Abstract
Voxel-based morphometry can be used to quantitatively compare structural differences and func-tional changes of gray matter in subjects. In the present study, we compared gray matter images of 32 patients with Parkinson's disease and 25 healthy controls using voxel-based morphometry based on 3.0 T high-field magnetic resonance T1-weighted imaging and clinical neurological scale scores. Results showed that the scores in Mini-Mental State Examination and Montreal Cognitive Assessment were lower in patients compared with controls. In particular, the scores of visuospa-tial/executive function items in Montreal Cognitive Assessment were significantly reduced, but mean scores of non-motor symptoms significantly increased, in patients with Parkinson's disease. In dition, gray matter volume was significantly diminished in Parkinson's disease patients compared with normal controls, including bilateral temporal lobe, bilateral occipital lobe, bilateral parietal lobe, bilateral frontal lobe, bilateral insular lobe, bilateral parahippocampal gyrus, bilateral amygdale, right uncus, and right posterior lobe of the cerebellum. These findings indicate that voxel-based phometry can accurately and quantitatively assess the loss of gray matter volume in patients with Parkinson' disease, and provide essential neuroimaging evidence for multisystem pathological mechanisms involved in Parkinson's disease.
Keywords: neural regeneration, neuroimaging, neurodegeneration, voxel-based morphometry, Parkinson's disease, MRI, dopamine, non-motor symptoms, gray matter abnormality, region of interest, Mini-Mental State Examination, Montreal Cognitive Assessment, neurodegenerative disease, grants-supported paper, neuroregeneration
Research Highlights
(1) Motor symptoms can affect the quality of life in patients with Parkinson's disease, although non-motor symptoms have the greatest impact on quality of life.
(2) Gray matter images of 32 Parkinson's disease patients and 25 healthy controls were compared using voxel-based morphometry to investigate the correlation between brain structural loss and non-motor symptoms in Parkinson's disease.
(3) Comparison of gray matter images showed that gray matter volume was significantly diminished in patients with Parkinson's disease compared with normal controls, including the bilateral temporal lobe, bilateral occipital lobe, bilateral parietal lobe, bilateral frontal lobe, bilateral insular lobe, teral parahippocampal gyrus, bilateral amygdale, right uncus, and right posterior lobe of cerebellum.
(4) Voxel-based morphometry can accurately and quantitatively display the loss of gray matter vo-lume in patients with Parkinson's disease.
INTRODUCTION
Parkinson's disease is a selective degenerative disorder of dopamine-generating cells in the substantia nigra (a region of the midbrain), significantly reduced dopamine in the corpus striatum, and Lewy bodies in the substantia nigra and locus ceruleus, resulting in functional abnormalities in the basal ganglia and cortical circuits[1,2,3]. However, the precise mechanisms of Parkinson's disease remain poorly understood. In addition to typical motor symptoms such as shaking, slowness of movement, hypermyotonia, and difficulty with walking and gait, complex non-motor symptoms, including abnormalities in cognition, mental behaviors, autonomic nerves and sensory perception, are also evident in Parkinson's disease[4,5].
In particular, cognitive disturbances such as executive dysfunction, memory loss, depression, indifference, anxiety and somnipathy are important clinical manifestations. In some patients with Parkinson's disease, some non-motor symptoms even appear earlier than motor symptoms or in the early stage of Parkinson's disease, and the non-motor symptoms have the greatest effect on the quality of patient life[6,7,8].
The diagnosis of Parkinson's disease is mainly based on clinical motor system criteria and the Levodopa challenge test. However, shaking, slowness of movement and muscle rigidity can also be found in secondary Parkinson's disease and Parkinsonism-Plus. As the clinical manifestations are similar in early Parkinson's disease patients, the incidence of misdiagnosis is high. Nevertheless, because treatment and prognosis of these disorders are different, correct diagnosis is important.
Because of the strong compensatory ability of the substantia nigra-corpus striatum dopamine system, symptoms are not obvious in patients with dopaminergic neuron degeneration or loss less than 50%. However, obvious motor symptoms in patients indicate that the dopaminergic neuron loss has reached 70–80%[9], implying that the disease has progressed to the middle to advanced stage. Early definite diagnosis and appropriate neuroprotection may delay disease progression. Thus, a specific, objective and sensitive examination method for early diagnosis of Parkinson's disease is very important for treatment and delaying progression.
Voxel-based morphometry can be used to quantitatively compare brain gray matter structures across subjects, and display the volume of gray matter loss in abnormal brain areas to reflect possible functional changes[10]. Voxel-based morphometry is a statistical analysis of the entire brain with no bias, and enables in vivo study of brain structures in the absence of evaluation of structural and morphological changes in brain areas.
Gray matter abnormalities have been found in the temporal lobe, occipital lobe, frontal lobe, parietal lobe and limbic system in patients with Parkinson's disease. Previous studies of voxel-based morphometry mainly investigated the pattern of gray matter loss between patients with Parkinson's disease and normal controls, but with differing results. For example, gray matter atrophy was only observed in the left caudate nucleus in one study[11], but was also found in the superior temporal gyrus in a separate study[12].
