptic AMPA receptor targeting. Commun Integr Biol. 2010;3(4):347–349. doi: 10.4161/cib.3.4.11765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Eroglu C, Allen NJ, Susman MW, et al. Gabapentin receptor alpha2delta-1 is a neuronal thrombospondin receptor responsible for excitatory CNS synaptogenesis. Cell. 2009;139(2):380–392. doi: 10.1016/j.cell.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Blake SM, Strasser V, Andrade N, et al. Thrombospondin-1 binds to ApoER2 and VLDL receptor and functions inpostnatal neuronal migration. EMBO J. 2008;27(22):3069–3080. doi: 10.1038/emboj.2008.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lu Z, Kipnis J. Thrombospondin 1--a key astrocyte- derived neurogenic factor. FASEB J. 2010;24(6):1925–1943. doi: 10.1096/fj.09-150573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Liauw J, Hoang S, Choi M, et al. Thrombospondins 1 and 2 are necessary for synaptic plasticity and functional recovery after stroke. J Cereb Blood Flow Metab. 2008;28(10):1722–1732. doi: 10.1038/jcbfm.2008.65. [DOI] [PubMed] [Google Scholar]
- [22].Ikeda H, Miyatake M, Koshikawa N, et al. Morphine modulation of thrombospondin levels in astrocytes and its implications for neurite outgrowth and synapse formation. J Biol Chem. 2010;285(49):38415–38427. doi: 10.1074/jbc.M110.109827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Pfrieger FW, Barres BA. New views on synapse-glia interactions. Curr Opin Neurobiol. 1996;6(5):615–621. doi: 10.1016/s0959-4388(96)80093-6. [DOI] [PubMed] [Google Scholar]
- [24].Pfrieger FW, Barres BA. Synaptic efficacy enhanced by glial cells in vitro. Science. 1997;277(5332):1684–1687. doi: 10.1126/science.277.5332.1684. [DOI] [PubMed] [Google Scholar]
- [25].Christopherson KS, Ullian EM, Stokes CC, et al. Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell. 2005;120(3):421–433. doi: 10.1016/j.cell.2004.12.020. [DOI] [PubMed] [Google Scholar]
- [26].Xu J, Xiao N, Xia J. Thrombospondin 1 accelerates synaptogenesis in hippocampal neurons through neuroligin 1. Nat Neurosci. 2010;13(1):22–24. doi: 10.1038/nn.2459. [DOI] [PubMed] [Google Scholar]
- [27].Garcia O, Torres M, Helguera P, et al. A role for thrombospondin-1 deficits in astrocyte-mediated spine and synaptic pathology in Down's syndrome. PLoS One. 2010;5(12):e14200. doi: 10.1371/journal.pone.0014200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Lin TN, Kim GM, Chen JJ, et al. Differential regulation of thrombospondin-1 and thrombospondin-2 after focal cerebral ischemia/reperfusion. Stroke. 2003;34(1):177–186. doi: 10.1161/01.str.0000047100.84604.ba. [DOI] [PubMed] [Google Scholar]
- [29].Valder CR, Liu JJ, Song YH, et al. Coupling gene chip analyses and rat genetic variances in identifying potential target genes that may contribute to neuropathic allodynia development. J Neurochem. 2003;87(3):560–573. doi: 10.1046/j.1471-4159.2003.02016.x. [DOI] [PubMed] [Google Scholar]
- [30].Conget PA, Minguell JJ. Phenotypical and functional properties of human bone marrow mesenchymal progenitor cells. J Cell Physiol. 1999;181(1):67–73. doi: 10.1002/(SICI)1097-4652(199910)181:1<67::AID-JCP7>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- [31].Qu D, Li J, Li Y, et al. Ectopic osteochondral formation of biomimetic porous PVA-n-HA/PA6 bilayered scaffold and BMSCs construct in rabbit. J Biomed Mater Res B Appl Biomater. 2011;96(1):9–15. doi: 10.1002/jbm.b.31697. [DOI] [PubMed] [Google Scholar]
- [32].Choi YA, Lim J, Kim KM, et al. Secretome analysis of human BMSCs and identification of SMOC1 as an important ECM protein in osteoblast differentiation. J Proteome Res. 2010;9(6):2946–2956. doi: 10.1021/pr901110q. [DOI] [PubMed] [Google Scholar]
- [33].Afizah H, Yang Z, Hui JH, et al. A comparison between the chondrogenic potential of human bone marrow stem cells (BMSCs) and adipose-derived stem cells (ADSCs) taken from the same donors. Tissue Eng. 2007;13(4):659–666. doi: 10.1089/ten.2006.0118. [DOI] [PubMed] [Google Scholar]
- [34].Woodbury D, Schwarz EJ, Prockop DJ. Adult rat and human bone marrow stromal cells differentiate into neurons. Neurosci Res. 2000;61(4):364–370. doi: 10.1002/1097-4547(20000815)61:4<364::AID-JNR2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- [35].Deng W, Obrocka M, Fishcher I, et al. In vitro differentiation of human marrow stromal cells into early progenitors of neural cells by conditions that increase intracellular cyclic AMP. Biochem Biophysic Res Commun. 2001;282(1):148–152. doi: 10.1006/bbrc.2001.4570. [DOI] [PubMed] [Google Scholar]
