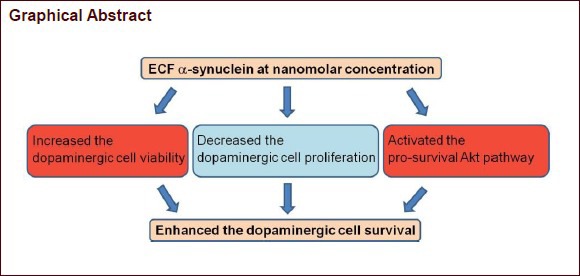
Keywords: neural regeneration, alpha-synuclein, neuronal survival, nanomolar, extracellular, phosphorylated Akt, SH-SY5Y cell, neuronal differentiation, proliferation, dopaminergic, 5-bromo-2’-deoxyuridine, grants-supported paper, neuroregeneration
Abstract
Although alpha-synuclein is generally thought to have a pathological role in Parkinson's disease, accumulative evidence exists that alpha-synuclein has a neuroprotective effect. The aim of this study was to evaluate the effect of extracellular alpha-synuclein on dopaminergic cell survival. We assessed cell viability using the 3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyltertazolium bromide (MTT) assay both in undifferentiated SH-SY5Y (SHSY) cells and neuronally-differentiated SH-SY5Y (ndSHSY) cells after 24 hour treatment with monomeric alpha-synuclein at various concentrations (0 [control], 50, 100 nmol/L, 1 μmol/L). To determine whether cell viability assessed by MTT assay was affected by cell proliferation, 5-bromo-2’-deoxyuridine (BrdU) incorporation assay was performed. Level of both Akt and phosphorylated Akt was measured using western blot method in ndSHSY cells with or without 24 hour alpha-synuclein treatment. Cell viability was increased in ndSHSY cells at the nanomolar concentration of alpha-synuclein, but not in SHSY cells. Proportion of BrdU-positive ndSHSY cells was decreased in alpha-synuclein-treated group compared with control group. Level of phosphorylated Akt in alpha-synuclein-treated group was higher compared with the control group. Our study shows that extracellular alpha-synuclein at nanomolar concentration benefits dopaminergic cell survival via Akt pathway.
INTRODUCTION
Alpha-synuclein is a principal component of Lewy bodies and Lewy neurites, which are pathologic hallmarks of Parkinson's disease[1,2]. Alpha-synuclein is generally considered to play a role in synaptic activity[3], although its function remains largely unknown. Mutations in three loci (A53T, A30P, and E46K)[4,5,6] and multiplication[7,8] in the alpha-synuclein encoding gene have been recognized as causes of the inherited form of Parkinson's disease. Therefore, it is proposed that alpha-synuclein contributes to the pathogenesis of Parkinson's disease.
Alpha-synuclein is conventionally known as an intraneuronal protein. However, a series of studies demonstrated the presence of alpha-synuclein in extracellular fluids, possibly as a result of the unconventional exocytosis of intravesicular alpha-synuclein[9]. The collected data suggest cytotoxic effects of the fibrillar aggregates[10,11] or the protofibrillar or oligomeric aggregates[12] of extracellular alpha-synuclein. In addition, it has been shown that aggregated extracellular alpha-synuclein fibrils can be internalized in the cells and enhance the intracellular formation of protein inclusions, leading to cell death[13]. Therefore, extracellular alpha-synuclein has been indicated as a treatment target of Parkinson's disease[14], and passive immunization for alpha-synuclein has recently been attempted[15,16].
Conversely, there is emerging evidence suggesting that alpha-synuclein has also neuroprotective effects. In one study, extracellular alpha-synuclein treatment at nanomolar concentrations protected neurons against cellular stresses such as serum deprivation, oxidative stress, and excitotoxicity through the PI3/Akt signaling pathway[17]. In a transgenic mouse model, the increased expression of alpha-synuclein prevented paraquat-induced dopaminergic cell degeneration[18]. Endogenous alpha-synuclein induction by valproic acid showed protective effects against glutamate-induced excitotoxicity in rat cerebellar granule cells[19]. Wild-type alpha-synuclein rescued dopaminergic cells from both acute and chronic cytotoxicity induced by rotenone and maneb whereas mutant alpha-synuclein did not[20].
