Abstract
In this study, we aimed to explore the role of ursolic acid in the neural regeneration of the injured sciatic nerve. BALB/c mice were used to establish models of sciatic nerve injury through unilateral sciatic nerve complete transection and microscopic anastomosis at 0.5 cm below the ischial tube-rosity. The successfully generated model mice were treated with 10, 5, or 2.5 mg/kg ursolic acid via intraperitoneal injection. Enzyme-linked immunosorbent assay results showed that serum S100 protein expression level gradually increased at 1–4 weeks after sciatic nerve injury, and significantly decreased at 8 weeks. As such, ursolic acid has the capacity to significantly increase S100 protein expression levels. Real-time quantitative PCR showed that S100 mRNA expression in the L4–6 segments on the injury side was increased after ursolic acid treatment. In addition, the muscular mass index in the soleus muscle was also increased in mice treated with ursolic acid. Toluidine blue staining revealed that the quantity and average diameter of myelinated nerve fibers in the injured sciatic nerve were significantly increased after treatment with ursolic acid. 10 and 5 mg/kg of ursolic acid produced stronger effects than 2.5 mg/kg of ursolic acid. Our findings indicate that ursolic acid can dose-dependently increase S100 expression and promote neural regeneration in BALB/c mice following sciatic nerve injury.
Keywords: neural regeneration, traditional Chinese medicine, ursolic acid, triterpenoid, sciatic nerve, peripheral nerve injury, S100, muscular atrophy, nerve myelin sheath, grants-supported paper, neuroregeneration
Research Highlights
(1) Ursolic acid has anti-oxidative, anti-inflammatory, anti-apoptotic, and anti-scarring effects, and it regulates the immune system and promotes the repair of injured neurons. However, no reports have explored its role in peripheral nerve injury.
(2) This study is the first to demonstrate a role of ursolic acid in repair and regeneration following peripheral nerve injury. Ursolic acid promoted the regeneration of injured nerve myelin sheaths and reconstructed muscular functions. All experimental findings provide favorable evidence for the ap-plication of ursolic acid following peripheral nerve injury.
INTRODUCTION
Neural regeneration following peripheral nerve injuries has attracted increasing attention from neurosurgical scholars. Although surgery and drug therapy are the two most commonly used treatment schemes, neither achieves precise effects. Growing evidence has confirmed that traditional Chinese medicine and its extracts can stimulate nerve growth factor expression and Schwann cell proliferation after nerve injury, thus promoting neural regeneration and functional recovery[1,2,3].
Ursolic acid (chemical name 3-hydroxy-12-ursen-28-oic acid) is a triterpenoid extracted from natural plant-based drugs, with a formula of C30H48O3 and molecular weight of 456.68. Its structural formula is shown in Figure 1.
Figure 1.
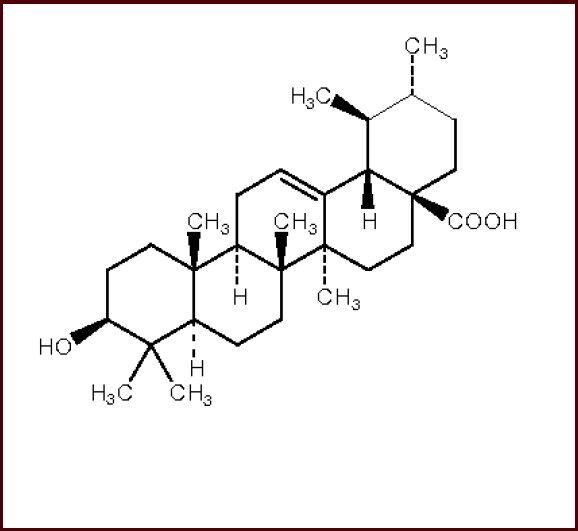
Structure formula of ursolic acid.
Ursolic acid has anti-cancer, anti-tumor, and anti-inflammatory effects. It also treats diabetes mellitus[4,5] and has neuroprotective effects[5,6]. In the central nervous system, Taviano et al[6] showed that ursolic acid antagonized Nepeta-induced injuries, protected excitability, and relieved Nepeta-induced depression. Shih et al[7] showed that kainic acid is excitotoxic to hippocampal neurons, and ursolic acid protects and maintains hippocampal neurons in a normal state. Pemminati et al[8] reported that ursolic acid can relax nerves and long-term use can alleviate haloperidol-induced catalepsy. Kunkel et al[9] found that ursolic acid extracted from apple peel could prevent muscular atrophy caused by muscle damage or malnutrition, indicating that ursolic acid can promote the repair of peripheral nerves in muscle tissue and provide nutrition. These evidences suggest that ursolic acid can repair damaged neurons and promote neural regeneration after peripheral nerve injury.
In this study, we observed nerve myelin sheath loss, muscular atrophy, and changes of S100 protein and mRNA expression in the peripheral blood and L4–6 segments of the vertebral column in BALB/c mice with peripheral nerve injury following different dose ursolic acid intervention. The injury model was produced through complete transection and microscopic anastomosis of the unilateral sciatic nerve. The aim of this study was to explore the promoting effects of ursolic acid on neural regeneration following peripheral nerve injury.
RESULTS
Quantitative analysis of experimental animals
One hundred and sixty mice were included in this study. They underwent unilateral sciatic nerve transection and microscopic anastomosis at 0.5 cm below ischial tuberosity. The mice with sciatic nerve injuries were equally and randomly divided into four groups: a model group (rats were injected intraperitoneally with physiological saline) and high-, medium- and low-dose ursolic acid groups (rats were injected intraperitoneally with 10, 5, 2.5 mg/kg ursolic acid). Ten mice were selected at 1, 2, 4, and 8 weeks after injury and 160 mice were involved in the final analysis.
Ursolic acid increased serum levels of S100 in mice with sciatic nerve injury
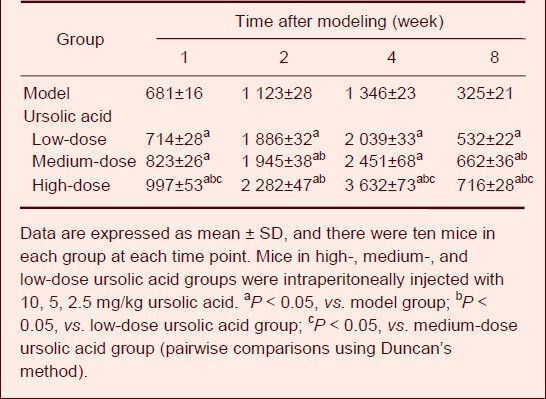
Enzyme-linked immunosorbent assay results showed that serum S100 protein concentrations gradually increased after sciatic nerve injury and peaked at 4 weeks. They then significantly decreased at 8 weeks. Compared with the model group, serum levels of S100 significantly increased after high-, medium- and low-dose ursolic acid interventions (P < 0.05), and the high dose engendered the strongest effects (Table 1).