Gray matter atrophy was also reported in regions in the frontal lobe including the superior frontal gyrus, middle frontal gyrus, inferior frontal gyrus and medial frontal gyrus[13,14,15], and in the superior temporal gyrus[12,14,15], parahippocampal gyrus[13] and hippocampus[16]. Evidence of frontal lobe and parahippocampal gyrus gray matter atrophy in the limbic system during Parkinson's disease progression (Hoehn & Yahr scores > 2) was also reported[13], in addition to atrophy in the parietal lobe[17], cingulate gyrus[16] and caudate nucleus[11]. By contrast, other studies have reported no evidence of regions of gray matter loss in patients with Parkinson's disease compared with controls[11,17]. Ding et al[18] also used voxel-based morphometry to assess white matter changes in Parkinson's disease patients with dysosmia, and found an increase in white matter intensity in the right occipital lobe, left posterior part of cingulate gyrus, left occipital lobe and left paracentral lobule, and an increase in white matter volume in the bilateral occipital lobe, left posterior part of cingulate gyrus and left paracentral lobule; olfactory dysfunction was not correlated with age or course of disease. These findings indicate that dysosmia is the result of central neurodegeneration in patients with Parkinson's disease.
The variation in these studies may be a result of the number of diseases, course of disease and severity of disease. Moreover, different voxel-based morphometry techniques were applied. There are two main types of voxel-based morphometry techniques: standard voxel-based morphometry and optimized voxel-based morphometry prior to statistical parametric mapping 5[19,20]. The standard voxel-based morphometry uses a built-in template of statistical parametric mapping, while the optimized morphometry uses a self-designed template to reduce the influence on the results. In the present study, we used the template from Montreal Neurological Institute for registration[21], and used statistical parametric mapping 5 software to compare the gray matter structure of the entire brain between patients with Parkinson's disease and normal controls using voxel-based morphometry. We also analyzed the morphological changes in the brain of patients based on their clinical neurological scale scores to investigate the correlation between complex clinical symptoms and neuroimaging in Parkinson's disease patients. These data provide essential neuroimaging evidence for potential pathological multisystem mechanisms involved in Parkinson's disease.
RESULTS
Quantitative analysis of participants
A total of 32 patients with Parkinson's disease and 25 healthy controls were included. All subjects completed the experiments and were included in the final analysis. The patients received oral administration of benzhexol (2 mg) at 1 hour prior to scanning to control tremor.
Baseline data of participants
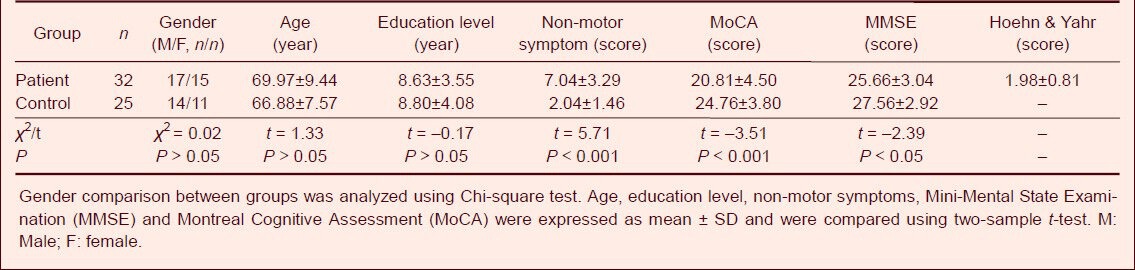
The patients and controls were matched in terms of age, gender and education background. The mean scores of Montreal Cognitive Assessment and Mini-Mental State Examination were significantly lower in patients compared with normal controls (P < 0.001 and P < 0.05, respectively), while the scores of non-motor symptoms were significantly higher in patients compared with normal controls (P < 0.05; Table 1).
Table 1.
Comparison of clinical baseline data among subjects
Comparison of Mini-Mental State Examination and Montreal Cognitive Assessment between patients and controls
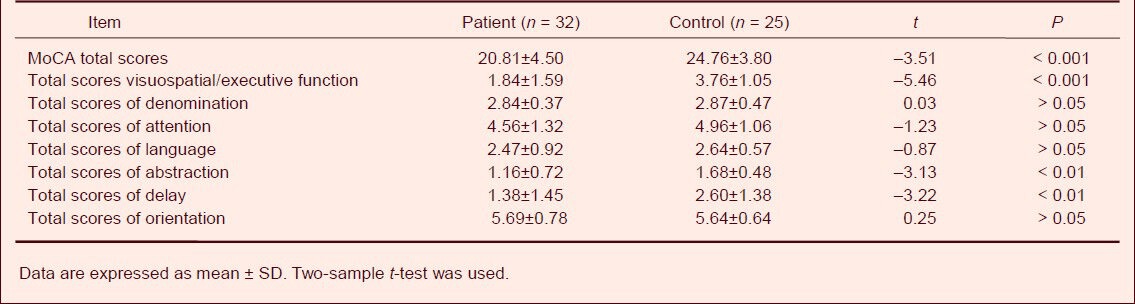
Analysis of Montreal Cognitive Assessment item scores showed that the mean of total scores of visuospatial/executive function and the mean of total scores of abstraction and delay were significantly lower in patients compared with normal controls (P < 0.001, P < 0.01, respectively). However, there were no differences between two groups in total scores of denomination, attention, language and orientation (Table 2).