Thus far, it is uncertain as to whether alpha-synuclein plays a favorable role for neurons at physiologic concentrations, which are estimated to be from picomolar to nanomolar levels in cerebrospinal fluid[9,10]. In this study, we attempted to assess the effect of extracellular alpha-synuclein at nanomolar concentrations on neuronal survival in cultured dopaminergic cell lines.
RESULTS
Effect of extracellular alpha-synuclein on cell viability in neuronally-differentiated SH-SY5Y (ndSHSY) cells
The effect of monomeric alpha-synuclein on the viability of undifferentiated SH-SY5Y (SHSY) cells and ndSHSY cells is shown in Figure 1. In ndSHSY cells, viability was increased when they were treated with alpha-synuclein for 24 hours at 50 nmol/L (from 100% to 126.8%, P < 0.001, data were presented as percentage of control) and at 100 nmol/L (from 100% to 120.2%, P = 0.001), while cell viability did not change significantly in cells treated with 1 μmol/L alpha-synuclein (P = 0.128). The change in cell viability was not observed in alpha-synuclein-treated SHSY cells (Figure 1).
Figure 1.
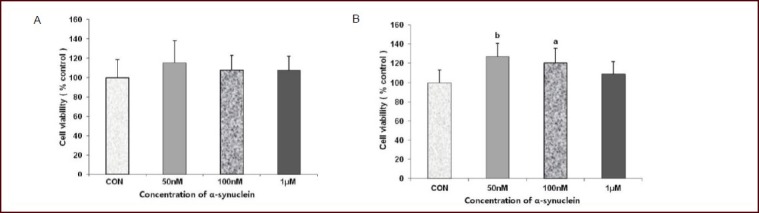
Different effects of alpha-synuclein on cell viability in undifferentiated and neuronally-differentiated SH-SY5Y cells.
Cell viability was not significantly increased in undifferentiated SH-SY5Y cells treated with alpha-synuclein (A) but significantly increased in neuronally-differentiated SH-SY5Y cells treated with nonomolar (50 and 100 nmol/L [M]) alpha-synuclein (B). Values are represented as mean ± SD for three independent experiments. aP < 0.05, bP < 0.01, vs. control (CON) group (Mann-Whitney U test).
ndSHSY cell proliferation after alpha-synuclein treatment
To see whether cell viability increased after alpha-synuclein treatment with increased cellular proliferation, cell proliferation was measured by determining the proportion of number of both 5-bromo-2’-deoxyuridine (BrdU) and 4’,6-diamidino-2-phenylindole (DAPI)-positive cells to number of DAPI-positive cells in 100-fold microscopic filed (Figures 2, 3). The proportion of BrdU-positive cells was decreased after addition of alpha-synuclein for 24 hours compared to control (Figures 2, 3).
Figure 2.
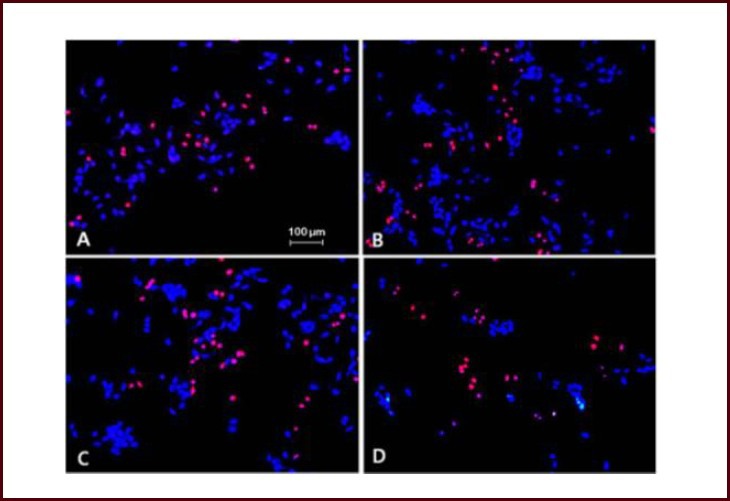
Cell proliferation after treatment with different concentrations of alpha-synuclein.
