Abstract
Reward-based decision-making has been found to activate several brain areas, including the ventrolateral prefrontal lobe, orbitofrontal cortex, anterior cingulate cortex, ventral striatum, and mesolimbic dopaminergic system. In this study, we observed brain areas activated under three degrees of uncertainty in a reward-based decision-making task (certain, risky, and ambiguous). The tasks were presented using a brain function audiovisual stimulation system. We conducted brain scans of 15 healthy volunteers using a 3.0T magnetic resonance scanner. We used SPM8 to analyze the location and intensity of activation during the reward-based decision-making task, with respect to the three conditions. We found that the orbitofrontal cortex was activated in the certain reward condition, while the prefrontal cortex, precentral gyrus, occipital visual cortex, inferior parietal lobe, cerebellar posterior lobe, middle temporal gyrus, inferior temporal gyrus, limbic lobe, and midbrain were activated during the ‘risk’ condition. The prefrontal cortex, temporal pole, inferior temporal gyrus, occipital visual cortex, and cerebellar posterior lobe were activated during ambiguous decision-making. The ventrolateral prefrontal lobe, frontal pole of the prefrontal lobe, orbitofrontal cortex, precentral gyrus, inferior temporal gyrus, fusiform gyrus, supramarginal gyrus, inferior parietal lobule, and cerebellar posterior lobe exhibited greater activation in the ‘risk’ than in the ‘certain’ condition (P < 0.05). The frontal pole and dorsolateral region of the prefrontal lobe, as well as the cerebellar posterior lobe, showed significantly greater activation in the ‘ambiguous’ condition compared to the ‘risk’ condition (P < 0.05). The prefrontal lobe, occipital lobe, parietal lobe, temporal lobe, limbic lobe, midbrain, and posterior lobe of the cerebellum were activated during decision-making about uncertain rewards. Thus, we observed different levels and regions of activation for different types of reward processing during decision-making. Specifically, when the degree of reward uncertainty increased, the number of activated brain areas increased, including greater activation of brain areas associated with loss.
Keywords: neural regeneration, neuroimaging, decision-making, reward, uncertainty, cognitive processing, functional magnetic resonance imaging, brain, grants-supported paper, neuroregeneration
Research Highlights
-
(1)
Compared with decision-making in the ‘certain’ condition, the ventrolateral prefrontal lobe, frontal pole of the prefrontal lobe, orbitofrontal cortex, precentral gyrus, inferior temporal gyrus, fusiform gyrus, supramarginal gyrus, inferior parietal lobule, and cerebellar posterior lobe exhibited greater activation in the ‘risk’ condition. Compared with decision-making in the ‘certain’ condition, the frontal pole of the prefrontal lobe was strongly activated in the ‘ambiguous’ condition. Compared with the ‘risk’ condition, the dorsolateral prefrontal lobe and cerebellar posterior lobe showed significantly greater activation in the ‘ambiguous’ condition.
-
(2)
The activation of brain areas related to the processing information about loss increased with the degree of uncertainty.
INTRODUCTION
Each day, people are required to make decisions with varying degrees of risk. In cognitive neuroscience, decision-making is known as the cognitive process underlying the selection of a course of action among several alternative choices. The options are characterized by risk and ambiguity regarding different types of rewards and losses[1].
Although decision-making research originated in the early 1900s, since the 1940s, scholars in this field have mainly taken a cognitive psychology approach[2]. Recently, the mechanisms underlying decision-making have been explored using techniques in neuropsychology and cognitive neuroscience, with a focus on functional brain activities related to evaluation of risk, leading to a decision[3,4,5]. Previous studies have identified several brain regions involved in decision-making, including the orbital and frontal cortex, prefrontal lobe, anterior cingulate cortex, amygdale, hippocampus, limbic system, parietal lobe, cerebellum, and midbrain[6,7]. These regions can be divided into two functions in terms of decision-making: “loss utility calculation” and “reward utility calculation”. Under different decision-making conditions, people analyze, judge, and distinguish advantages and disadvantages of a choice, while brain areas related to “loss” and “reward” are activated. Ultimately, a choice indicating tendency or avoidance is made. Primary studies have shown that the ventrolateral prefrontal lobe, orbitofrontal lobe, anterior cingulate, ventral striatum, and mesolimbic dopaminergic system are involved in reward-based decision-making. When an expected gain is received or a practical outcome results in a gain, reward-related brain areas are activated, resulting in a tendency behavior[8,9,10,11]. However, several questions about decision-making remain. For instance, the role of degrees of uncertainty and different levels of reward/loss in decision-making is unclear, as is the neurophysiological underpinnings of decision-making behavior.
In this study, we used fMRI to study functional alterations of brain activation specific to different decision-making tasks[11,12]. Specifically, we investigated whether activation in brain areas involved in reward-based decision-making would vary with degrees of uncertainty. and assessed the intensity of activation in brain regions related to gain/reward and loss.