Table 1.
Effect of ursolic acid on serum S100 concentrations (μg/L) in mice following sciatic nerve injury (enzyme-linked immunosorbent assay)
Ursolic acid promoted S100 mRNA expression in the L4–6 spinal segments of mice with sciatic nerve injury
Real-time quantitative PCR analysis showed that in the model and low-dose ursolic acid groups S100 mRNA expression in the L4–6 segments of the spinal cord gradually increased after sciatic nerve injury and peaked at 4 weeks, and then significantly decreased at 8 weeks. Compared with the model group, S100 mRNA expression in the high- and medium-dose ursolic acid groups was significantly higher and peaked at 1 week (P < 0.05), especially in the high-dose ursolic acid group (Figure 2).
Figure 2.
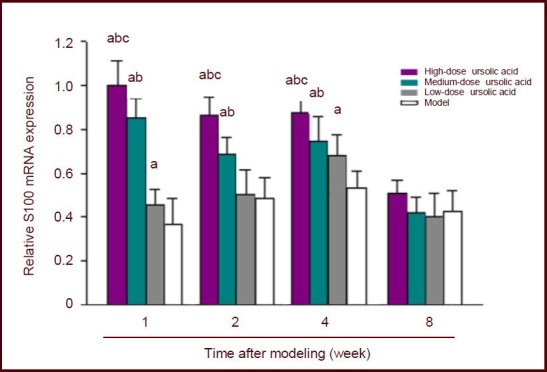
Effects of ursolic acid on S100 mRNA expression in L4–6 spinal segments of mice following sciatic nerve injury (real-time quantitative PCR).
Data (fluorescent density [dRn] of S100 to GAPDH) are expressed as mean ± SD, and there were five mice in each group at each time point. Mice in high-, medium-, and low-dose ursolic acid groups were intraperitoneally injected with 10, 5, 2.5 mg/kg ursolic acid. aP < 0.05, vs. model group; bP < 0.05, vs. low-dose ursolic acid group; cP < 0.05, vs. medium-dose ursolic acid group (pairwise comparisons were performed using Duncan's method).
Ursolic acid promoted remyelination in mice following sciatic nerve injury
After sciatic nerve injury, toluidine blue staining showed disordered myelin sheaths, significantly proliferated fibrous connective tissue, and slow neural regeneration that did not change significantly over time. Compared with the model group, neural regeneration was more apparent in different dose ursolic acid groups at each time point, especially the high- and medium-dose groups (Figure 3).
Figure 3.
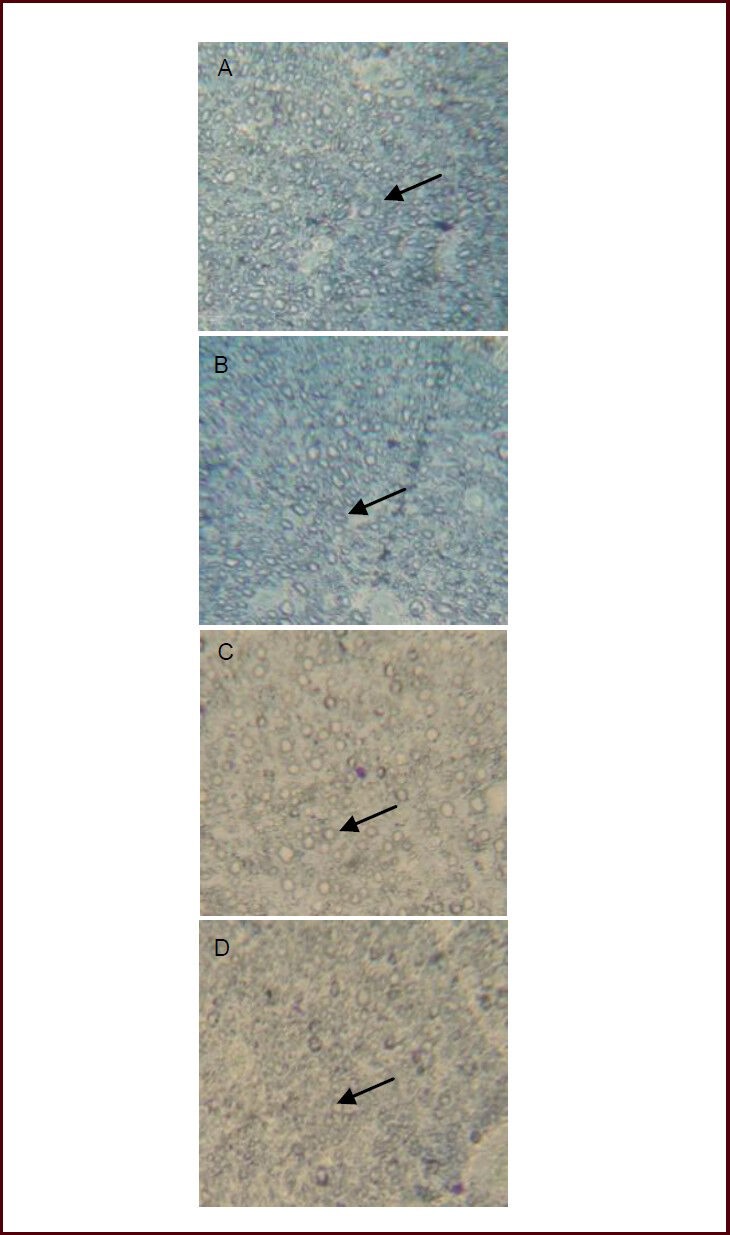
Effects of ursolic acid on neural regeneration in mice following sciatic nerve injury (toluidine blue staining, × 400). Arrows indicate the myelin sheath.
At 8 weeks, as seen under the light microscope, the myelin sheath was disordered and there was proliferated fibrous connective tissue in the model group (D); the high- (A; 10 mg/kg) and medium-dose (5 mg/kg) ursolic acid groups (B) showed regular arrangement of the sheath myelin, at uniform thickness and clearly visible boundary, and no fibrous tissue hyperplasia; in the low-dose (2.5 mg/kg) ursolic acid group (C), the morphology and thickness of myelin sheath were irregular, and its outline was still clear, hyperplasia of connective tissue was apparent.