- [36].Kurshan PT, Oztan A, Schwarz TL. Presynaptic alpha2delta-3 is required for synaptic morphogenesis independent of its Ca2+-channel functions. Nat Neurosci. 2009;12(11):1415–1423. doi: 10.1038/nn.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Murphy-Ullrich JE. The de-adhesive activity of matricellular proteins: is intermediate cell adhesion an adaptive state? J Clin Invest. 2001;107(7):785–790. doi: 10.1172/JCI12609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Zhang HW, Zhang YZ, Zhang HX, et al. Electrophysiological functions of adult rat marrow stromal cells before and after differentiating into neurons induced by astrocytes. Zhonghua Shenjing Yixue Zazhi. 2004;3(5):324–326. [Google Scholar]
- [39].Bal R, Erdogan S, Theophilidis G, et al. Assessing the effects of the neonicotinoid insecticide imidacloprid in the cholinergic synapses of the stellate cells of the mouse cochlear nucleus using whole-cell patch-clamp recording. Neurotoxicology. 2010;31(1):113–120. doi: 10.1016/j.neuro.2009.10.004. [DOI] [PubMed] [Google Scholar]
- [40].Kawa K. Glycine facilitates transmitter release at developing synapses: a patch clamp study from Purkinje neurons of the newborn rat. Brain Res Dev Brain Res. 2003;144(1):57–71. doi: 10.1016/s0165-3806(03)00159-7. [DOI] [PubMed] [Google Scholar]
- [41].Brumback AC, Lieber JL, Angleson JK, et al. Using FM1-43 to study neuropeptide granule dynamics and exocytosis. Methods. 2004;33(4):287–294. doi: 10.1016/j.ymeth.2004.01.002. [DOI] [PubMed] [Google Scholar]
- [42].McCoole MD, D’Andrea BT, Baer KN, et al. Genomic analyses of gas (nitric oxide and carbon monoxide) and small molecule transmitter (acetylcholine, glutamate and GABA) signaling systems in daphnia pulex. Comp Biochem Physiol Part D Genomics Proteomics. 2012;7(2):124–160. doi: 10.1016/j.cbd.2012.01.001. [DOI] [PubMed] [Google Scholar]
- [43].Huang YA, Grant J, Roper S. Glutamate may be an efferent transmitter that elicits inhibition in mouse taste buds. PLoS One. 2012;7(1):e30662. doi: 10.1371/journal.pone.0030662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Okubo Y, Iino M. Visualization of glutamate as a volume transmitter. J Physiol. 2011;589(Pt 3):481–488. doi: 10.1113/jphysiol.2010.199539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci. 2002;25:103–126. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- [46].Isaac JT. Postsynaptic silent synapses: evidence and mechanisms. Neuropharmacology. 2003;45(4):450–460. doi: 10.1016/s0028-3908(03)00229-6. [DOI] [PubMed] [Google Scholar]
- [47].Zhang P, Lisman JE. Activity-dependent regulation of synaptic strength by PSD-95 in CA1 neurons. J Neurophysiol. 2012;107(4):1058–1066. doi: 10.1152/jn.00526.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Zeng HC, Li YY, Zhang L, et al. Prenatal exposure to Perfluorooctanesulfonate in rat resulted in long-lasting changes of expression of synapsins and synaptophysin. Synapse. 2011;65(3):225–233. doi: 10.1002/syn.20840. [DOI] [PubMed] [Google Scholar]
- [49].The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals 2006-09-30 [Google Scholar]
- [50].Huang TF, Chen YT, Yang TH, et al. Isolation and characterization of mesenchymal stromal cells from human anterior cruciate ligament. Cytotherapy. 2008;8:806–814. doi: 10.1080/14653240802474323. [DOI] [PubMed] [Google Scholar]
- [51].Reyes M, Verfaillie CM. Characterization of multipotent adult progenitor cells, a subpopulation of mesenchymal stem cells. Ann N Y Acad Sci. 2001;938:231. doi: 10.1111/j.1749-6632.2001.tb03593.x. [DOI] [PubMed] [Google Scholar]
- [52].Sarugaser R, Ennis J, Stanford WL, et al. Isolation, propagation, and characterization of human umbilical cord perivascular cells (HUCPVCs) Methods Mol Biol. 2009;482:269–279. doi: 10.1007/978-1-59745-060-7_17. [DOI] [PubMed] [Google Scholar]
- [53].Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- [54].Kang SK, Putnam LA, Ylostalo J, et al. Neurogenesis of Rhesus adipose stromal cells. J Cell Sci. 2004;117(Pt 18):4289–4299. doi: 10.1242/jcs.01264. [DOI] [PubMed] [Google Scholar]
- [55].Bisping G, Kropff M, Wenning D, et al. Targeting receptor kinases by a novel indolinone derivative in multiple myeloma: abrogation of stroma-derivedinterleukin-6 secretion and induction of apoptosis in cytogenetically defined subgroups. Blood. 2006;107(5):2079–2089. doi: 10.1182/blood-2004-11-4250. [DOI] [PubMed] [Google Scholar]
- [56].Sahoo S, Ang LT, Cho-Hong Goh J, et al. Bioactive nanofibers for fibroblastic differentiation of mesenchymal precursor cells forligament/tendon tissue engineering applications. Differentiation. 2010;79(2):102–110. doi: 10.1016/j.diff.2009.11.001. [DOI] [PubMed] [Google Scholar]
- [57].Chen Q, Fu Y, Wang X, et al. The study on growth and proliferation of neural stem cells from rats in vitro. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2008;22(16):747–750. [PubMed] [Google Scholar]