RESULTS
Quantitative analysis of participants
A total of 15 healthy participants were included in the final analysis.
Baseline data
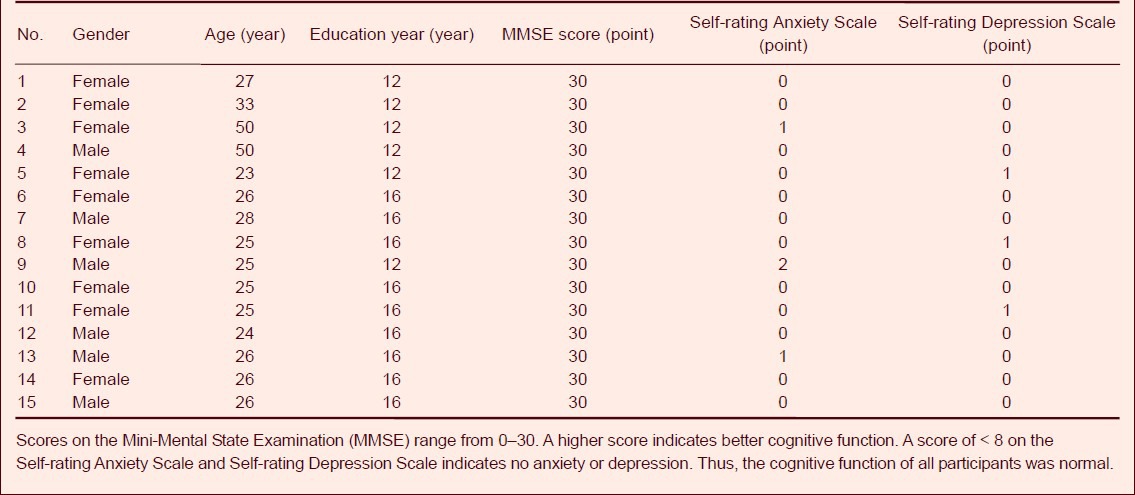
All 15 participants were right handed, with normal cognitive function. Detailed baseline data are listed in Table 1.
Table 1.
Baseline data of healthy participants
Brain areas activated by different reward-based decision-making conditions and activation intensity
Our experimental methods were controlled using E-Prime 2.0 software. Using an audiovisual stimulation system, we presented a decision-making task with three conditions: certainty, risk, and ambiguity. We used fMRI to observe the activated brain areas and activation intensity under the different task conditions. The results showed that the orbitofrontal cortex was activated during decision-making trials in the ‘certain’ condition, with an average activation intensity of 2.432 8 ± 0.194 9 (P < 0.05). In the ‘risk’ and ‘ambiguity’ trials, we observed activation in the prefrontal lobe (dorsolateral and ventrolateral prefrontal lobe, frontal pole of prefrontal lobe, and orbitofrontal cortex), precentral gyrus, limbic lobe, inferior parietal lobe, middle temporal gyrus, temporal pole, occipital lobe and visual cortex, cerebellar posterior lobe, and midbrain Tables 2, 3, Figure 1A–C).
Table 2.
Activated brain areas and activation intensity in healthy participants during risky decision-making
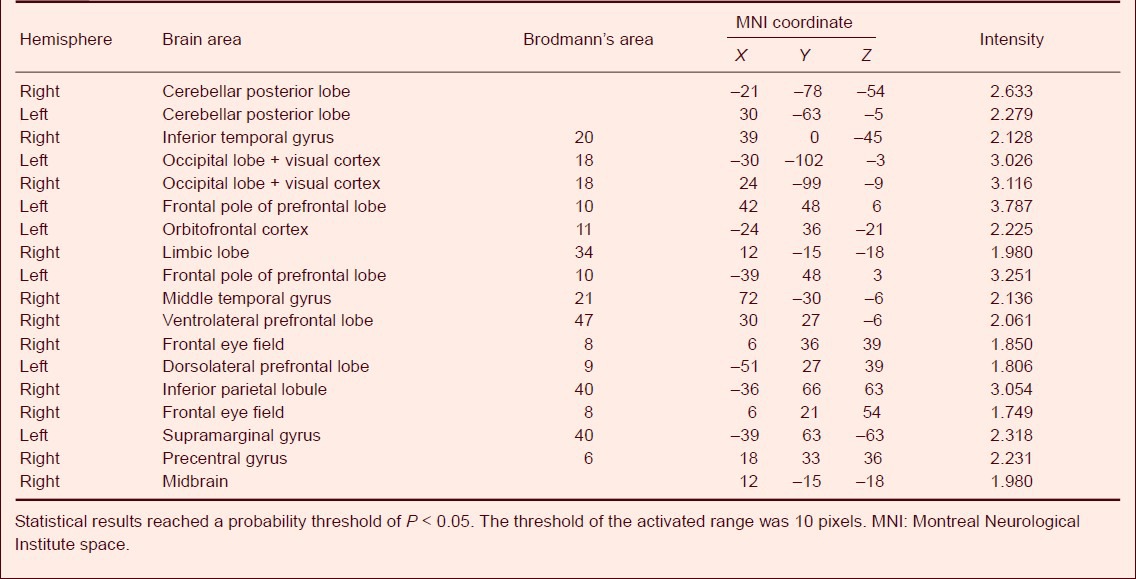
Table 3.