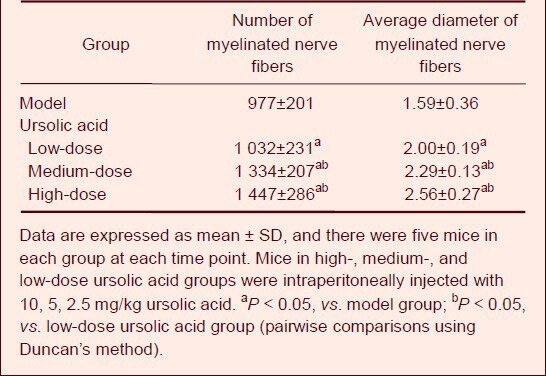
Image analysis showed that the numbers and average diameters of myelinated nerve fibers in the high- and medium-dose ursolic acid groups were significantly higher than in the low-dose ursolic acid and model groups (P < 0.05). Moreover, the low-dose ursolic acid group showed better effects than the model group (P < 0.05; Table 2).
Table 2.
Effects of ursolic acid on the quantity (n/mm2) and average diameter (μm) of myelinated nerve fiber in mice at 8 weeks after sciatic nerve injury
Ursolic acid increased soleus muscle mass in mice following sciatic nerve injury
At 8 weeks, the muscular mass index in soleus muscle was significantly higher in the high-, medium-, and low-dose ursolic acid groups compared with model group (P < 0.05), and the high- and medium-dose ursolic acid groups showed higher indices (P < 0.05; Figure 4) than the low-dose ursolic acid group.
Figure 4.
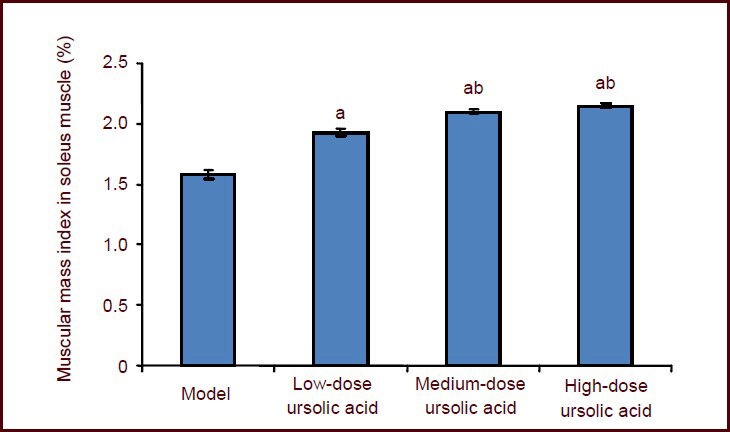
Effects of ursolic acid on soleus muscle atrophy in mice at 8 weeks after sciatic nerve injury.
Data are expressed as mean ± SD, and there were ten mice in each group. Mice in high-, medium-, and low-dose ursolic acid groups were injected intraperitoneally with 10, 5, 2.5 mg/kg ursolic acid. aP < 0.05, vs. model group; bP < 0.05, vs. low-dose ursolic acid group (pairwise comparisons using Duncan's method).
Muscular mass index = soleus muscle mass/body mass × 100%.
DISCUSSION
It is widely recognized that the peripheral nerve is an immune-privileged site, as because of the blood-nerve barrier, lymphocytes and antibodies cannot enter the nervous parenchyma, and no antigen-presenting cells and major histocompatibility complex II has been detected. In 1978, Saida et al[10] first proposed that, after peripheral nerve injury, the blood-nerve barrier in the perineurium and intima was destroyed, resulting in leakage of nerve antigens and invasion into blood circulation, where neurologic antigens induce immune responses. Subsequently, growing evidence has supported this proposal. Schwartz et al[11] detected anti-myelin antibodies and anti-ganglioside antibodies in peripheral blood of rats following sciatic nerve injury. Ansselin et al[12] found major histocompatibility complex expression and T lymphocyte aggregation following nerve transplantation. After in vitro cultured Schwann cells were induced with interferon-γ, it expressed the major histocompatibility complex class II antigens and activates T cells through antigen presentation, which disagrees with previous reports insisting that the peripheral nerve is an immune-privileged site. Subsequent to peripheral nerve injury, the induced immune response may inhibit nerve repair and regeneration, as the local immune response intensity is proportional to the severity of neurological impairment, with stronger immune response predicting more severe damage and poor neurological regeneration and functional recovery[13].
The peripheral nervous system, similar to other tissue, has an anatomical environment used to generate immune responses. A vascular network within nerve parenchyma contains immune response factors, such as antibodies, immune cells, and plasma proteins. Because of the existence of the blood-nerve barrier, the aforementioned immune response factors cannot enter nervous tissue.
In our study, we speculated that peripheral nerve blood-nerve barrier damage is caused by the following factors: nerve traction injury[14], nerve compression injury[15], chronic entrapment injury[16], traumatic nerve injury[17], clinical surgery or nerve graft[18], and other physical and chemical factors[19]. When the blood-brain barrier is damaged or neurological dysfunction occurs, the immune response factors in blood circulation enter nerve parenchyma and induce a series of immune responses after injury. Ansselin et al[12] also found marked T lymphocyte aggregation after peripheral nerve injury, indicating that nerve injury may trigger cellular immune responses. The interferon-γ-activated T lymphocytes can cross through the blood-nerve barrier and further trigger allergic neuritis, and some cytokines may also produce immune reactions in peripheral nerve cells even when they remain undamaged[20].
After peripheral nerve injury, the resultant Wallerian degeneration causes macrophage accumulation, and macrophages secrete interleukin-1 after activation of phagocytosis nerve antigen. Then, interleukin-1 activates T cells and T cells proliferate to produce interleukin-2. Interleukin-2 acts on T and B lymphocytes, triggering cellular and humoral immune responses. Interleukin-1β can stimulate macrophages and Schwann cells in nerve parenchyma to secrete interleukin-1 and to exacerbate immune responses. Interleukin-4 can induce macrophage expression of major histocompatibility complex class II antigens and enhance their phagocytosis. In contrast, interleukin-10 inhibits macrophage expression of major histocompatibility complex II antigens after nerve injury[21].