Table 2.
Comparison of Montreal Cognitive Assessment (MoCA) scores between patient and control groups
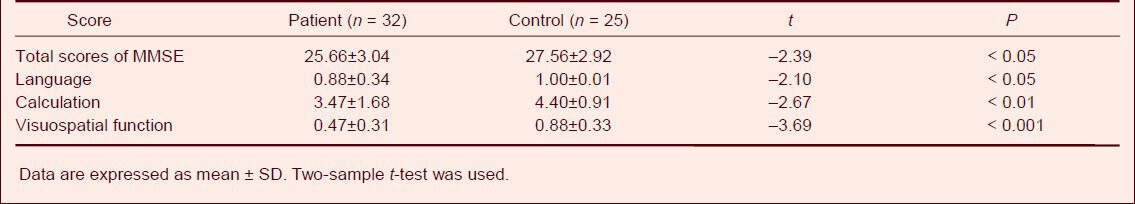
Analysis of Mini-Mental State Examination items showed that the mean scores of visuospatial function (0.47 ± 0.51; P < 0.001), calculation (3.47 ± 1.68; P < 0.01) and language (0.88 ± 0.34; P < 0.05) were all significantly lower than the normal controls (Table 3).
Table 3.
Comparison of Mini-Mental State Examination (MMSE) scores between patient and control groups
Comparison of non-motor symptoms between patients and normal controls
The scores of non-motor symptoms were significantly higher in patient groups compared with controls (P < 0.001). All 32 patients presented typical non-motor symptoms of 2–13 items, while the controls exhibited 0-3 items of non-motor symptoms. In the patient group, frequent micturition (71.9%, n = 23), constipation (65.6%, n = 21), slowed attention (56.2%, n =18), dyssomnia (53.1%, n = 17), recent memory loss (46.9%, n = 15) and illusion (6.3%, n = 2) were found. By contrast, in the normal group the incidence of recent memory loss (44%, n = 11) was the highest, followed by nocturia increase (31.2%, n = 10), slowed attention (21.9%, n = 7) and dizziness (21.9%, n = 7).
Gray matter abnormalities in the entire brain of Parkinson's disease patients
The gray matter volume was significantly diminished in patients with Parkinson's disease compared with normal controls, including the bilateral temporal lobe (right inferior, middle and superior temporal gyrus, right middle temporal gyrus), bilateral occipital lobe (right cuneate lobe, lingual gyrus, fusiform gyrus and left middle occipital gyrus), bilateral parietal lobe (right precuneus, left postcentral gyrus), bilateral frontal lobe (inferior frontal gyrus, medial frontal gyrus, orbital gyri, and gyrus rectus), bilateral insular lobe, bilateral parahippocampal gyrus, bilateral amygdale, right uncus and right posterior lobe of the cerebellum.
The loss of hemisphere volume was asymmetric, and the volume of the gray matter in the right hemisphere was significantly diminished (Table 4, Figure 1). No brain areas with increased gray matter volume were found.
Table 4.
Brain areas with abnormal gray matter volume in patients with Parkinson's disease
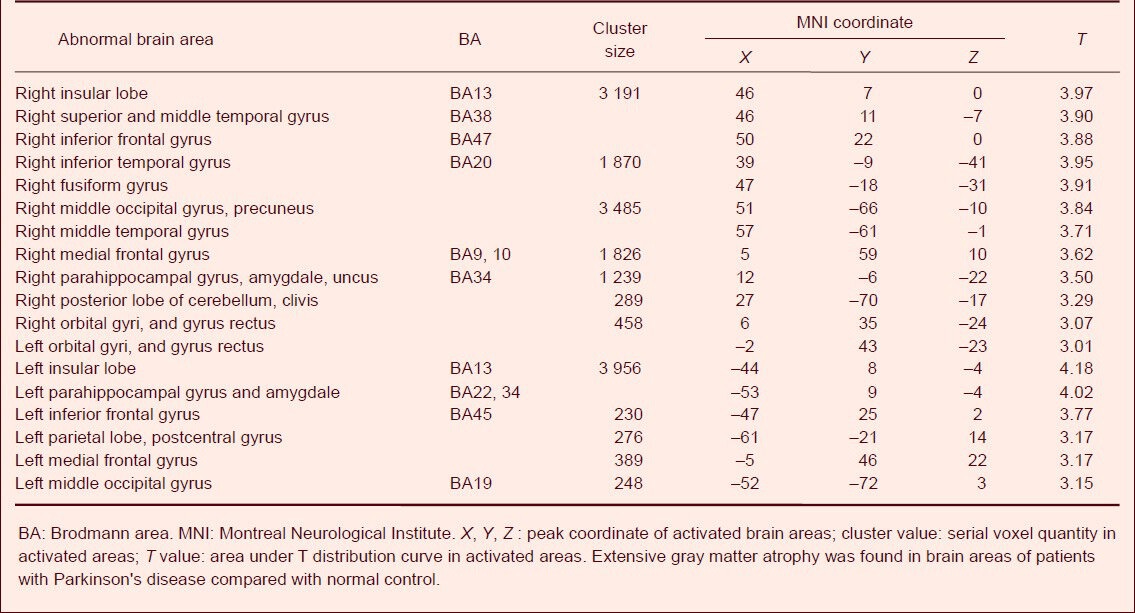
Figure 1.