Activated brain areas and activation intensity in healthy participants during reward-based decision-making in the ‘ambiguous’ condition
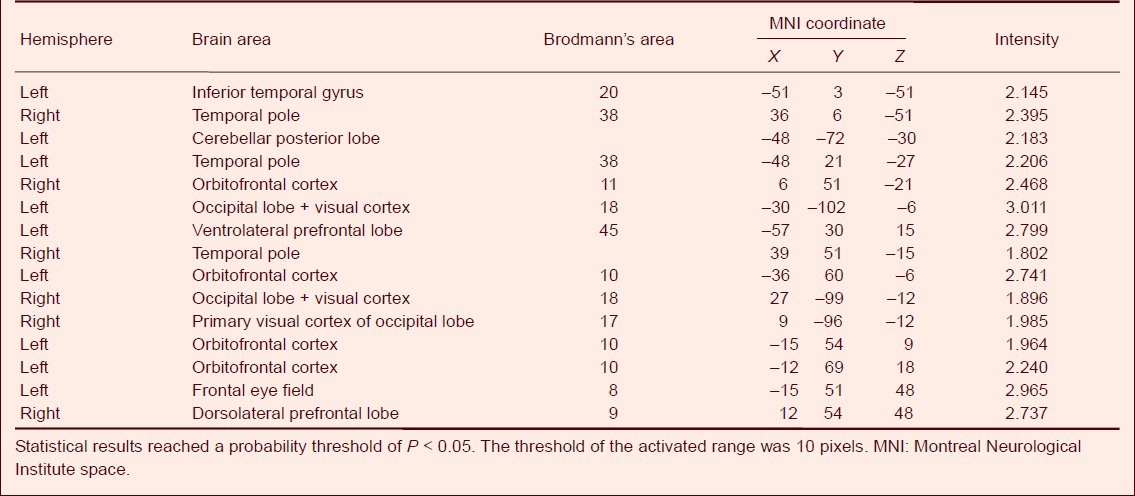
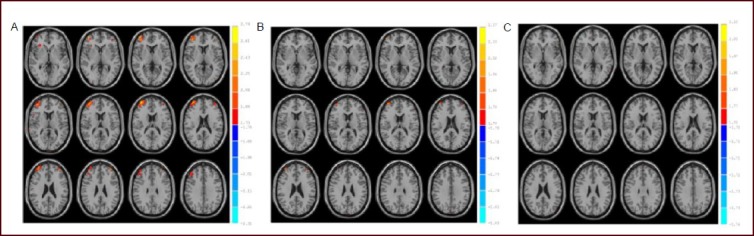
Figure 1.
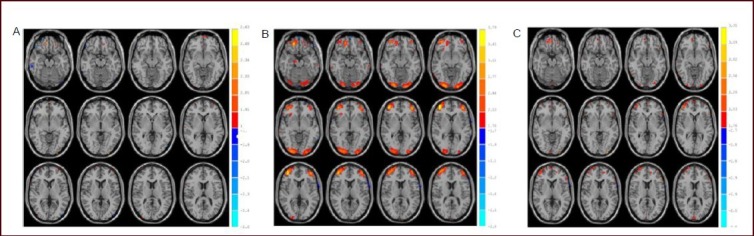
Activated brain areas of healthy participants in the ‘certain’ (A), ‘risk’ (B) and ‘ambiguous’ (C) conditions by functional MRI.
Statistical results reached a probability threshold of P < 0.05. The threshold of the activated range was 10 pixels. Red: Activated areas.
Difference of activated brain areas in reward-based decision-making under uncertainty
Compared with the reward-based decision-making in the ‘certain’ condition, the orbitofrontal cortex (BA11), ventrolateral prefrontal lobe (BA47), frontal pole of prefrontal lobe (BA10), precentral gyrus (BA6), inferior temporal gyrus and fusiform gyrus (BA20), inferior parietal lobule (BA40), supramarginal gyrus (BA40) and cerebellar posterior lobe showed significantly greater activation in the ‘risk’ condition (P < 0.05). Compared with the reward-based decision-making in the ‘certain’ condition, the frontal pole of the prefrontal lobe (BA10) showed significantly greater activation in the ‘ambiguity’ condition (P < 0.05). Compared with decision-making in the ‘risk’ condition, the dorsolateral prefrontal lobe and the cerebellar posterior lobe showed significantly greater activation in the ‘ambiguity’ condition (P < 0.05; Table 4, Figure 2A–C).
Table 4.
