
Keywords: neural regeneration, brain injury, hyperbaric oxygen, magnetic resonance spectroscopy, astrocytes, immunohistochemistry, choline, creatine, N-acetylaspartate, CA3 region, Morris water maze, hippocampus, neuroregeneration
Abstract
Hyperbaric oxygen therapy has been widely applied and recognized in the treatment of brain injury; however, the correlation between the protective effect of hyperbaric oxygen therapy and changes of metabolites in the brain remains unclear. To investigate the effect and potential mechanism of hyperbaric oxygen therapy on cognitive functioning in rats, we established traumatic brain injury models using Feeney's free falling method. We treated rat models with hyperbaric oxygen therapy at 0.2 MPa for 60 minutes per day. The Morris water maze test for spatial navigation showed that the average escape latency was significantly prolonged and cognitive function decreased in rats with brain injury. After treatment with hyperbaric oxygen therapy for 1 and 2 weeks, the rats’ spatial learning and memory abilities were improved. Hydrogen proton magnetic resonance spectroscopy analysis showed that the N-acetylaspartate/creatine ratio in the hippocampal CA3 region was significantly increased at 1 week, and the N-acetylaspartate/choline ratio was significantly increased at 2 weeks after hyperbaric oxygen therapy. Nissl staining and immunohistochemical staining showed that the number of nerve cells and Nissl bodies in the hippocampal CA3 region was significantly increased, and glial fibrillary acidic protein positive cells were decreased after a 2-week hyperbaric oxygen therapy treatment. Our findings indicate that hyperbaric oxygen therapy significantly improves cognitive functioning in rats with traumatic brain injury, and the potential mechanism is mediated by metabolic changes and nerve cell restoration in the hippocampal CA3 region.
INTRODUCTION
Traumatic brain injury (TBI) is one of the contributory reasons for death and disability[1]. Survivors of severe TBI often develop a variety of disorders in cognition, neurophysiological function, psychology and emotion, and behavioral function, which seriously affects their quality of life[2,3,4]. The hippocampus is the main brain center for learning and memory ability in animals. Are there any changes to nerve cells in the hippocampus after brain injury? What happens to its metabolic function?
Hyperbaric oxygen therapy (HBOT) has been recognized as an effective treatment for brain injury[5], but its potential mechanism remains unclear. The goals of this study are threefold: to observe the influence of HBOT on cognitive function in TBI rats; to explore the changes of hippocampal structure and function after HBOT; to investigate the mechanism of HBOT involved in cognitive function.
In this study, the Morris water maze navigation task was applied to observe the change in cognitive functions immediately after TBI and at different periods after HBOT. Previous studies addressing the HBOT mechanism have been limited to the observation of the animal brain morphology, apoptosis, pathophysiology, cytokines and neurotrophic factors at lesions after animals were killed at a certain time point[6,7,8]. However, there has been no investigation concerning the in vivo evidence at different time points.
Functional MRI can provide pathophysiological and functional information after brain injury in a non-invasive manner. It can also assist in assessing brain injury severity, cognitive dysfunction, structural and functional reorganization, hemorrhage focus and prognosis[9]. Among the functional MRI techniques, hydrogen proton magnetic resonance spectroscopy (1H-MRS) is the only non-invasive imaging technique for revealing tissue metabolism in vivo. It allows continuous dynamic observation of metabolic changes at the lesion site and provides a new means for understanding the disease pathogenesis[10]. Currently, 1H-MRS has been used for observing brain metabolic changes in multiple sclerosis and Parkinson's disease, and for exploring the correlation with cognitive function[11,12,13,14]. However, little evidence is available regarding the dynamic observation of HBOT for brain injury. Churchill et al[15] found that 51% of brain injury patients experienced improvement in memory and attention after 1-year of HBOT, leaving neuroimaging changes unstudied. To explore the effect of HBOT on cognitive function in rats, we used 1H-MRS to conduct continuous observations of hippocampal tissue metabolism change in TBI rats at different time points after treatment, and ratios of N-acetylaspartate/creatine and N-acetylaspartate/choline in the rat hippocampal CA3 region at the same time point after HBOT.