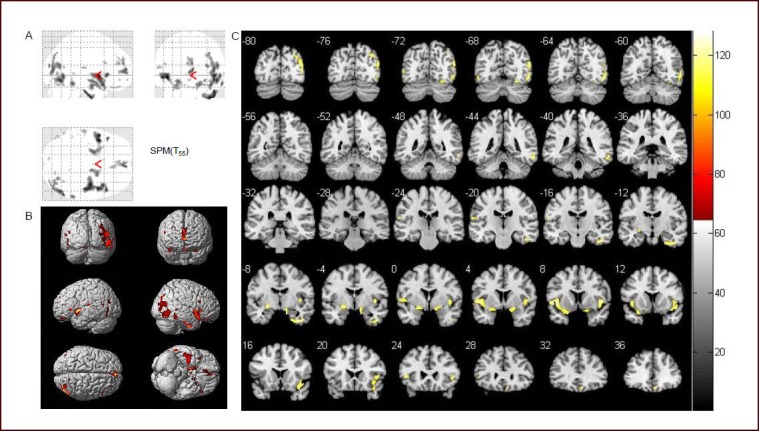
Statistical parameter map (two-sample t-test) of gray matter volume between patient (n = 32) and control (n = 25) groups (volume of gray matter was reduced in the patient group).
(A) Three-dimensional brain images of comparison between patient and control groups. Shadow represents atrophic brain areas in Parkinson's disease patients.
(B) Three-dimensional coronal image in MNI standard coordinate of statistical analysis results using pseudo-color. The pseudo-color represents atrophic brain areas in the patient group.
(C) Coronal template map in MNI standard coordinates of the statistical analysis results using pseudo-color. Shadow and color represent atrophic brain areas in the patient group. MNI: Montreal Neurological Institute.
DISCUSSION
Voxel-based morphometry
Voxel-based morphometry is an automatic measurement technique for the brain. It can objectively, accurately and quantitatively investigate the structure of the entire brain and show the relationships between different brain areas and abnormal brain structures. This technique is useful for investigating structural changes in the brain following nervous system disease with uncertain pathological mechanisms[25]. Moreover, it is highly reproducible. Voxel-based morphometry is strongly influenced by variability. Variation among individuals in samples and errors in pretreatment can reduce the sensitivity of differences between groups. Segmentation error may also occur in tissues with displacement, and the gray matter structure of the cortex adjacent to the brain ventricle may migrate due to extension of the ventricular system. In addition, more attention should be paid in smoothing as high-level smoothing can improve the ability of voxel-based morphometry to detect gray matter differences, although redundant smoothing can reduce the ability to accurately localize the changes. A smoothing kernel of 4–12 mm is generally used[26,27]. The sample sizes for group analysis using voxel-based morphometry are typically 14–20[28]; large samples sizes can increase the reliability of results.
Abnormality in gray matter structure
In the present study, we observed significant differences between patients and controls in scores of non-motor symptoms, Montreal Cognitive Assessment and Mini-mental State Examination.
Extensive gray matter loss was previously reported in brain areas of Parkinson's disease patients compared with normal controls, including the bilateral temporal lobe, occipital lobe, parietal lobe, frontal lobe and limbic system. Further, these reductions in gray matter volume were asymmetric in both hemispheres; i.e., the gray matter volume was significantly reduced in the right hemisphere, indicating a correlation with complex clinical symptoms in Parkinson's disease patients with multisystem effects. There is also neuropathological evidence that that following neuronal Lewy body formation, local neuronal loss, necrosis, gliosis and cortical atrophy gradually occur in the limbic system and frontal, parietal, occipital and temporal lobes, resulting in a series of complex clinical symptoms, including non-motor systems[29].