Three degrees of uncertainty in decision-making in healthy participants
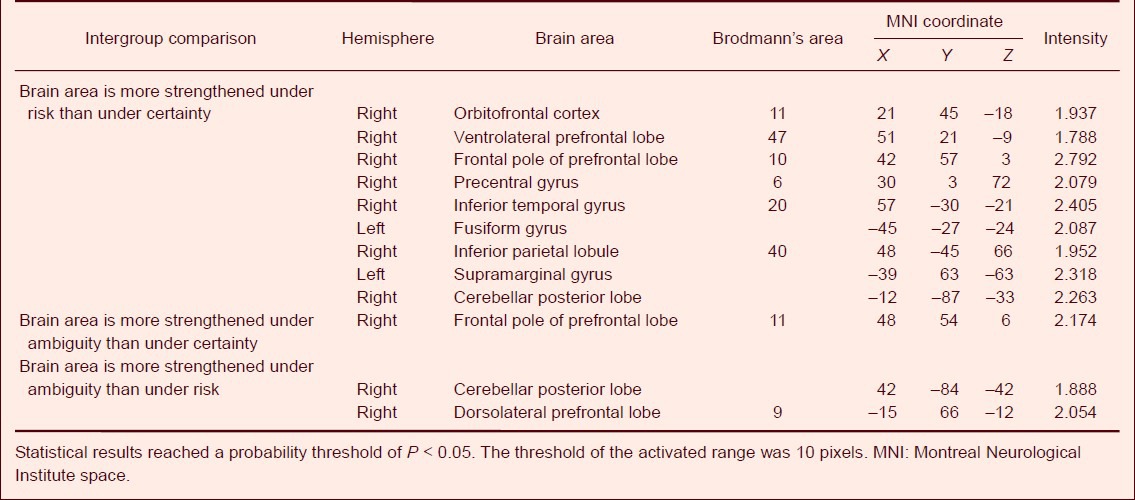
Figure 2.
Difference of activated brain areas in reward-based decision-making under uncertainty by functional MRI.
(A) Activation in the ‘risk’ condition was greater than that in the ‘certain’ condition. (B) Activation was greater in the ‘ambiguous’ than the ‘certain’ condition in healthy participants. (C) Activation was greater in the “ambiguous’ than the ‘risk’ condition in healthy participants. Red: Activated brain areas. Statistical results reached a probability threshold of P < 0.05. The threshold of the activated range was 10 pixels.
DISCUSSION
Decision-making encompasses certainty and uncertainty. Uncertain decisions can be sorted according to levels of risk and ambiguity, depending on the level of knowledge about the probability a particular result[2,13]. Risk decision-making refers to the possible states and corresponding outcomes of a choice. People can predict the probability of each state and the weight of its corresponding outcome. Under ambiguous decision-making conditions, it can be difficult to estimate the future probability of the appearance of certain states.
The cognitive processes underlying decision-making center around three main factors: perception and calculation of external information, evaluation of gains or losses, and plan implementation. Thus, decision-making requires the coordination of multiple brain areas. Previous studies have demonstrated that many brain regions are involved in decision-making, including the orbitofrontal cortex, medial prefrontal cortex, anterior cingulate cortex, amygdale, and corpus striatum (including nucleus accumbens)[14,15,16]. Different elements of decision-making, such as the evaluation and calculation of expected utility, have been associated with activation of specific brain regions[14,15,16]. Our experimental results indicated that the orbitofrontal cortex was activated during reward-based decision-making under ‘certain’ conditions. In the certain condition, precise and reliable information was available to the participant, and so we expected to see activation in reward-related brain areas[14,15,16].
Rolls and colleagues[17] suggested that activation of the orbitofrontal cortex is positively correlated with gain number and expected value. During risky decision-making, the uncertainty of the utility and expected outcomes is increased. Our results indicated that the dorsolateral and ventrolateral prefrontal lobe, frontal pole of prefrontal lobe, orbitofrontal cortex, precentral gyrus, limbic lobe, inferior parietal lobe, middle temporal gyrus, temporal pole, occipital lobe and visual cortex, cerebellar posterior lobe, and midbrain were activated during risky decision-making. Thus, it appears that these areas play a role in the calculation of utility, and thus perhaps these areas comprise a neural feedback loop in the brain regarding risk-reward decision-making. In previous research, the orbitofrontal cortex has been associated with gain[18]. The occipital lobe is known to be activated when calculating visual arabic numbers, and is involved in primary information encoding, digital computing, and logical reasoning[19]. The parietal cortex has been found to encode the probability of a possible occurrence of a visual stimulus-coupled reward in economic decision-making[19]. Activity in midbrain dopaminergic neurons, as well as the orbitofrontal cortex, insular cortex, and cingulate cortex, has been associated with probability and quantity of reward, such that activity increases with gain[17]. Tom et al[20] verified that during a gambling task, when the probability of either a loss or a gain was 50%, an increase in the frequency of gains was associated with enhanced midbrain dopaminergic activity, and vice versa. Another study found that losses were associated with activity in the inferior parietal lobule and cerebellum[18]. We found activation in the cerebellar posterior lobe and inferior parietal lobule, which appeared to be related to the uncertainty of a rewarding opportunity when making a risky decision. The sense of uncertainty may have resulted in a negative perception about the outcome. The above-mentioned processing may have activated the cerebellar posterior lobe and inferior parietal lobule, as these regions are known to process negative events and calculate outcomes[21].