The emergency of nerve immune responses allows for increasing attention on the role of immunosuppressants in the nervous system and their application in experimental research and clinical treatment of neurological diseases. In 1994, Gold et al[22] found that the immunosuppressant tacrolimus can improve axonal regeneration rates after sciatic nerve crush injury, and promote the recovery of nerve morphology and functions. After pre-denaturated rats were transplanted with autologous spinal cord into the tibial nerve, administration of tacrolimus promoted axonal growth and rubrospinal tract regeneration[23]. Tacrolimus also promoted in vitro axonal growth in cultured PC12 cells and dorsal ganglion neurons[24]. Furthermore immunosuppressants such as tacrolimus, cyclosporin A and rapamycin were involved in the repair and regeneration of injured peripheral neurons[25]. Jost et al[26] found that tacrolimus was superior to cyclosporine in promoting neural regeneration and repair. Tacrolimus's neurotrophic effects were associated with the dose used[27], and 5 mg/kg was the optimal dose. In the rats with sciatic nerve crush injuries, delayed (9–17 days after nerve injury) and immediate (0–8 days) administration of tacrolimus had the same effects[28], so tacrolimus treatment is insensitive to timing, thus expanding the range of clinical indications.
Short-term systemic tacrolimus intervention may promote the functional recovery of hind limbs after peripheral nerve injury[29]. After rats with brachial plexus nerve root avulsion injuries were replanted with nerve roots and treated with tacrolimus, the biceps muscle action potential, wet weight, and other indicators were significantly higher than in the blank control group[30]. However, immunosuppressive agents produce adverse reactions including nervous system disorders[31]. Animal toxicology studies have shown that different doses of tacrolimus may cause focal cerebral spinal meningitis, optic neuritis, radiculitis, tremor, and ataxia[32]. Organ transplant patients present with insomnia, tremor, headache, photophobia, clouding of consciousness, epileptic seizures, coma, or articulatory disorders after treatment with tacrolimus[33,34]. This evidence suggests that tacrolimus and other immunosuppressive agents have a two-sided effect on the nervous system, both neurotrophic and neurotoxic. The balance between these two roles may depend on drug dose, duration, and other unknown factors, thus requiring further studies.
Immunosuppressive natural drugs are characterized by few side effects and complex mechanisms, with an underlying mechanism associated with the immune system and neuroendocrine immune regulation networks[35]. A series of efficient, low-toxicity Chinese herbs have been regarded as potential immunosuppressants[36,37,38], such as ursolic acid. In our experiments, mice with sciatic nerve injury were treated with ursolic acid and observed for 8 weeks. Enzyme-linked immunosorbent assay and real-time quantitative PCR results showed that S100 protein in serum and spinal cord L4–6 segments was activated and expressed in the high- and medium-dose ursolic acid groups compared with low-dose ursolic acid group and model group, and the expression was sustained for 2 weeks. S100 protein family is an acidic calcium-binding protein consisting of 16 members. It only contains amino acid sequences that are highly conserved in vertebrates[39,40,41], and most of them serve as a calmodulin and target protein[42]. After calcium ions bind, the S100 protein undergoes conformational changes, exposing the target protein binding site, and then acts with the corresponding target proteins to produce biological effects[43,44,45,46,47,48,49,50]. S100 protein concentrations in biological fluids can be used as a marker of functional activation after nerve injury[51,52,53,54]. Nerve injury may trigger the repair system in vivo, including activation of S100 protein overexpression and promotion of neural regeneration[55,56,57,58]. In our experiments, S100 protein and mRNA expression levels were significantly increased after treatment with ursolic acid at the high- and medium-dose, suggesting that ursolic acid can activate S100 protein overexpression, thus promoting neural regeneration.
The toluidine blue staining results showed that the number, integrity, and thickness of the myelin sheaths increased during the regeneration of the damaged nerves. The number of myelinated nerve fibers and their average diameter in the high- and medium-dose ursolic acid groups were significantly higher than in the low-dose ursolic acid and model groups, and the low-dose ursolic acid group showed stronger effects than the model group. This is evidence that ursolic acid can strongly promote the growth of damaged nerve myelin. Ursolic acid at different dosages could significantly promote S100 protein expression and there were significant differences compared with the model group. Nerve injury is accompanied with partial muscle atrophy. When nerve regeneration occurs and neurological functions recover, muscle atrophy also improves. Our experimental findings showed that soleus muscle cells in the high- and medium-dose ursolic acid groups were in good physiological condition, and the recovery from muscle atrophy was significantly stronger than in the low-dose ursolic acid and model groups. These results suggest that nerve regeneration and functional recovery were improved following ursolic acid intervention. In addition, after ursolic acid given for 8 weeks, S100 mRNA expression levels showed no significant differences in the high- and medium-dose ursolic acid groups compared with low-dose ursolic acid and model groups, whereas myelinated nerve and recovery of muscular atrophy showed significant differences. These data suggested that nerve repair and regeneration have been completed at 8 weeks, and then S100 expression levels decreased, which was close to what occurring in the low-dose ursolic acid and model groups, and nerves and muscles had undergone good recovery.
Repair and regeneration after nerve injury is a complex process involving inflammation, adhesion, synergistic effects of the extracellular matrix, regulation of neurotrophic factors, neurotransmitter synthesis and release, growth cone formation and extension, and neural regeneration[59,60,61,62]. Nerve injury leads to Wallerian degeneration in nerve fibers. After neuronal cell bodies and axons appear to fracture, nerve fibers at the distal end and some axons and the myelin sheath at the proximal end will gradually rupture, disintegrate, and undergo phagocytosis. Nerve injury elicits immune responses that aggravate Wallerian degeneration, resulting in secondary nerve injury and hindering nerve repair and regeneration after injury. Meanwhile, excessive immune responses may trigger the expression of inflammatory mediators surrounding the injured tissue, and promote local scar formation and hyperplasia, which directly affects conduction recovery after nerve injury[63]. Excessive immune inflammatory response will inhibit S100 protein expression[51]. Therefore, we suggest that it is reasonable to apply immunosuppressive agents to regulate inflammatory responses, which will reduce scar formation after nerve injury and provide a favorable external environment for nerve repair and regeneration.
Ursolic acid is a pentacyclic triterpenoid that is widely found in bear berry, Ilex rotunda Thunb and other natural medicines. Ursolic acid has antioxidant and anti-inflammatory effects, promotes tumor cell apoptosis, and regulates immune functions[64,65], as well as having anti-viral and anti-scarring effects[66,67]. Our findings showed that ursolic acid promoted S100 protein expression in a dose-dependent manner after peripheral nerve injury in mice. Toluidine blue staining revealed that myelin sheath recovery after ursolic acid interventions was significantly better than in the model group. The assessments of soleus muscle mass index showed that ursolic acid reduced muscle atrophy after injury. Thus, ursolic acid may inhibit inflammatory immune response following nerve injury, thereby promoting sustained expression of S100 in spinal cord cells, reducing scar proliferation, preventing nerve demyelination, and providing favorable conditions for nerve repair and regeneration. However, we did not detect local immune responses after injury and this needs further confirmation. In addition, growth associated protein 43 expression and inflammation-related cell signaling pathways also require more attention.