Parkinson's disease not only involves the substantia nigra-corpus striatum dopamine system, but also alters the distribution of the dopamine system at other sites, as well as non-dopamine systems such as the serotonergic and noradrenergic systems[30]. This may explain the occurrence of extensive brain area atrophy in Parkinson's disease patients. Previous studies have also demonstrated a reduction in metabolism in the bilateral parietal lobe, bilateral occipital lobe, frontal lobe and temporal lobe[31,32,33,34,35].
Voxel-based morphometry was previously used to investigate brain anatomical structural changes related to special clinical symptoms in Parkinson's disease patients. For example, Feldmann et al[36] found that the volume of the bilateral orbital and frontal cortex and gyrus rectus were significantly reduced in Parkinson's disease patients with depression, indicating that regional dysfunction is a major cause of the depression symptoms. Poor memory was also reported to be associated with gray matter atrophy in the left hemisphere (uncus, middle temporal and fusiform gyri), left putamen and right temporal lobes[37].
In addition, dopamine non-responsive motor signs and executive function were associated with caudate atrophy, and executive function was associated with gray matter atrophy in the middle temporal gyri, the left precuneus and the cerebellum[37]. Kassubek et al[38] found that gray matter was increased in the nucleus ventralis intermedius of the thalamus contralateral to the tremor side in unilateral Parkinsonian tremor patients, and that the volume reductions were significantly correlated with tremor amplitudes, suggesting that the thalamus is important for tremor generation. Ramirez-Ruiz et al[39] also reported a progressive gray matter volume decrease in Parkinson's disease patients without dementia in the limbic system (hippocampus, parahippocampal gyrus) and paralimbic regions (insular lobe and cingulate gyrus), while patients with dementia exhibited gray matter volume reductions predominantly in the neocortical regions. These data suggest that cortical atrophy is a substrate for dementia in Parkinson's disease.
Because of the complex and varied clinical manifestations of Parkinson's disease and different clinical data in studies, there are often varying results. Nevertheless, the atrophic brain areas in Parkinson's disease patients observed in the present study were consistent with previous studies. Thus, we conclude that extensive brain morphological changes in Parkinson's disease patients form part of a complex pathological mechanism in Parkinson's disease. In addition, we found a reduction in the volume of the bilateral amygdala in Parkinson's disease patients.
The afferent and efferent fibers of the amygdala are complex and provide association between sensory and emotion-behavior executive systems. The major function of the amygdala is related to emotion and motivation generation, internal organ activity, learning and memory formation and sleep and wakefulness. The loss of bilateral amygdala volume may contribute to cognitive disturbances in our patients, and suggests that some Parkinson's disease patients may develop cognitive impairment in the early stage.
In summary, we found extensive loss of gray matter volume in Parkinson's disease patients, resulting in damage in the function of corresponding brain areas (based on neurological scale results), consistent with clinical characteristics of Parkinson's disease. In addition, this study provided important neuroimaging evidence for in vivo observation of the distribution abnormal gray matter features in Parkinson's disease.
Further investigation and wide application of voxel-based morphometry can provide a greater understanding of brain morphological abnormalities in Parkinson's disease, as well as potential mechanisms.
SUBJECTS AND METHODS
Design
A case-control study.
Time and setting
The experiments were conducted in the Department of Radiology, Affiliated Hospital of Nantong University in China, and data processing and analysis was conducted in the Department of Medical Image Engineering, Nantong University, China from January 2011 to April 2012.
Subjects
Patients with Parkinson's disease were recruited from the Affiliated Hospital of Nantong University in China between January and December 2011.
Diagnostic criteria of Parkinson's disease
All patients were diagnosed by an physician engaging in extrapyramidal disease in the Department of Neurology, according to the diagnostic criteria of Parkinson's Disease Society Brain Bank, London: overview and research[40] (at least three of the following): unilateral onset; rest tremor present; progressive disorder; persistent asymmetry affecting side of onset most; excellent response (70–100%) to levodopa; severe levodopa-induced chorea; levodopa response for 5 years or longer; and clinical course of 10 years or longer. In addition, patients with Parkinson's syndrome or Parkinsonism-Plus were excluded.
Inclusion criteria of Parkinson's disease
All patients who met the diagnostic criteria were assessed using the Mini-Mental State Examination and Montreal Cognitive Assessment to eliminate those with dementia. Moreover, the Geriatric Depression Scale and Activities of Daily Living were used to evaluate and exclude those subjects with severe depression or poor activities of daily life.
Non-motor symptoms were assessed using the Non-motor Symptoms Questionnaire. Hoehn & Yahr staging was used for Parkinson's disease staging. In addition, patients with other diseases that can affect evaluation of brain function were excluded. Finally, 32 patients were included, comprising 17 males and 15 females, aged 51–87 years, with education for 0–15 years and course of disease for 1–11 years. All subjects were right handed.
The normal control group comprised 25 healthy people, aged 53–79 years, with education for 0–14 years. They were all right handed, and matched for age and gender with the patient group. Subjects were selected from retired employees of the hospital and families of the included patients. They had no definite nervous system disease, no dental prosthesis that may have affected image quality, and no abnormalities in routine brain MRI examination. The normal control group was subjected to Mini-Mental State Examination, Montreal Cognitive Assessment and Non-motor Symptoms examinations.