Our experimental results demonstrated that decision-making under ambiguous conditions mainly activated the frontal pole of the prefrontal lobe, the orbitofrontal cortex, dorsolateral and ventrolateral prefrontal lobe, temporal pole of the temporal lobe, inferior temporal gyrus, primary visual cortex of the occipital lobe, the visual cortex, and the cerebellar posterior lobe. A previous study reported that the orbitofrontal cortex, amygdale, and prefrontal cortex were related to decision-making under ambiguous condition[3]. Ambiguity in decision-making, that is, the uncertainty of an unknown probability, has been positively correlated with activation of the amygdale and orbitofrontal cortex, and negatively correlated with the activity in the striatal system[22]. This increased activity in the amygdale and orbitofrontal area may be related to fear and negative emotions associated with ambiguity. Likewise, the benefit-driving function of the striatal system decreases with ambiguity, along with positive perceptions and emotions[22]. Simultaneous activation of the amygdala, orbitofrontal area, and striatal system has been associated with escape behavior[23]. Therefore, ambiguity in decision-making increases avoidance behavior[23,24]. Under conditions of ambiguity, individuals may exhibit avoidance behavior, regardless of the probability of gains or losses[23,24]. Temporal lobe activation has been associated with the processing of large rewards through feedback learning and strategy transformation[25]. A previous study demonstrated that the degrees of ambiguity in an option affected decision-making behavior, and, when faced with several different ambiguous choices, people commonly exhibited anxiety, doubt, and aversive behavior[26]. The higher the degree of ambiguity, the more complicated the process of decision-making, and the greater the number of activated brain areas[27]. This was consistent with our results. Activation of the cerebellum was associated with losses[20]. In sum, decision-making under ambiguous conditions appeared to elicit activation of the cerebellar posterior lobe, which was likely associated with an increase in negative perceptions induced by ambiguity[24,27].
The ventrolateral prefrontal lobe, frontal pole of the prefrontal lobe, cerebellar posterior lobe, inferior temporal gyrus, fusiform gyrus, inferior parietal lobule, precentral gyrus, and supramarginal gyrus were more strongly activated during decision-making in risky compared with certain conditions. The dorsolateral prefrontal lobe and cerebellar posterior lobe exhibited significantly greater activation in the ‘ambiguous’ compared with the ‘risky’ condition. A previous study demonstrated that the frontal lobe is involved in information encoding, and is associated with reward/punishment processing, emotion, and motivation[27]. The prefrontal cortex has been found to play a role in the judgment component of decision-making[28]. The lateral prefrontal lobe is important for calculating future utility during decision-making[29]. It is possible that we observed greater activation of the inferior parietal lobule and cerebellar posterior lobe in the ‘ambiguous’ condition, because ambiguity can induce fear and behavioral avoidance[21]. Our results indicate that the different degrees of uncertainty in our task activated several brain areas that play distinct roles in processing the return probability of decisions. Much of this processing appears to emerge from reward-related neural structures[20]. However, the precise mechanisms underlying reward-based decision-making require further investigation. The probability of a reward, degree of ambiguity, and size of a reward contribute to the degree of uncertainty in reward-related decision-making. Thus, we believe that our task simulated a real decision-making process. The present study utilized a GE 3.0T magnetic resonance scanner and brain function audiovisual stimulation system (SA-9800 system), which are advanced methods and instruments in China.
SUBJECTS AND METHODS
Design
A block design, random sampling study.
Time and setting
Experiments were conducted at the Department of Medical Imaging, Affiliated Hospital of Medical College, Qingdao University, China, from October 2011 to August 2012.
Subjects
A total of 15 healthy individuals who underwent a medical examination at the Affiliated Hospital of the Medical College, Qingdao University, China, between October 2011 and August 2012 were randomly recruited for this study. There were 7 males and 8 females. The participants were right handed, 20–55 years of age (average 29.3 ± 8.7 years), and had an average of 14.4 ± 2.0 years of education. Cognitive ability was normal, and all participants received a score of at least 30 on the Mini-Mental State Examination (MMSE)[30]. There was no history of heart, brain, liver, or kidney disease. The participants were emotionally stable, and did not suffer from depression or anxiety, as measured by the Self-rating Anxiety Scale and Self-rating Depression Scale[31]. All participants reported no family history of mental illness. All participants provided written informed consent. Before the experiment, we obtained demographic and contact information for each participant.