(A) Control, (B) alpha-synuclein 50 nmol/L, (C) alpha-synuclein 100 nmol/L, (D) alpha-synuclein 1 μmol/L. 4’,6-Diamidino-2-phenylindole (DAPI) staining (blue), 5-bromo-2’-deoxyuridine (BrdU) staining (red), × 100. Proportion of BrdU-positive cells was decreased after 24 hour alpha-synuclein treatment in neuronally-differentiated SH-SY5Y cells.
Figure 3.
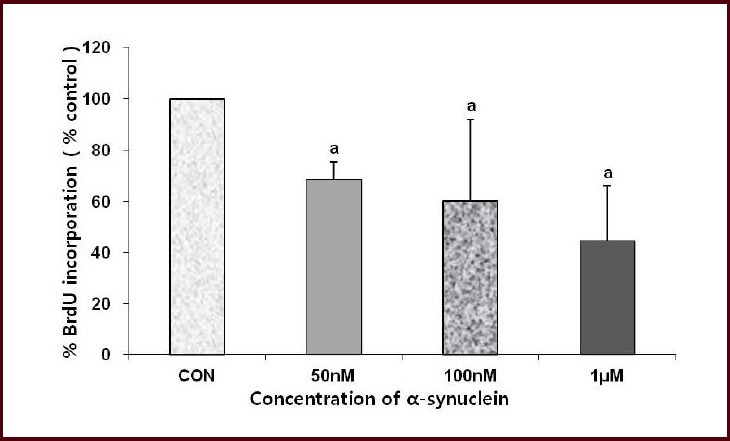
Effect of alpha-synuclein on cell proliferation in neuronally-differentiated SH-SY5Y cells.
%BrdU incorporation was assessed by percentage of number of BrdU(+)/DAPI(+) cells and represented as percentage of control. %BrdU in corporation was decreased after alpha-synuclein treatment. Cell count was done in 100-fold microscopic field and values are represented as mean ± SD of three individual experiments. aP < 0.05, vs. control (CON) group (Mann-Whitney U test). BrdU: 5-Bromo-2’-deoxyuridine; DAPI: 4’,6-diamidino-2-phenylindole.
Involvement of Akt pathway in alpha-synuclein related ndSHSY cell survival
To identify the mechanisms underlying the effects of nanomolar extracellular alpha-synuclein on cell survival, we measured the level of both Akt and phosphorylated Akt in ndSHSY cells after treatment with 100 nmol/L alpha-synuclein for 24 hours. Results showed that, compared to control, level of phosphorylated Akt was up-regulated after treatment with 100 nmol/L alpha-synuclein for 24 hours (Figure 4).
Figure 4.
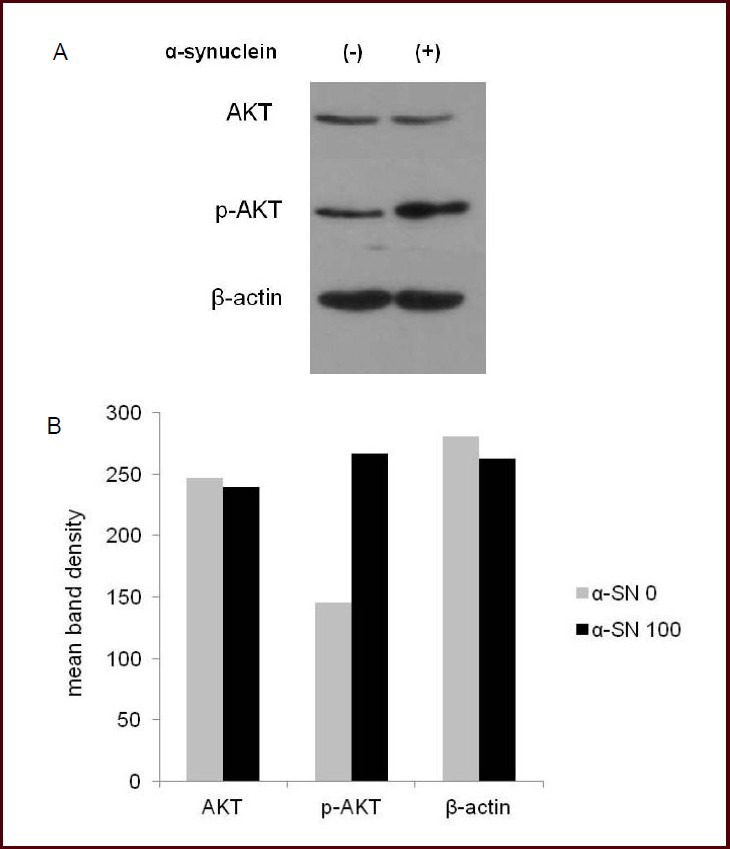
Western blot analysis for AKT and phosphorylated AKT (p-AKT) after alpha-synuclein (α-SN) treatment.