Written informed consent was obtained from all subjects according to the Administrative Regulations on Medical Institution, issued by the State Council of China[41].
Methods
Magnetic resonance examination
A GE HDX3.0T superconducting magnetic resonance scanner (GE Healthcare, Fairfield, CT, USA) with an 8-channel head coil was used for the examination.
The 3D T1 images of subjects were acquired by a 3D fast spoiled gradient echo sequence, with the following parameters: repetition time 7.3 ms; echo time 3.4 ms; inversion time 450.0 ms; flip angle 12°; number of excitations 1; slice thickness 1.2 mm; slice gap 0; field of view 24 cm × 24 cm; matrix 256 × 256; voxel 0.47 mm × 0.47 mm × 1.20 mm; and 124 slice axial images in total. In addition, conventional magnetic resonance scanning was conducted in all subjects (axial T2WI, fluid-attenuated inversion recovery imaging, diffusion weighted imaging). A physician from the Department of Radiology read the acquired images, and no morphological abnormalities were found.
Data processing
All data were processed in Matlab 7.1 platform (Department of Medical Imaging Engineering of Nantong University; MathWorks, Natick, MA, USA) using the voxel-based morphometry 5 tool box (http://dbm.neuro.unijena.de/vbm/) in the statistical parametric mapping 5 software (SPM5, http//www.fil.ion.ucl.ac.uk/spm/software/spm5).
Acquired three-dimensional data were preprocessed as follows: (1) image inversion: the raw data in DICOM format was transmitted to NIFTI format; (2) registering and correction: the transmitted images were registered to the standard template of Montreal Neurological Institute using the voxel-based morphometry 5 software package, and the registered images were corrected to 1 mm3 pixels; (3) segmentation: the 3D images were segmented into gray matter, white matter and cerebrospinal fluid, and the segmented gray matter images were subjected to volume modulation, equal to the tissue volume of the original images; (4) smoothing: the modulated gray matter and white matter were smoothed using an 8 mm full width at half maximization Gaussian kernel to improve signal to noise ratio of images.
Gray matter structures in the entire brain were compared between the groups using a two-sample t-test from the SPM software. A P < 0.005 (non-corrected) and connected brain areas > 200 voxel were considered statistically significant. Brain areas with statistically significant voxel, activation volume (cluster size), activation intensity (t value from the two-sample t-test; T value was positively correlated with intensity) and Montreal Neurological Institute coordinates were recorded using xjView8.2 software (http://www.alivelearn.net/xjview8/). Corresponding Brodmann areas of gray matter structures were confirmed.
Statistical analysis
Measurement data were expressed as mean ± SD. The data were analyzed using SPSS 17.0 software (SPSS, Chicago, IL, USA). Differences in gender between groups were compared using chi-square test, and comparison of the other measurement data was conducted using two sample t-test. A value of P < 0.05 was considered statistically significant.
Acknowledgments:
We thank Professor Wang for providing the information of Parkinson's disease patients, and the staff from the Radiology Department of Affiliated hospital of Nantong University, China for technical support.
Footnotes
Funding: This work was supported by the Priority Academic Program Development of Jiangsu Higher Education Institutions, the Medical Clinical Science and Technology Developemnt Fund of Jiangsu University, No. JLY20120122; Innovative Climb Program of Natural Science Foundation of Jiangsu Province, No. BK2008010; and the Natural Science Foundation of Nantong University, No.11Z001.
Conflicts of interest: None declared.
Ethical approval: This study received permission from the Ethics Committee of Nantong University, China.