Methods
Experimental tasks
Study design: in accordance with previously published methods[32,33], stimuli were presented on a computer screen as participants lay in the fMRI machine. A box on the screen contained 10 poker cards, which included both diamonds and clubs. The cards randomly appeared on either the left or right side of the screen. The quantity of each kind of poker card in the box was indicated in the certain and risk conditions, but not indicated in the ambiguous condition. In each trial, the participant was asked to draw a poker card from the box according to the predilection. If the participants selected the card on the left side, they pressed a button marked “1”. If the participants selected the card on the right side, they pressed a button marked “4”. After selection, the computer automatically displayed whether the participant had obtained the poker card that they had expected. If the participant obtained the poker card that he/she expected, they were given a reward of “+10”. If not, the participant was not given a reward or punishment, only a score of 0 for that trial. Scores were automatically recorded by the computer. Following this task, the participants were given the amount of money that they had won in RMB.
There were three decision-making situations. (1) The box contained 10 clubs and 0 diamonds (reward-based decision-making in ‘certain’ condition). (2) The box contained six diamonds and four clubs (reward-based decision-making in the ‘risk’ condition). (3) The box contained four clubs and three diamonds, and the remaining three poker cards were uncertain, either three diamonds, three clubs, two diamonds and one club, or one club and two diamonds (reward-based decision-making in the ‘ambiguous’ condition). A control task was designed to assess activation caused by visual recognition of the clubs and diamonds: the box contained two kinds of poker cards (diamonds and clubs), which randomly appeared on both sides of the screen. The participant always was asked to draw a diamonds card from the box, but no points were scored, and no reward or punishment in this condition. All the subjects were informed the above-mentioned requirement, scoring and reward conditions.
Experimental design
Each task contained a stimulation, feedback, and break component. The results of each trial were shown during the feedback component, and the screen displayed a fixation cross (“+”) during the break. Each task consisted of stimulation for 2 000 ms, feedback for 1 000 ms, and break for 1 000 ms. Each task was conducted five times, followed by a resting period of 12 000 ms. The control task contained the stimulation component for 2 000 ms and the break component 2 000 ms, and had no feedback component. The experiment was conducted in three BLOCK cycles with two condition tasks followed by one control task in each cycle.
Experimental procedure
Experimental procedures were controlled using E-Prime 2.0 software (PST, Pittsburgh, Pennsylvania, USA). The data were loaded into a brain function audiovisual stimulation system (SA-9800 system; Shenzhen Sinorad Medical Electronics Inc., Shenzhen, Guangdong Province, China) and presented to the participants. Simultaneously, we obtained data from the functional MRI. After receiving an explanation of the experimental procedure, the participants completed a practice session, and then completed the experiment. During the experiment, the participant was placed in a horizontal position on the bed of a GE 3.0T magnetic resonance scanner (GE Healthcare, Bethesda, MD, USA), where they were able to see the screen, and give their response via a handheld controller. The brain function audiovisual stimulation system automatically recorded the participant responses, including reaction time, choice, and score after each selection.
The GE 3.0T magnetic resonance scanner applied echo planar imaging and BOLD imaging. Scanning parameters were as follows: repetition time/echo time 2 000 ms/30 ms, flip angle 75°, field of view 230 × 230 mm, slice thickness 4 mm, yielding 33 slices in total with 205 sets of images. Total scanner time was 410 seconds. We used Excel and SPSS database for data analysis.
Analysis of functional MRI data
A total of 602 trials were included in our analysis. We obtained 33 slices (no interval, whole brain) every 2 seconds. Using the Matlab platform (The MathWorks, Natick, MA, USA), the functional images were preprocessed and individually analyzed using SPM8 (Hammersmith hospital, Hammersmith, UK). Preprocessing of image space was conducted using time correction, head motion correction, spatial normalization, and Gaussian smoothing. The original data were screened, and data that did not exceed motor correction normalization (three-dimensional panning did not exceed 0.5 mm, and three-dimensional rotation did not exceed 0.5°) were statistically analyzed[11].
Individual analysis
Linear regression analysis of the matrix and real functional MRI data from each condition were performed using SPM8. Activated brain areas were obtained for each participant: activated area in the condition task (region of interest-condition), and activated area in the control task (region of interest-control). We used a t-test to compare the region of interest-task condition and the region of interest-control. The activated brain areas in each task were obtained for each participant during decision-making. The value range was identified using a t-test, with a significance threshold of P < 0.001. The threshold of the activated range was five pixels. That is, significantly activated areas comprised regions where five or more pixels were continuously activated.
Group analysis
Using REST software (Beijing Normal University, Beijing, China), we analyzed the data for all participants for each task. The statistical significance threshold was P < 0.05. The threshold of the activated range was 10 pixels. The mean activated map was obtained, and overlaid on the Talairaeh template to localize the activated areas in the three task conditions.