MATERIALS AND METHODS
Design
A randomized, controlled animal experiment.
Time and setting
Experiments were performed in Experimental Animal Center, Norman Bethune College of Medicine, Jilin University, China, from June to August in 2011.
Materials
One hundred and sixty healthy BALB/c male mice, aged 4–6 weeks, weighing 20 ± 2 g, were provided by the Experimental Animal Center of Jilin University, China (license No. SCXK (Ji) 2007-0003). All mice were housed in a breeding room and allowed free access to water and food. All procedures were in accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals, formulated by the Ministry of Science and Technology of China[68].
Methods
Establishment of a sciatic nerve injury model in mice
Mice were intraperitoneally anesthetized with 1% thiopental (100 mg/kg) and fixed in the prone position. After the surgical site was disinfected, a 1.5 cm longitudinal incision was made on the posterior femur of a unilateral lower limb. Then, the sciatic nerve starting at the lower edge of the piriformis muscle was bluntly exposed, the surrounding tissues were stripped, and the sciatic nerve was transected at 0.5 cm below the ischial tuberosity. Five minutes later, sciatic nerve anastomosis was performed under a 100-fold stereo microscope (Nikon SM645S, Tokyo, Japan). The fascia, subcutaneous tissue, and skin wound were sutured.
Drug interventions
Ursolic acid was heated and dissolved in a 0.9% NaCl solution (purity > 90%; Aldrich U6753, Sigma, St. Louis, MO, USA). Mice in the high-, medium- and low-dose ursolic acid groups were injected intraperitoneally with 10, 5, 2.5 mg/kg ursolic acid per day[69], until specimen collection. The model group were given 25 mL/kg of 0.9% NaCl solution.
Enzyme-linked immunosorbent assay of serum S100 protein content in mice following sciatic nerve injury
At 1, 2, 4, and 8 weeks after sciatic nerve injury, ten mice in each group were selected to harvest eyeball blood samples. Blood samples were placed in sterile EP tube at room temperature for 2 hours, centrifuged at 5 000 r/min for 3 minutes, and the supernatant was collected. Serum S100 protein contents were measured using enzyme-linked immunosorbent assay in strict accordance with kit instructions (mouse (S-100) enzyme-linked immunosorbent assay kit 96T/48T, KB3773; Kaibo, Shanghai, China). Absorbance at 490 nm was measured and standard curve was plotted.
Real-time quantitative PCR assay of S100 mRNA expression in L4–6 spinal segments of mice with sciatic nerve injury
At 1, 2, 4, and 8 weeks after sciatic nerve injury, five mice in each group were intraperitoneally anesthetized with 1% sodium thiopental (100 mg/kg), and the sciatic nerve was cut and isolated, the vertebral plate was bitten to expose the spinal segments, and the L4–6 segments on the ipsilateral side to the injury were cut (Figure 5).
Figure 5.
Spinal segments associated with the sciatic nerve.
A longitudinal incision, 1.5 cm long, was made on the posterior femur in unilateral lower limbs. Then, the sciatic nerve at the lower edge of the piriformis muscle was bluntly exposed and isolated. The vertebral plate was bitten and spinal segments associated with the sciatic nerve were exposed.
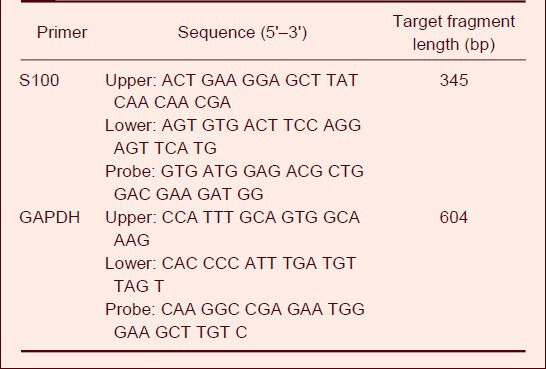
Total RNA was extracted using TRIzol reagent according to the total RNA extraction kit (Invitrogen, Carlsbad, CA, USA), and reversely transcribed into cDNA for real-time quantitative PCR amplification. PCR reaction contained 40 cycles of 95°C for 30 seconds, 58°C for 60 seconds, and 72°C for 60 seconds. 2 × SYBR real-time PCR kit was purchased from Roche (Shanghai, China). S100 primer sequences were designed with Beacon Designer™ 7 software (PREMIER Biosoft, Palo Alto, CA, USA) according to NCBI GenBank. GAPDH served as a reference (Table 3).
Table 3.
S100 and GAPDH primer sequences
Toluidine blue staining of sciatic nerve myelin changes in mice
At 1, 2, 4, and 8 weeks after sciatic nerve injury, five mice in each group were anesthetized, and neural stems 0.5 cm away from sciatic nerve anastomosis was resected and fixed in formalin for 72 hours, dehydrated in ethanol and paraffin embedded, cut into 3-μm-thick sections, then dewaxed and rinsed three times with distilled water. Toluidine blue staining: specimens were soaked in 0.1% toluidine blue solution (0.1 g toluidine blue was dissolved in 100 mL of 0.1 mol/L acetate buffer) for 10 minutes, dehydrated in ethanol, put through xylene transparency, and mounted with neutral gum. Specimens were observed under a microscope (Eclipse TE-2000-U, Nikon, equipped with an attached camera, digital camera SXM1200F) and the number of myelinated nerve fibers and their average diameter were measured.
Determination of muscular mass index in the soleus muscle
At 8 weeks after sciatic nerve injury, the triceps surae were cut and weighed on an electronic analytical balance (FA214, Haikang Shanghai Scientific Instrument Co., Ltd., Shanghai, China). The muscular mass index in the soleus muscle was calculated with the Cuadros method, according to the formula: muscle mass / body mass × 100%[70].
Statistical analysis
All data are expressed as mean ± SD and were analyzed using SPSS 12.0 software (SPSS, Chicago, IL, USA). Multi-group comparisons were performed using one-way analysis of variance, and pairwise comparisons using Duncan's method. A P < 0.05 level was considered statistically significant.
Footnotes
Funding: This study was financially sponsored by the Science and Technology Ministry of Jilin Province, No. 200705318.
Conflicts of interest: None declared.
Ethical approval: This study was approved by the Animal Ethics Committee of Jilin University, China.