(Reviewed by Dean J, Raye W, Bai H, Cui GY)
(Edited by Wang J, Su LL, Li CH, Song LP, Liu WJ, Zhao M)
REFERENCES
- [1].Stoessl AJ. Neuroimaging in Parkinson's Disease. Neurotherapeutics. 2011;8(1):72–81. doi: 10.1007/s13311-010-0007-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Wirdefeldt K, Adami HO, Cole P, et al. Epidemiology and etiology of Parkinson's disease: a review of the evidence. Eur J Epidemiol. 2011;26(Suppl 1):S1–58. doi: 10.1007/s10654-011-9581-6. [DOI] [PubMed] [Google Scholar]
- [3].Lynch-Day MA, Mao K, Wang K, et al. The role of autophagy in Parkinson's disease. Cold Spring Harb Perspect Med. 2012;2(4):a009357. doi: 10.1101/cshperspect.a009357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Olanow CW, McNaught K. Parkinson's disease, proteins, and prions: milestones. Mov Disord. 2011;26(6):1056–1071. doi: 10.1002/mds.23767. [DOI] [PubMed] [Google Scholar]
- [5].Schapira AH. Etiology and pathogenesis of Parkinson disease. Neurol Clin. 2009;27(3):583–603. doi: 10.1016/j.ncl.2009.04.004. [DOI] [PubMed] [Google Scholar]
- [6].Qin ZH, Chen B, Zhang LY, et al. Study on non-motor symptoms impacting on health related quality of life in early Parkinson disease randomized controlled clinical trial. Zhongguo Xiandai Shenjing Jibing Zazhi. 2009;9(3):246–251. [Google Scholar]
- [7].Shulman LM, Taback RL, Bean J, et al. Comorbidity of the nonmotor symptoms of Parkinson's disease. Mov Disord. 2001;16(3):507–510. doi: 10.1002/mds.1099. [DOI] [PubMed] [Google Scholar]
- [8].Weintraub D, Moberg PJ, Duda JE, et al. Effect of psychiatric and other nonmotor symptoms on disability in Parkinson's diseases. J Am Geriatr Soc. 2004;52(5):784–788. doi: 10.1111/j.1532-5415.2004.52219.x. [DOI] [PubMed] [Google Scholar]
- [9].Fearnley JM, Lees A. Ageing and Parkinson's disease: sustantia nigra regional selectivity. Brain. 1991;114(5):2283–2301. doi: 10.1093/brain/114.5.2283. [DOI] [PubMed] [Google Scholar]
- [10].Ashburner J, Friston KJ. Voxel-based morphometry: the methods. Neuroimage. 2000;11(6):805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- [11].Brenneis C, Seppi K, Schocke MF, et al. Voxel-based morphometry detects cortical atrophy in the Parkinson variant of multiple system atrophy. Mov Disord. 2003;18(10):1132–1138. doi: 10.1002/mds.10502. [DOI] [PubMed] [Google Scholar]
- [12].Beyer MK, Janvin CC, Larsen JP, et al. A magnetic resonance imaging study of patients with Parkinson's disease with mild cognitive impairment and dementia using voxel-based morphometry. J Neurol Neurosurg Psychiatry. 2007;78(3):254–259. doi: 10.1136/jnnp.2006.093849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Nagano-Saito A, Washimi Y, Arahata Y, et al. Cerebral atrophy and its relation to cognitive impairment in Parkinson disease. Neurology. 2005;64(2):224–229. doi: 10.1212/01.WNL.0000149510.41793.50. [DOI] [PubMed] [Google Scholar]
- [14].Burton EJ, McKeith IG, Burn DJ, et al. Cerebral atrophy in Parkinson's disease with and without dementia: a comparison with Alzheimer's disease, dementia with Lewy bodies and controls. Brain. 2004;127(Pt 4):791–800. doi: 10.1093/brain/awh088. [DOI] [PubMed] [Google Scholar]
- [15].Ramirez-Ruiz B, Marti MJ, Tolosa E, et al. Cerebral atrophy in Parkinson's disease patients with visual hallucinations. Eur J Neurol. 2007;14(7):750–756. doi: 10.1111/j.1468-1331.2007.01768.x. [DOI] [PubMed] [Google Scholar]
- [16].Summerfield C, Junque C, Tolosa E, et al. Structural brain changes in Parkinson disease with dementia: a voxel- based morphometry study. Arch Neurol. 2005;62(2):281–285. doi: 10.1001/archneur.62.2.281. [DOI] [PubMed] [Google Scholar]
- [17].Cordato NJ, Duggins AJ, Halliday GM, et al. Clinical deficits correlate with regional cerebral atrophy in progressive supranuclear palsy. Brain. 2005;28(Pt 6):1259–1266. doi: 10.1093/brain/awh508. [DOI] [PubMed] [Google Scholar]
- [18].Ding H, Wu XL, Zhang KY, et al. Change of olfactory function associated structures in Parkinson's disease: a voxel-based morphometry study. Zhongguo Xiandai Shenjing Jibing Zazhi. 2011;11(2):54–59. [Google Scholar]
- [19].Good CD, Johnsrude IS, Ashburner J, et al. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14(1 Pt 1):21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- [20].Mechelli A, Price CJ, Friston KJ, et al. Voxel-based morphometry of the human brain: methods and applications. Curr Med Imaging Rev. 2005;1(1):105–113. [Google Scholar]