Level of p-AKT (60 kDA) increased in neuronally-differentiated SH-SY5Y cells treated with 100 nmol/L α-SN than in control (A). The mean band density of two independent experiments (B).
DISCUSSION
In the present study, nanomolar concentrations of alpha-synuclein increased ndSHSY cell survival, which was accompanied by an increase in Akt phosphorylation.
Cell viability was increased after treatment with nanomolar alpha-synuclein (at 50 and 100 nmol/L) in ndSHSY cells. The increased cell viability induced by alpha-synuclein could be attributed to the increased cell proliferation or cell protection against death. A BrdU immunoassay revealed a significant decrease in the proportion of BrdU-positive cells after the alpha-synuclein treatment at all concentrations (0 [control], 50, 100 nmol/L, 1 μM)). The decreased proportion of BrdU-positive proliferating cells might be caused by the increased proportion of BrdU-negative non-proliferating surviving cells. As a result, we could exclude a bias caused by the cell proliferation from the increased ndSHSY cell viability. However, the exact mechanism of the decrease in the proportion of proliferating cells remains as a subject for future research.
Cell viability in SHSY cells showed an uptrend at 50 nmol/L but the change was not statistically significant. However, Seo et al[17] showed that extracellular alpha-synuclein plays a protective role in SHSY cells as well. Therefore, further studies under a stress condition using SHSY cells are required.
The Akt pathway is a major neuronal pro-survival pathway which is activated in response to growth factor, oxidative stress and nutrient deprivation. Some neuroprotective agents, such as caffeine and insulin-like growth factor 1, have been shown to afford significant anti-apoptotic effects in Parkinson's disease cellular models via the activation of PI3/Akt pathways[21,22]. The Akt pathway was also linked to the survival of various neurons via Bcl-2 and Bax[17]. The Akt pathway is involved in the modulation of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) toxicity on dopaminergic neurons lead by alpha-synuclein in the ventral midbrain[23]. In our study, the increased p-Akt levels after the alpha-synuclein treatment indicated that the increased neuronal survival rate after the alpha-synuclein treatment is likely to be associated with the activation of the Akt pathway.
A nanomolar concentration of extracellular alpha-synucl-ein enhanced dopaminergic neuronal survival in our study; this effect disappeared when the level of alpha-synuclein reached a micromolar concentration. Our result is in line with a previous report which showed that a low concentration of alpha-synuclein protected neurons against various toxic insults such as hypoxia, oxidative stress, and glutamate toxicity via the PI3/Akt pathway, whereas a high concentration or overexpression of alpha-synuclein had a cytotoxic effect on neurons[17].
The role of extracellular alpha-synuclein remains to be elucidated as regards the pathogenesis of Parkinson's disease. Although alpha-synuclein clearing by immunization was recently attempted[15,16,24] as a novel treatment, our results allude to the possibility of an undesirable effect of alpha-synuclein clearing therapy on dopaminergic neurons.
Our study has still several limitations. First, it involved an in vitro condition, and the SHSY and ndSHSY cells we used could not precisely mirror real dopaminergic neurons in vivo. The effect of a nanomolar concentration of extracellular alpha-synuclein may be different in an in vivo condition, where neurons and glial cells interact with each other. Second, only the effect of the monomeric form of alpha-synuclein was evaluated in this study. In addition to the monomeric form of alpha-synuclein, various forms of alpha-synuclein such as the oligomer, fibril and aggregated alpha-synuclein exist in extracellular fluid. In fact, only a small portion of alpha-synuclein remains in the monomeric form in body fluids. Furthermore, modifications of alpha-synuclein such as truncation, ubiquitination, nitration and phosphorylation can have effects that differ from those in our results[25]. Third, the internalization of extracellular alpha-synuclein into dopaminergic neuronal cells was not assessed in our study. Therefore, it was unclear whether the enhanced cell survival by alpha-synuclein was mediated by some form of interaction between extracellular alpha-synuclein and certain receptors of cells or by the internalization of extracellular alpha-synuclein into the cells.