In 2007, Huang et al[16] proposed that glial scar hyperplasia was highly involved in spatial learning and memory defects in newborn rats with hypoxic-ischemic brain injury, and that HBOT reduced hippocampal neuronal loss and improved cognitive function in rats. Using Nissl staining and immunofluorescence staining, we observed hippocampal CA3 neurons and astrocytes in TBI rats after HBOT treatment for 2 weeks. This was a broad attempt to analyze the link between hippocampal neurons and cognitive functions and to explore the HBOT actions on hippocampal neurons.
RESULTS
Quantitative analysis of experimental animals
Thirty-three Sprague-Dawley rats were randomly divided into either the sham surgery group (n = 10, only exposing the dura mater) or the model group (n = 23, TBI models were established using the Feeney's free falling method). After three rats were excluded from the model group due to modeling failure, the remaining 20 successful models were further assigned into the HBOT group or TBI group, with ten rats in each group. The HBOT group was given HBOT, once per day for 2 weeks. A total number of 30 rats were included in the analysis.
Effect of HBOT on cognitive function of TBI rats
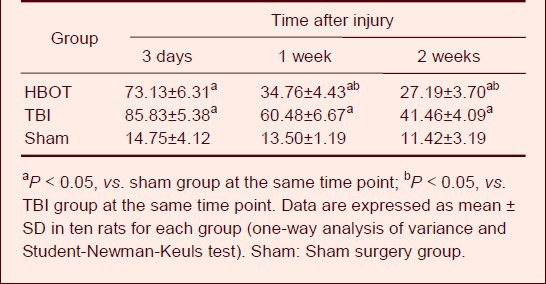
Test results from the Morris water maze task showed that the average escape latency of rats in the TBI and HBOT groups at 3 days, 1 week and 2 weeks after treatment was increased compared with the sham surgery group (P < 0.05). This demonstrates that cognitive functions were significantly decreased after brain injury in rats. At 1 and 2 weeks, the HBOT group had better cognitive function than the TBI group (P < 0.05; Table 1).
Table 1.
Effect of hyperbaric oxygen therapy (HBOT) on the average escape latency (s) of traumatic brain injury (TBI) rats in the Morris water maze
Effect of HBOT on brain metabolism in the hippocampal CA3 region of TBI rats
1H-MRS analysis showed that there was no significant difference in the N-acetylaspartate/choline and N-acetylaspartate/creatine ratios in the contralateral hippocampal CA3 region of rats in the TBI and HBOT groups, when compared with the sham surgery group at each time point (Table 2).
Table 2.
Effect of hyperbaric oxygen therapy (HBOT) on N-acetylaspartate/choline (NAA/Ch) and N-acetylaspartate/creatine (NAA/Cr) ratios in the hippocampal CA3 region of traumatic brain injury (TBI) rats at different time points
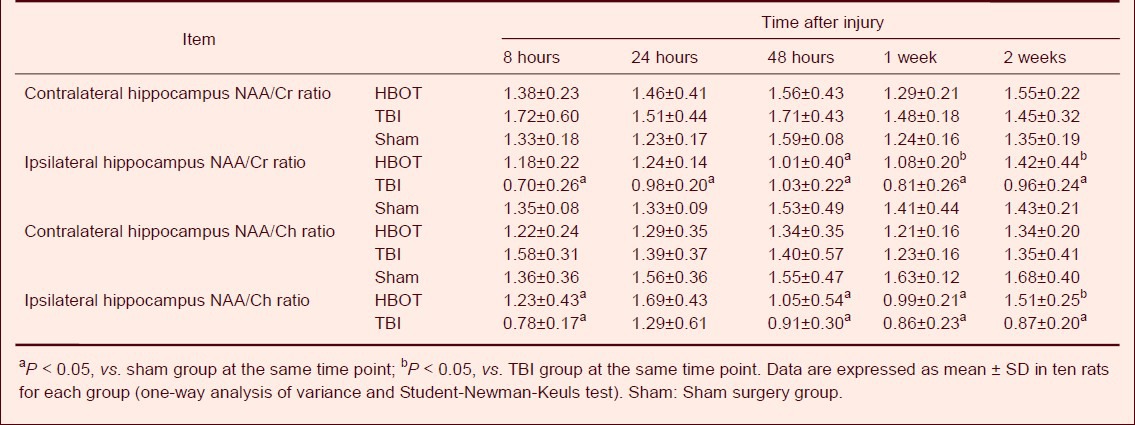
Compared with the sham surgery group, the N-acetylaspartate/creatine ratio in the ipsilateral hippocampal CA3 region was significantly decreased in the TBI group (P < 0.05). After 48 hours of treatment, the decreased ratio was more apparent in the TBI and HBOT groups (P < 0.05). After 1 and 2 weeks of treatment, the N-acetylaspartate/creatine ratio in the HBOT group was significantly increased compared with the TBI group (P < 0.05; Table 2, Figure 1).