(Reviewed by Murnane K, Norman C, Meng QG, Ge JZ)
(Edited by Wang LM, Yang Y, Li CH, Song LP, Liu WJ, Zhao M)
REFERENCES
- [1].Qiu D, Zhao G, Aoki Y, et al. Immunosuppressant PG490 (triptolide) inhibits T-cell interleukin-2 expression at the level of purine-box/nuclear factor of activated T-cells and NF-kappaB transcriptional activation. J Biol Chem. 1999;274(19):13443–13450. doi: 10.1074/jbc.274.19.13443. [DOI] [PubMed] [Google Scholar]
- [2].Xue B, Jiao J, Zhang L, et al. Triptolide upregulates NGF synthesis in rat astrocyte cultures. Neurochem Res. 2007;32(7):1113–1119. doi: 10.1007/s11064-006-9253-1. [DOI] [PubMed] [Google Scholar]
- [3].Ye M, Xie WD, Lei F, et al. Brazilein, an important immunosuppressive component from Caesalpinia sappan L. Int Immunopharmacol. 2006;6(3):426–432. doi: 10.1016/j.intimp.2005.09.012. [DOI] [PubMed] [Google Scholar]
- [4].Ikeda Y, Murakami A, Ohigashi H. Ursolic acid: an anti- and pro-inflammatory triterpenoid. Mol Nutr Food Res. 2008;52(1):26–42. doi: 10.1002/mnfr.200700389. [DOI] [PubMed] [Google Scholar]
- [5].Sultana N. Clinically useful anticancer, antitumor, and antiwrinkle agent, ursolic acid and related derivatives as medicinally important natural product. J Enzyme Inhib Med Chem. 2011;26(5):616–642. doi: 10.3109/14756366.2010.546793. [DOI] [PubMed] [Google Scholar]
- [6].Taviano MF, Miceli N, Monforte MT, et al. Ursolic acid plays a role in Nepeta sibthorpii Bentham CNS depressing effects. Phytother Res. 2007;21(4):382–385. doi: 10.1002/ptr.2076. [DOI] [PubMed] [Google Scholar]
- [7].Shih YH, Chein YC, Wang JY, et al. Ursolic acid protects hippocampal neurons against kainate-induced excitotoxicity in rats. Neurosci Lett. 2004;362(2):136–140. doi: 10.1016/j.neulet.2004.03.011. [DOI] [PubMed] [Google Scholar]
- [8].Pemminati S, Gopalakrishna HN, Varma A, et al. Effect of chronic administration of ursolic acid on haloperidol induced catalepsy in albino mice. Drug Invention Today. 2011;3(6):83–85. [Google Scholar]
- [9].Kunkel SD, Suneja M, Ebert SM, et al. mRNA expression signatures of human skeletal muscle atrophy identify a natural compound that increases muscle mass. Cell Metab. 2011;13(6):627–638. doi: 10.1016/j.cmet.2011.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Saida T, Saida K, Silberberg DH, et al. Transfer of demyelination by intraneural injection of experimental allergic neuritis serum. Nature. 1978;272(5654):639–641. doi: 10.1038/272639a0. [DOI] [PubMed] [Google Scholar]
- [11].Schwartz M, Sela BA, Eshhar N. Antibodies to gan-gliosid and myelin autoantigens are produced in mice following sciatic nerve injury. J Neurochem. 1982;38(5):1192–1195. doi: 10.1111/j.1471-4159.1982.tb07890.x. [DOI] [PubMed] [Google Scholar]
- [12].Ansselin AQ, Pollard JD. Immunopathological factors in peripheral nerve allograft rejection: quantification of lymphocyte invasion and major histocompatility complex expression. J Neurol Sci. 1990;96(1):75–88. doi: 10.1016/0022-510x(90)90058-u. [DOI] [PubMed] [Google Scholar]
- [13].Pei FX, Yang ZM, Huang FG, et al. Study of immunoreaction and regeneration of peripheral nerve following trauma. Zhonghua Xianwei Waike Zazhi. 1995;18(2):146–148. [Google Scholar]
- [14].DeLano H, Fun FY, Zinreich SJ. Relationship of the optic nerve to the posterior paranasal sinuses: A CT anatomic study. AJNR Am J Neuroradiol. 1996;17(4):669–675. [PMC free article] [PubMed] [Google Scholar]
- [15].Kainz J, Stammberger H. Danger areas of the posterior rhinobasis. An endoscopic and anatomical-surgical study. Acta Otolaryngol. 1992;112(5):852–861. doi: 10.3109/00016489209137484. [DOI] [PubMed] [Google Scholar]
- [16].Uemura T, Iisaka Y, Kazuno T, et al. Optic canal decompression--the significance of the simultaneous optic canal sheath incision (author's transl) Neurol Med Chir (Tokyo) 1978;18(2 Pt 2):151–157. doi: 10.2176/nmc.18pt2.151. [DOI] [PubMed] [Google Scholar]
- [17].Frattini F, Lopes FR, Almeida FM, et al. Mesenchymal stem cells in a polycaprolactone conduit promote sciatic nerve regeneration and sensory neuron survival after nerve injury. Tissue Eng Part A. 2012;18(19-20):2030–2039. doi: 10.1089/ten.TEA.2011.0496. [DOI] [PubMed] [Google Scholar]
- [18].Willis CL, Leach L, Clarke GJ, et al. Reversible disruption of tight junction complexes in the rat blood-brain barrier, following transitory focal astrocyte loss. Glia. 2004;48(1):1–13. doi: 10.1002/glia.20049. [DOI] [PubMed] [Google Scholar]
- [19].Kanda T, Yamawaki M, Mizusawa H. Sera from Guillain-Barré patients enhance leakage in blood-nerve barrier model. Neurology. 2003;60(2):301–306. doi: 10.1212/01.wnl.0000041494.70178.17. [DOI] [PubMed] [Google Scholar]
- [20].Kanda T. Review: Biology of the blood-nerve barrier and its alteration in immune mediated neuropathies. J Neurol Neurosurg Psychiatry. 2013;84(2):208–212. doi: 10.1136/jnnp-2012-302312. [DOI] [PubMed] [Google Scholar]
- [21].Smith ME, McFarlin DE, Dhib-Jalbut S. Differential effect of interleukin-1 beta on Ia expression in astrocytes and microglia. J Neuroimmunol. 1993;46(1-2):97–104. doi: 10.1016/0165-5728(93)90238-t. [DOI] [PubMed] [Google Scholar]
- [22].Gold BG, Storm-Dickerson T, Austin DR. The immunosuppressant FK506 increases functional recovery and nerve regeneration following peripheral nerve injury. Restor Neurol Neurosci. 1994;6(4):287–296. doi: 10.3233/RNN-1994-6404. [DOI] [PubMed] [Google Scholar]
- [23].Wang MS, Gold BG. FK506 increases the regeneration of spinal cord axons in a predegenerated peripheral nerve autograft. J Spinal Cord Med. 1999;22(4):287–296. doi: 10.1080/10790268.1999.11719582. [DOI] [PubMed] [Google Scholar]