In this study, we provide evidence that extracellular alpha-synuclein enhances neuronal survival at a nanomolar concentration and this effect is most likely mediated by the Akt pathway. To the best of our knowledge, this is the first report describing a beneficial effect of extracellular alpha-synuclein on neuronal survival excluding the effect of alpha-synuclein on cell proliferation. Our study can update the role of alpha-synuclein in neuronal cells and its involvement in Parkinson's disease and related diseases.
MATERIALS AND METHODS
Design
This is an in vitro study using human dopaminergic cell line (SHSY cells and ndSHSY cells).
Time and setting
This experiment was performed at the Neurology Laboratory of Biomedical Research Institute, Seoul National University Hospital, South Korea, from March to December in 2012.
Materials
Monomeric alpha-synuclein 1:1 000 (ATGen, Seongnam, South Korea), 3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenylt-ertazolium bromide (MTT) solution (Sigma-Aldrich, St. Louis, MO, USA), dimethyl sulfoxide (DMSO; Sigma-Aldrich), DAPI (1:300) vector and anti-β actin (1:500) (Sigma-Aldrich) were used. Primary antibodies include mouse anti-BrdU (1:300) (BD Biosciences, San Jose, CA, USA), rabbit anti-Akt (1:1 000) (Cell Signaling Technology, Boston, MA, USA), and rabbit anti-phosphorylated Akt (1:500) (Cell Signaling Technology). Secondary antibodies include Cy3 conjugated goat anti-mouse (1:100) antibody and anti-rabbit IgG (1:5 000) antibody.
Methods
Cell culture and treatment
The human SH-SY5Y neuroblastoma cell line was purchased from ATCC and they were maintained in Dulbecco's modified Eagles medium (DMEM) supplemented with 10% fetal bovine serum (FBS; Hyclone, Logan, UT, USA), 100 U/mL penicillin, 100 μg/μL streptomycin and 2 mL glutamine (Gibco BRL, Gaithersburg, MD, USA) at 37°C in 95% air/5% CO2. SHSY cells were treated with 50 μmol/L retinoic acid and cultured for 5 days for neuronal differentiation. Both SHSY and ndSHSY cells were treated with 50, 100 nmol/L and 1 μmol/L concentration of alpha-synuclein for 24 hours.
Viability assay
Cell viability was determined by a mitochondrial enzyme-dependent reaction of MTT (Sigma-Aldrich). The cell suspensions (5 × 104 cells/well) were maintained for 24 hours and then treated with 0, 50, 100 nmol/L, 1 μmol/L monomeric alpha-synuclein for 24 hours. The media was removed and MTT solution (200 μL/well) was added to each well. After 2.5 hours of incubation, the MTT solution was removed. The formazan precipitate was dissolved in DMSO and was shaken for 5 minutes in room air. The absorbance was measured at 540 nm (Beckman spectrophotometer) using PBS as a blank. Cell survival was expressed as percentage of control.
BrdU incorporation and immunocytochemistry
After treatment with monomeric alpha-synuclein for 24 hours, the ndSHSY cells were labeled with 10 μmol/L BrdU solution and incubated for an additional 3 hours at 37°C. After the labeling medium was removed from the plates, the cells were fixed with 4% paraformaldehyde, MgCl2 and CaCl2 for 20 minutes. Subsequently, cells were treated with 1.5 mol/L HCl for 30 minutes, fixed with 4% paraformaldehyde in PBS for 20 minutes at room temperature, treated in 4% normal goat serum in PBS with 0.2% Triton X-100 for 1 hour at room temperature, and then incubated with a mouse anti-BrdU antibody (1:300; BD Biosciences) at 4°C overnight. PBS washes were performed between each step. After washing the plates, cells were incubated with goat anti-mouse IgG conjugated to a fluorescein Cy3 label (1:100, Vector) for 1 hour at 37°C. The nuclei were counterstained with DAPI. The number of cells was counted under × 100 magnification. The images were photographed using confocal microscopy (Leica Microsystems, Wetzlar, Germany).