Figure 1.
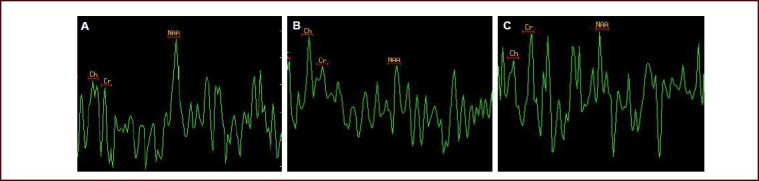
Magnetic resonance spectroscopy images of the rat hippocampal CA3 at the injury side after hyperbaric oxygen therapy for 2 weeks.
(A) In the sham-surgery group, the NAA peak value was significantly higher than Cr and Ch values.
(B) In the traumatic brain injury group, the NAA value was significantly decreased, while the Cr and Ch values were elevated.
(C) In the hyperbaric oxygen therapy group, the NAA value was higher than that in the traumatic brain injury group. Both the NAA/Ch and NAA/Cr ratios were increased.
NAA: N-acetylaspartate; Cr: creatine; Ch: choline.
At 8 hours, 48 hours, 1 week, and 2 weeks after treatment, the N-acetylaspartate/choline ratio in the ipsilateral hippocampal CA3 region was significantly decreased in the TBI group compared with the sham surgery group (P < 0.05). At 2 weeks after treatment, the HBOT group had significantly higher N-acetylaspartate/choline ratios than the TBI group (P < 0.05; Table 2, Figure 1).
Effect of HBOT on histological change of the hippocampal CA3 region in TBI rats
Nissl staining showed that the hippocampal CA3 neurons were tightly and neatly arranged, demonstrating intact morphology and multilateral shape in the sham-operated rats. The nucleolus was distributed in the center and blue plaques or granular Nissl bodies were visible in the cytoplasm. In the TBI group, the hippocampal CA3 neurons were sparse and disorderly arranged; cell spacing was widened, a large number of pyknotic and necrotic neurons demonstrated atrophy, and Nissl bodies were decreased or had disappeared. In the HBOT group, the number of nerve cells in the hippocampal CA3 region was significantly increased at 2 weeks after treatment. The neurons were tightly distributed and Nissl bodies were increased compared with the TBI group (Figure 2).
Figure 2.
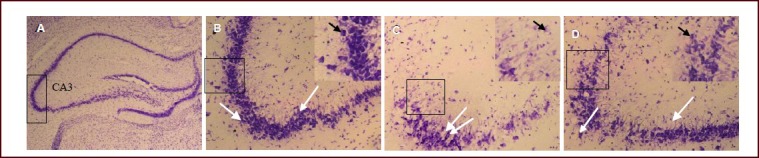
Histological changes in the hippocampal CA3 region on the injured side of traumatic brain injury rats after hyperbaric oxygen therapy for 2 weeks (Nissl staining, optical microscopy, scale bars: A, × 25; B–D, × 200).
White arrows indicate Nissl-positive cells, black arrows indicate Nissl bodies. The black squares in B, C and D are the areas enlarged in the insert at the right upper of each image.