Intergroup analysis
Individual decision-making data in the ‘certain’, ‘risk’ and ‘ambiguous’ conditions were grouped and compared using a two-sample t-test (P < 0.05). The relevant activated brain areas were reported using the “report” command in the xjView program (Beijing Normal University, China).
Statistical analysis
SPM8 software was utilized to analyze the functional images based on the MATLAB platform. REST software was employed to analyze and gain activated maps under the different decision-making conditions. The differences between the certain, risk, and ambiguous task conditions were compared using a two-sample t-test. Statistical results reached a probability threshold of P < 0.05. The threshold of the activated range was 10 pixels[33].
Acknowledgments
We are very grateful to Xu WJ from the Department of Medical Imaging, Affiliated Hospital of Medical College, Qingdao University, China, for assistance with the design of the methods.
Footnotes
Funding: This study was supported by the Science and Technology Development Project of Shandong Province, China, No. 2011YD18045; the Natural Science Foundation of Shandong Province, China, No. ZR2012HM049; the Health Care Foundation Program of Shandong Province, China, No. 2007BZ19; the Foundation Program of Technology Bureau of Qingdao, China; No. Kzd-03; 09-1-1-33-nsh.
Conflicts of interest: None declared.
Ethical approval: This study was approved by the Ethics Committee at the Affiliated Hospital of the Medical College of Qingdao University, China.
(Reviewed by Koke S, Raye W, Zhao XP, Zhao Y)
(Edited by Wang LM, Qiu Y, Li CH, Song LP, Liu WJ, Zhao M)
REFERENCES
- [1].Zhang K, Qi W, Guo AK. Towards decision-making theories: the interface of neuroscience and economics. Science. 2008;60(3):5–9. [Google Scholar]
- [2].Kahneman D, Tversky A. Prospect theory: An analysis of decision under risk. In: Kahneman D, editor. Choices, Values, and Frames. New York: Cambridge University Press; 2000. [Google Scholar]
- [3].Huettel SA, Stowe CJ, Gordon EM, et al. Neural signatures of economic preferences for risk and ambiguity. Neuron. 2006;49(5):765–775. doi: 10.1016/j.neuron.2006.01.024. [DOI] [PubMed] [Google Scholar]
- [4].Wang L, Shen XY, Lin ZP. The research development of risk and ambugity decision making under the science of decision making. Dongnan Daxue Xuebao: Yixue Ban. 2010;29(4):473–476. [Google Scholar]
- [5].Lubman DI, Yücel M, Pantelis C. Addiction, a condition of compulsive behaviour? Neuroimaging and neuropsychological evidence of inhibitory dysregulation. Addiction. 2004;99(12):1491–1502. doi: 10.1111/j.1360-0443.2004.00808.x. [DOI] [PubMed] [Google Scholar]
- [6].Paulus MP, Rogalsky C, Simmons A, et al. Increased activation in the right insula during risk-taking decision making is related to harm avoidance and neuroticism. Neuroimage. 2003;19(4):1439–1448. doi: 10.1016/s1053-8119(03)00251-9. [DOI] [PubMed] [Google Scholar]
- [7].Sanfey AG, Loewenstein G, McClure SM, et al. Neuroeconomics: cross-currents in research on decision-making. Trends Cogn Sci. 2006;10(3):108–116. doi: 10.1016/j.tics.2006.01.009. [DOI] [PubMed] [Google Scholar]
- [8].Knutson B, Adams CM, Fong GW, et al. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neurosci. 2001;21(16):RC159. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Erk S, Spitzer M, Wunderlich AP, et al. Cultural objects modulate reward circuitry. Neuroreport. 2002;13(18):2499–2503. doi: 10.1097/00001756-200212200-00024. [DOI] [PubMed] [Google Scholar]
- [10].Shin R, Ikemoto S. Administration of the GABAA receptor antagonist picrotoxin into rat supramammillary nucleus induces c-Fos in reward-related brain structures. Supramammillary picrotoxin and c-Fos expression. BMC Neurosci. 2010;11:101. doi: 10.1186/1471-2202-11-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Forbes EE, Christopher May J, Siegle GJ, et al. Reward-related decision-making in pediatric major depressive disorder: an fMRI study. J Child Psychol Psychiatry. 2006;47(10):1031–1040. doi: 10.1111/j.1469-7610.2006.01673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Domenech P, Dreher JC. Decision threshold modulation in the human brain. J Neurosci. 2010;30(43):14305–14317. doi: 10.1523/JNEUROSCI.2371-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Trepel C, Fox CR, Poldrack RA. Prospect theory on the brain. Toward a cognitive neuroscience of decision under risk? Brain Res Cogn Brain Res. 2005;23(1):34–50. doi: 10.1016/j.cogbrainres.2005.01.016. [DOI] [PubMed] [Google Scholar]