Western blot analysis for Akt and phosphorylated Akt
The cells were washed with ice-cold PBS and protein was extracted in a lysis buffer [50 mmol/L Tris-HCl, pH 7.4, containing 150 mmol/L NaCl, 5 mmol/L ethylenediamine tetraacetic acid, 10% glycerol, 1% Triton X-100, 0.1% sodium dodecyl sulfate, 1% NP-40, protease inhibitor cocktail (Sigma-Aldrich), tris-buffered saline, 8% sodium dodecyl sulphate, 8 mol/L urea]. Protein concentration was determined with BCA Protein Assay Kit (Pierce, Rocford, Illinois, USA). Protein 20 μg was loaded onto sodium dodecyl sulfate polyacrylamide gel electropheresis and transferred to a nitrocellulose membrane. After blocking with 5% non-fat dried milk, the membrane was incubated with the primary antibodies including rabbit anti-Akt (1:1 000), rabbit anti-p-Akt (1:500) and anti-β-actin (1:500) overnight at 4°C. After washes, the membrane was incubated with a horseradish peroxidase conjugated goat anti-rabbit IgG antibody (1:5 000) at room temperature for 1 hour. The desired target bands were exposed to X-ray film and then developed using ECL detection kit (BioRad, Seoul, South Korea). The band density was measured with a densitometer (GS700 model, BioRad, Seoul, South Korea).
Statistical analysis
Results were expressed as mean ± SD. Mann-Whitney U tests were applied to evaluate the differences among the variables. A value of P < 0.05 was considered to be statistically significant.
Footnotes
Funding: This work was supported by the Seoul National University Hospital (SNUH) Research Fund, No. 03-2010-0240.
Conflicts of interest: None declared.
(Reviewed by Chadchankar H, Zhang N, Wang LS)
(Edited by Li CH, Song LP, Liu WJ, Zhao M)
REFERENCES
- [1].Forno LS. Neuropathology of Parkinson's disease. J Neuropathol Exp Neurol. 1996;55:259–272. doi: 10.1097/00005072-199603000-00001. [DOI] [PubMed] [Google Scholar]
- [2].Spillantini MG, Crowther RA, Jakes R, et al. α-Synuclein in filamentous inclusions of Lewy bodies from Parkinson's disease and dementia with lewy bodies. Proc Natl Acad Sci U S A. 1998;95:6469–6473. doi: 10.1073/pnas.95.11.6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Burré J, Sharma M, Tsetsenis T, et al. Alpha-synuclein promotes SNARE-complex assembly in vivo and in vitro. Science. 2010;329:1663–1667. doi: 10.1126/science.1195227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Polymeropoulos MH, Lavedan C, Leroy E, et al. Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- [5].Krüger R, Kuhn W, Müller T, et al. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson's disease. Nat Genet. 1998;18:106–108. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- [6].Zarranz JJ, Alegre J, Gómez-Esteban JC, et al. The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann Neurol. 2004;55:164–173. doi: 10.1002/ana.10795. [DOI] [PubMed] [Google Scholar]
- [7].Chartier-Harlin MC, Kachergus J, Roumier C, et al. Alpha-synuclein locus duplication as a cause of familial Parkinson's disease. Lancet. 2004;364:1167–1169. doi: 10.1016/S0140-6736(04)17103-1. [DOI] [PubMed] [Google Scholar]
- [8].Singleton AB, Farrer M, Johnson J, et al. Alpha-synuclein locus triplication causes Parkinson's disease. Science. 2003;302:841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- [9].Lee SJ. Origins and effects of extracellular alpha-synuclein: implications in Parkinson's disease. J Mol Neurosci. 2008;34:17–22. doi: 10.1007/s12031-007-0012-9. [DOI] [PubMed] [Google Scholar]
- [10].Borghi R, Marchese R, Negro A, et al. Full length alpha-synuclein is present in cerebrospinal fluid from Parkinson's disease and normal subjects. Neurosci Lett. 2000;287:65–67. doi: 10.1016/s0304-3940(00)01153-8. [DOI] [PubMed] [Google Scholar]