- [24].Lyons WE, George EB, Dawson TM, et al. Immunosuppressant FK506 promotes neurite outgrowth in cultures of PC12 cells and sensory ganglia. Proc Natl Acad Sci U S A. 1994;91(8):3191–3195. doi: 10.1073/pnas.91.8.3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Sabatini DM, Lai MM, Snyder SH. Neural roles of immunophilins and their ligands. Mol Neurobiol. 1997;15(2):223–239. doi: 10.1007/BF02740635. [DOI] [PubMed] [Google Scholar]
- [26].Jost SC, Doolabh VB, Mackinnon SE, et al. Acceleration of peripheral nerve regeneration following FK506 administration. Restor Neurol Neurosci. 2000;17(1):39–44. [PubMed] [Google Scholar]
- [27].Wang MS, Zeleny-Pooley M, Gold BG. Comparative dose-dependence study of FK506 and cyclosporine A on the rate of axonal regeneration in the ratsciatic nerve. J Pharmacol Exp Ther. 1997;282(2):1084–1093. [PubMed] [Google Scholar]
- [28].Gold BG, Gordon HS, Wang MS. Efficacy of delayed or discontinuous FK506 administrations on nerve regeneration in the rat sciatic nerve crush model: lack of evidence for a conditioning lesion-like effect. Neurosci Lett. 1999;267(1):33–36. doi: 10.1016/s0304-3940(99)00333-x. [DOI] [PubMed] [Google Scholar]
- [29].Chen GF, Gu LQ. FK506 accelerates functional recovery following repair of peripheral nerve injury. Zhonghua Shou Waike Zazhi. 2002;18(2):51–53. [Google Scholar]
- [30].Xia PG, Gu LQ, Lin JM, et al. FK506 promotes nerve regeneration after nerve root replants to spinal cord. Guangdong Yaoxueyuan Xuebao. 2001;17(3):213–215. [Google Scholar]
- [31].Bösmüller C, Ollinger R, Sieb M, et al. Tacrolimus monotherapy following alemtuzumab induction in combined kidney-pancreas transplantation: Results of a prospective randomized trial. Ann Transplant. 2012;17(4):45–51. doi: 10.12659/aot.883693. [DOI] [PubMed] [Google Scholar]
- [32].Qin XL, Yu T, Li LJ, et al. Effect of long-term co-administration of Wuzhi tablet (Schisandra sphenanthera extract) and prednisone on the pharmacokinetics of tacrolimus. Phytomedicine. 2013;20(3-4):375–379. doi: 10.1016/j.phymed.2012.11.008. [DOI] [PubMed] [Google Scholar]
- [33].Kuypers DR, Peeters PC, Sennesael JJ, et al. Improved adherence to tacrolimus once-daily formulation in renal recipients: a randomized controlled trial using electronic monitoring. Transplantation. 2013;95(2):333–340. doi: 10.1097/TP.0b013e3182725532. [DOI] [PubMed] [Google Scholar]
- [34].Ghodsizad A, Koch A, Ungerer MN, et al. Immunosuppression with tacrolimus early after orthotopic heart transplantation: a comparison of prograf and advagraf. Heart Surg Forum. 2012;15(6):E307–309. doi: 10.1532/HSF98.20111145. [DOI] [PubMed] [Google Scholar]
- [35].Yang GZ. The trend of traditional Chinese medicine immunology. Zhongguo Zhongxiyi Jiehe Zazhi. 1999;11(5):4–5. [Google Scholar]
- [36].Lee HF, Lee TS, Kou YR. Anti-inflammatory and neuroprotective effects of triptolide on traumatic brain injury in rats. Respir Physiol Neurobiol. 2012;182(1):1–8. doi: 10.1016/j.resp.2012.01.016. [DOI] [PubMed] [Google Scholar]
- [37].Yang EB, Zhao YN, Zhang K, et al. Daphnetin, one of coumarin derivatives, is a protein kinase inhibitor. Biochem Biophys Res Commun. 1999;260(3):682–685. doi: 10.1006/bbrc.1999.0958. [DOI] [PubMed] [Google Scholar]
- [38].Niu SC, Zhang XP, Yang CH, et al. Therapeutic effect of Qufeng Xitong Wan on adjuvant arthritis in rats. Zhongguo Yaolixue yu Dulixue. 2007;21(5):422–426. [Google Scholar]
- [39].Vos MJ, Postma TJ, Martens F, et al. Serum levels of S-100B protein and neuron-specific enolase in glioma patients: a pilot study. Anticancer Res. 2004;24(4):2511–2514. [PubMed] [Google Scholar]
- [40].Quincozes-Santos A, Gottfried C. Resveratrol modulates astroglial functions: neuroprotective hypothesis. Ann N Y Acad Sci. 2011;1215:72–78. doi: 10.1111/j.1749-6632.2010.05857.x. [DOI] [PubMed] [Google Scholar]
- [41].D’Angelo L, De Girolamo P, Cellerino A, et al. Immunolocalization of S100-like protein in the brain of an emerging model organism: Nothobranchius furzeri. Microsc Res Tech. 2012;75(4):441–447. doi: 10.1002/jemt.21075. [DOI] [PubMed] [Google Scholar]
- [42].Moroz OV, Antson AA, Murshudov GN, et al. The three- dimensional structure of human S100A12. Acta Crystallogr D Biol Crystallogr. 2001;57(Pt 1):20–29. doi: 10.1107/s090744490001458x. [DOI] [PubMed] [Google Scholar]
- [43].Gruden MA, Sewell RD, Yanamandra K, et al. Immunoprotection against toxic biomarkers is retained during Parkinson's disease progression. J Neuroimmunol. 2011;233(1-2):221–227. doi: 10.1016/j.jneuroim.2010.12.001. [DOI] [PubMed] [Google Scholar]
- [44].Bianchi R, Kastrisianaki E, Giambanco I, et al. S100B protein stimulates microglia migration via RAGE-dependent up-regulation of chemokine expression and release. J Biol Chem. 2011;286(9):7214–7226. doi: 10.1074/jbc.M110.169342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Zhang L, Liu W, Alizadeh D, et al. S100B attenuates microglia activation in gliomas: Possible role of STAT3 pathway. Glia. 2011;59(3):486–498. doi: 10.1002/glia.21118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Streicher WW, Lopez MM, Makhatadze GI. Modulation of quaternary structure of S100 proteins by calcium ions. Biophys Chem. 2010;151(3):181–186. doi: 10.1016/j.bpc.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].van Dieck J, Lum JK, Teufel DP, et al. S100 proteins interact with the N-terminal domain of MDM2. FEBS Lett. 2010;584(15):3269–3274. doi: 10.1016/j.febslet.2010.06.024. [DOI] [PubMed] [Google Scholar]