- [21].Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26(3):839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- [22].Wright IC, McGuire PK, Poline JB, et al. A voxel based method for the statistical analysis of gray and white matter density applied to schizophrenia. Neurolmage. 1995;2(4):244–252. doi: 10.1006/nimg.1995.1032. [DOI] [PubMed] [Google Scholar]
- [23].Baron JC, Chetelat G, Desgranges B, et al. In vivo mapping of gray matter loss with voxel-based morphometry in mild Alzheimer's disease. Neuroimage. 2001;14(2):298–309. doi: 10.1006/nimg.2001.0848. [DOI] [PubMed] [Google Scholar]
- [24].Brenneis C, Seppi K, Schocke MF, et al. Voxel-based morphometry detects cortical atrophy in the Parkinson variant of multiple system atrophy. Mov Disord. 2003;18(10):1132–1138. doi: 10.1002/mds.10502. [DOI] [PubMed] [Google Scholar]
- [25].Giuliani NR, Calhoun VD, Pearlson GD, et al. Voxel-based morphometry versos region of interest: a comparison of two methods for analyzing gray matter differences in schizophrenia. Schizophr Res. 2005;74(2-3):135–147. doi: 10.1016/j.schres.2004.08.019. [DOI] [PubMed] [Google Scholar]
- [26].Wilke M, Kassubek J, Ziyeh S, et al. Automated detection of gray matter malformations using optimized voxel-based morphometry: a systematic approach. Neuroimage. 2003;20(1):330–343. doi: 10.1016/s1053-8119(03)00296-9. [DOI] [PubMed] [Google Scholar]
- [27].Ibarretxe-Bilbao N, Ramirez-Ruiz B, Junque C, et al. Differential progression of brain atrophy in Parkinson's disease with and without visual hallucinations. J Neurol Neurosurg Psychiatry. 2010;81(6):650–657. doi: 10.1136/jnnp.2009.179655. [DOI] [PubMed] [Google Scholar]
- [28].Friston KJ, Holmes AP, Worsley KJ. How many subjects constitute a study? Neuroimage. 1999;10(1):1–5. doi: 10.1006/nimg.1999.0439. [DOI] [PubMed] [Google Scholar]
- [29].Braak H, Del Tredici K, Rub U, et al. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging. 2003;24(2):197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- [30].Zhu HC. Awareness and prospect of non motor symptoms in Parkinson's Disease. Shenjing Sunshang yu Gongneng Chongjian. 2006;1(4):227–230. [Google Scholar]
- [31].Zhao P. Tianjin: Tianjin Medical University; 2008. The Clinical and ~(18)F-FDG PET study on idiopathic parkinson disease and several parkinsonism-plus syndromes. [Google Scholar]
- [32].Hu MT, Taylor-Robinson SD, Chaudhuri KR, et al. Conical dysfunction in non-demented Parkinson's disease patients: a combined(31)P-MRS and (18)FDG-PET study. Brain. 2000;123(Pt 2):340–352. doi: 10.1093/brain/123.2.340. [DOI] [PubMed] [Google Scholar]
- [33].Hosokai Y, Nishio Y, Himyanm K, et al. Distinct patterns of regional cerebral glucose metabolism in Parkinson's disease with and without mild cognitive impairment. Mov Disord. 2009;24(6):854–862. doi: 10.1002/mds.22444. [DOI] [PubMed] [Google Scholar]
- [34].Rinne JO, Portin R, Ruottinen H, et al. Cognitive impairment and the brain dopaminergic system in Parkinson disease: [18.F] fluorodopa positron emission tomographic study. Arch Neurol. 2000;57(4):470–475. doi: 10.1001/archneur.57.4.470. [DOI] [PubMed] [Google Scholar]
- [35].Nobili F, Abbruzzese G, Morbelli S, et al. Amnestic mild cognitive impairment in Parkinson's disease: a brain perfusion SPECT study. Mov Disord. 2009;24(3):414–421. doi: 10.1002/mds.22381. [DOI] [PubMed] [Google Scholar]
- [36].Feldmann A, Illes Z, KosZtolanyi P, et al. Morphometric changes of gray matter in Parkinson's disease with depression: a voxel-based morphometry study. Mov Disord. 2008;23(1):42–46. doi: 10.1002/mds.21765. [DOI] [PubMed] [Google Scholar]
- [37].Camicioli R, Gee M, Bouchard TP, et al. Voxel-based morphometry reveals extra-nigral atrophy pattern associated with dopamine refractory cognitive and motor impairment in parkinsonism. Parkinsonism Relat Disord. 2009;15(3):187–195. doi: 10.1016/j.parkreldis.2008.05.002. [DOI] [PubMed] [Google Scholar]
- [38].Kassubek J, Juengling FD, Hellwig B, et al. Thalamic gray matter changes in unilateral Parkinsonian resting tremor: a voxel-based morphometric analysis of 3-dimensional magnetic resonance imaging. Neurosci Lett. 2002;323(1):29–32. doi: 10.1016/s0304-3940(02)00111-8. [DOI] [PubMed] [Google Scholar]
- [39].Ramirez-Ruiz B, Marti MJ, Tolosa E, et al. Longitudinal evaluation of cerebral morphological changes in Parkinson's disease with and without dementia. J Neurol. 2005;252(11):1345–1352. doi: 10.1007/s00415-005-0864-2. [DOI] [PubMed] [Google Scholar]
- [40].Daniel SE, Lees AJ. Parkinson's Disease Society Brain Bank, London: overview and research. J Neural Transm Suppl. 1993;39:165–172. [PubMed] [Google Scholar]
- [41].State Council of the People's Republic of China. Administrative Regulations on Medical Institution; 1994. Sep 01, [Google Scholar]