- [14].Christakou A, Brammer M, Giampietro V, et al. Right ventromedial and dorsolateral prefrontal cortices mediate adaptive decisions under ambiguity by integrating choice utility and outcome evaluation. J Neurosci. 2009;29(35):11020–11028. doi: 10.1523/JNEUROSCI.1279-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Shimizu K, Udagawa D. How can group experience influence the cue priority? A re-examination of the ambiguity-ambivalence hypothesis. Front Psychol. 2011;2:265. doi: 10.3389/fpsyg.2011.00265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Chiu YC, Lin CH, Huang JT, et al. Immediate gain is long-term loss: Are there foresighted decision makers in the Iowa Gambling Task? Behav Brain Funct. 2008;4:13. doi: 10.1186/1744-9081-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Rolls ET, McCabe C, Redoute J. Expected value, reward outcome, and temporal difference error representations in a probabilistic decision task. Cereb Cortex. 2008;18(3):652–663. doi: 10.1093/cercor/bhm097. [DOI] [PubMed] [Google Scholar]
- [18].Dickhaut J, McCabe K, Nagode JC, et al. The impact of the certainty context on the process of choice. Proc Natl Acad Sci U S A. 2003;100(6):3536–3541. doi: 10.1073/pnas.0530279100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Yang T, Shadlen MN. Probabilistic reasoning by neurons. Nature. 2007;447(7148):1075–1080. doi: 10.1038/nature05852. [DOI] [PubMed] [Google Scholar]
- [20].Tom SM, Fox CR, Trepel C, et al. The neural basis of loss aversion in decision-making under risk. Science. 2007;315(5811):515–518. doi: 10.1126/science.1134239. [DOI] [PubMed] [Google Scholar]
- [21].Liu X, Powell DK, Wang H, et al. Functional dissociation in frontal and striatal areas for processing of positive and negative reward information. J Neurosci. 2007;27(17):4587–4597. doi: 10.1523/JNEUROSCI.5227-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hsu M, Bhatt M, Adolphs R, et al. Neural systems responding to degrees of uncertainty in human decision-making. Science. 2005;310(5754):1680–1683. doi: 10.1126/science.1115327. [DOI] [PubMed] [Google Scholar]
- [23].Aïte A, Cassotti M, Rossi S, et al. Is human decision making under ambiguity guided by loss frequency regardless of the costs? A developmental study using the Soochow Gambling Task. J Exp Child Psychol. 2012;113(2):286–294. doi: 10.1016/j.jecp.2012.05.008. [DOI] [PubMed] [Google Scholar]
- [24].Rode C, Cosmides L, Hell W, et al. When and why do people avoid unknown probabilities in decisions under uncertainty? Testing some predictions from optimal foraging theory. Cognition. 1999;72(3):269–304. doi: 10.1016/s0010-0277(99)00041-4. [DOI] [PubMed] [Google Scholar]
- [25].Song DH, Zhu YL, Xi CH. Deficit of decision making in patients with temporal lobe cerebral infarction. Anhui Yiyao. 2012;16(5):626–627. [Google Scholar]
- [26].Zhang L, Wang K, Zhu CY, et al. Trait anxiety has effect on decision making under ambiguity, but not decision making under risk. Zhonghua Xingwei Yixue yu Nao Kexue Zazhi. 2011;20(11):980–982. [Google Scholar]
- [27].Padoa-Schioppa C, Assad JA. Neurons in the orbitofrontal cortex encode economic value. Nature. 2006;441(7090):223–226. doi: 10.1038/nature04676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Han S, Huettel SA, Dobbins IG. Rule-dependent prefrontal cortex activity across episodic and perceptual decisions: an fMRI investigation of the criterial classification account. J Cogn Neurosci. 2009;21(5):922–937. doi: 10.1162/jocn.2009.21060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Zamarian L, Sinz H, Bonatti E, et al. Normal aging affects decisions under ambiguity, but not decisions under risk. Neuropsychology. 2008;22(5):645–657. doi: 10.1037/0894-4105.22.5.645. [DOI] [PubMed] [Google Scholar]
- [30].Xu XJ, Guo ZJ. The study of decision-making processing on patients with cerebral infraction in different regions. Zhonghua Xingwei Yixue yu Nao Kexue Zazhi. 2013;22(1):21–24. [Google Scholar]
- [31].Liu G, Lu Q, Zhang Q, et al. Preliminary exploration of the frontal-cingulate functional connection during identifying sad facial expression in depressed patients. Zhonghua Xingwei Yixue yu Nao Kexue Zazhi. 2010;19(12):1057–1059. [Google Scholar]
- [32].Ellsberg D. Risk, ambiguity, and the savage axioms. Quart J Econ. 1961;75(4):643–669. [Google Scholar]
- [33].Smoski MJ, Felder J, Bizzell J, et al. fMRI of alterations in reward selection, anticipation, and feedback in major depressive disorder. J Affect Disord. 2009;118(1-3):69–78. doi: 10.1016/j.jad.2009.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]