- [48].Lin J, Yang Q, Wilder PT, et al. The calcium-binding protein S100B down-regulates p53 and apoptosis in malignant melanoma. J Biol Chem. 2010;285(35):27487–27498. doi: 10.1074/jbc.M110.155382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Warth A, Krysa S, Zahel T, et al. S100 protein positive sustentacular cells in pulmonary carcinoids and thoracic paragangliomas: differential diagnostic and prognostic evaluation. Pathologe. 2010;31(5):379–384. doi: 10.1007/s00292-010-1293-2. [DOI] [PubMed] [Google Scholar]
- [50].Holland SK, Hessler RB, Reid-Nicholson MD, et al. Utilization of peripherin and S-100 immunohistochemistry in the diagnosis of Hirschsprung disease. Mod Pathol. 2010;23(9):1173–1179. doi: 10.1038/modpathol.2010.104. [DOI] [PubMed] [Google Scholar]
- [51].Michetti F, Gazzolo D. Review: S100B protein in biological fluids: a tool for perinatal medicine. Clin Chem. 2002;48(12):2097–2104. [PubMed] [Google Scholar]
- [52].Yuan XS, Bian XX. S100B protein and its clinical effect on craniocerebral injury. Linchuang Waike Zazhi. 2007;15(4):274–276. doi: 10.1016/s1008-1275(08)60012-7. [DOI] [PubMed] [Google Scholar]
- [53].Petzold A, Jenkins R, Watt HC, et al. Cerebrospinal fluid S100B correlates with brain atrophy in Alzheimer's disease. Neurosci Lett. 2003;336(3):167–170. doi: 10.1016/s0304-3940(02)01257-0. [DOI] [PubMed] [Google Scholar]
- [54].Zhao G, Yang YN, Su XC, et al. Determination of s-100 beta protein and neuron-specific enolase of cerebrospinal fluid in patients with central nervous system infections. Jiefangjun Yixue Zazhi. 2002;27(10):887–889. [Google Scholar]
- [55].Voronina TA, Belopol’skaya MV, Kheyfets IA, et al. Effect of ultralow doses of antibodies to S-100 protein in animals with impaired cognitive function and disturbedemotional and neurological status under conditions of experimental Alzheimer disease. Bull Exp Biol Med. 2009;148(3):533–535. doi: 10.1007/s10517-010-0757-y. [DOI] [PubMed] [Google Scholar]
- [56].Voronina TA, Kheyfets IA, Dugina YL, et al. Study of the effects of preparation containing ultralow doses of antibodies to S-100 protein in experimental hemorrhagic stroke. Bull Exp Biol Med. 2009;148(3):530–532. doi: 10.1007/s10517-010-0756-z. [DOI] [PubMed] [Google Scholar]
- [57].Voronina TA, Kheyfets IA, Molodavkin GM, et al. Antiaggressive activity of antibodies to S-100 protein in ultralow doses. Bull Exp Biol Med. 2009;148(3):527–529. doi: 10.1007/s10517-010-0755-0. [DOI] [PubMed] [Google Scholar]
- [58].Woischneck D, Schütze M, Peters B, et al. Cranial magnetic resonance imaging and serum marker S-100 for expert opinions in severe brain injuries. Versicherungsmedizin. 2010;62(1):20–24. [PubMed] [Google Scholar]
- [59].Chen Z, Pradhan S, Liu C, et al. Skin-derived precursors as a source of progenitors for cutaneous nerve regeneration. Stem Cells. 2012;30(10):2261–2270. doi: 10.1002/stem.1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Ozdemir M, Attar A, Kuzu I. Regenerative treatment in spinal cord injury. Curr Stem Cell Res Ther. 2012;7(5):364–369. doi: 10.2174/157488812802481481. [DOI] [PubMed] [Google Scholar]
- [61].Oh SH, Kim JR, Kwon GB, et al. Effect of surface pore structure of nerve guide conduit on peripheral nerve regeneration. Tissue Eng Part C Methods. Tissue Eng Part C Methods. 2013;19(3):233–243. doi: 10.1089/ten.tec.2012.0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Angius D, Wang H, Spinner RJ, et al. A systematic review of animal models used to study nerve regeneration in tissue-engineered scaffolds. Biomaterials. 2012;33(32):8034–8039. doi: 10.1016/j.biomaterials.2012.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Schachtrup C, Ryu JK, Helmrick MJ, et al. Fibrinogen triggers astrocyte scar formation by promoting the availability of active TGF-beta after vascular damage. J Neurosci. 2010;30(17):5843–5854. doi: 10.1523/JNEUROSCI.0137-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Grudniak AM, Kurek A, Szarlak J, et al. Oleanolic and ursolic acids influence affect the expression of the cysteine regulon and the stress response in escherichia coli. Curr Microbiol. 2011;62(4):1331–1336. doi: 10.1007/s00284-010-9866-0. [DOI] [PubMed] [Google Scholar]
- [65].Wu CR, Hseu YC, Lien JC, et al. Triterpenoid contents and anti-inflammatory properties of the methanol extracts of ligustrum species leaves. Molecules. 2010;16(1):1–15. doi: 10.3390/molecules16010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Lin CC, Huang CY, Mong MC, et al. Antiangiogenic potential of three triterpenic acids in human liver cancer cells. J Agric Food Chem. 2011;59(2):755–762. doi: 10.1021/jf103904b. [DOI] [PubMed] [Google Scholar]
- [67].Prasad S, Yadav VR, Kannappan R, et al. Ursolic acid, a pentacyclin triterpene, potentiates TRAIL-induced apoptosis through p53-independent up-regulation of death receptors: evidence for the role of reactive oxygen species and JNK. J Biol Chem. 2011;286(7):5546–5557. doi: 10.1074/jbc.M110.183699. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [68].The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006 Sep 30; [Google Scholar]
- [69].Huang JH, Huang XH, Chen ZY, et al. Dose conversion among different animals and healthy volunteers in pharmacological study. Zhongguo Linchuang Yaolixue yu Zhiliaoxue. 2004;9(9):1069–1072. [Google Scholar]
- [70].Liu M, Liu XY, Cheng JF. Advance in the pharmacological research on matrine. Zhongguo Zhongyao Zazhi. 2003;28(9):11–14. [PubMed] [Google Scholar]