- [11].El-Agnaf OM, Irvine GB. Aggregation and neurotoxicity of alpha-synuclein and related peptides. Biochem Soc Trans. 2002;30:559–565. doi: 10.1042/bst0300559. [DOI] [PubMed] [Google Scholar]
- [12].Du HN, Tang L, Luo XY, et al. A peptide motif consisting of glycine, alanine, and valine is required for the fibrillization and cytotoxicity of human alpha-synuclein. Biochemistry. 2003;42:8870–8878. doi: 10.1021/bi034028+. [DOI] [PubMed] [Google Scholar]
- [13].Luk KC, Song C, O’Brien P, et al. Exogenous alpha-synuclein fibrils seed the formation of Lewy body-like intracellular inclusions in cultured cells. Proc Natl Acad Sci U S A. 2009;106:20051–20056. doi: 10.1073/pnas.0908005106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Bae EJ, Lee HJ, Rockenstein E, et al. Antibody-aided clearance of extracellular α-synuclein prevents cell-to-cell aggregate transmission. J Neurosci. 2012;32(39):13454–69. doi: 10.1523/JNEUROSCI.1292-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Masliah E, Rockenstein E, Adame A, et al. Effects of alpha-synuclein immunization in a mouse model of Parkinson's disease. Neuron. 2005;46:857–868. doi: 10.1016/j.neuron.2005.05.010. [DOI] [PubMed] [Google Scholar]
- [16].Park SM, Kim KS. Proteolytic clearance of extracellular α-synuclein as a new therapeutic approach against Parkinson disease. Prion. 2012 doi: 10.4161/pri.22850. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Seo JH, Rah JC, Choi SH, et al. Alpha-synuclein regulates neuronal survival via Bcl-2 family expression and PI3/Akt pathway. FASEB J. 2002;16:1826–1828. doi: 10.1096/fj.02-0041fje. [DOI] [PubMed] [Google Scholar]
- [18].Manning-Bog AB, McCormack AL, Purisai MG, et al. Alpha-synuclein overexpression protects against paraquat-induced neurodegeneration. J Neurosci. 2003;23:3095–3099. doi: 10.1523/JNEUROSCI.23-08-03095.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Leng Y, Chuang DM. Endogenous alpha-synuclein is induced by valproic acid through histone deacetylase inhibition and participates in neuroprotection against glutamate-induced excitotoxicity. J Neurosci. 2006;26:7502–7512. doi: 10.1523/JNEUROSCI.0096-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Choong CJ, Say YH. Alpha-synuclein under acute and chronic rotenone and maneb treatment is abolished by its familial Parkinson's disease mutations A30P, A53T and E46K. Neurotoxicology. 2011;32:857–863. doi: 10.1016/j.neuro.2011.05.012. [DOI] [PubMed] [Google Scholar]
- [21].Nakaso K, Ito S, Nakashima K, et al. Caffeine activates the PI3K/Akt pathway and prevents apoptotic cell death in a Parkinson's disease model of SH-SY5Y cells. Neurosci Lett. 2008;432:146–150. doi: 10.1016/j.neulet.2007.12.034. [DOI] [PubMed] [Google Scholar]
- [22].Qin R, Li X, Li G, et al. Protection by tetrahydroxystilbene glucoside against neurotoxicity induced by MPP+: the involvement of PI3K/Akt pathway activation. Toxicol Lett. 2011;202:1–7. doi: 10.1016/j.toxlet.2011.01.001. [DOI] [PubMed] [Google Scholar]
- [23].Thomas B, Mandir AS, West N, et al. Resistance to MPTP-neurotoxicity in α-synuclein knockout mice is complemented by human α-synuclein and associated with increased β-synuclein and Akt activation. PLoS One. 2011;6:e16706. doi: 10.1371/journal.pone.0016706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Masliah E, Rockenstein E, Mante M, et al. Passive immunization reduces behavioral and neuropathological deficits in an alpha-synuclein transgenic model of Lewy body disease. PLoS One. 2011;6:e19338. doi: 10.1371/journal.pone.0019338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Beyer K, Ariza A. Alpha-synuclein posttranslational modification and alternative splicing as a trigger for neurodegeneration. Mol Neurobiol. doi: 10.1007/s12035-012-8330-5. in press. [DOI] [PubMed] [Google Scholar]