(A) Black square in A and B is the observed area.
(B) In the sham surgery group, hippocampal CA3 neurons were arranged tightly and the structure was intact.
(C) In the traumatic brain injury group, hippocampal CA3 neurons were arranged sparsely and disordered. A large number of pyknotic and necrotic neurons exhibited atrophy, and Nissl bodies decreased or disappeared.
(D) In the hyperbaric oxygen therapy group, the number of hippocampal CA3 neurons was increased significantly, and more compact compared with those in the traumatic brain injury group. The number of Nissl bodies was also increased.
Effect of HBOT on the glial fibrillary acidic protein-positive cells in the hippocampal CA3 region of TBI rats
An immunofluorescence assay showed that at 2 weeks after treatment, glial fibrillary acidic protein-positive cells in the hippocampal CA3 region exhibited intact structure and clearly visible protrusion in the sham surgery rats. In the TBI group, the number of glial fibrillary acidic protein-positive cells and protrusions in the hippocampal CA3 region was significantly increased compared with the sham-surgery group (P < 0.01). After 2 weeks of hyperbaric oxygen therapy, the number of glial fibrillary acidic protein-positive cells and protrusions in the hippocampal CA3 region was significantly decreased compared with the TBI group (P < 0.01). Data are shown in Figure 3.
Figure 3.
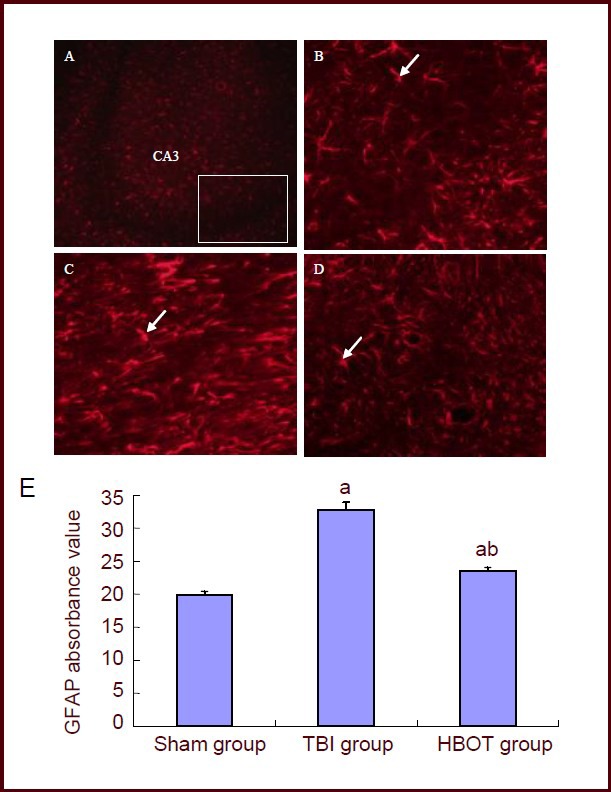
Glial fibrillary acidic protein (GFAP) expression in the hippocampal CA3 region of the injured side of traumatic brain injury (TBI) rats after hyperbaric oxygen therapy for 2 weeks (fluorescence microscopy, scale bars: A, × 100; B–D, × 200).
White arrows indicate GFAP-positive cells. (A) White block is the observed hippocampal CA3. (B) In the sham surgery group, the GFAP-positive cells in hippocampal CA3 showed an intact structure with clearly visible protrusions. (C) In the TBI group, the number of GFAP-positive cells and protrusions in the hippocampal CA3 region were significantly increased. (D) In the hyperbaric oxygen therapy (HBOT) group, the number of GFAP-positive cells and protrusions in the hippocampal CA3 region were significantly decreased.
(E) GFAP average absorbance values in the hippocampal CA3 region. Six sections of the CA3 region were selected to measure the absorbance value in eight fields of view. Then, the mean absorbance values in each group were calculated. aP < 0.01, vs. sham group; bP < 0.01, vs. TBI group. Data are expressed as mean ± SD in 10 rats for each group (one-way analysis of variance and Student-Newman-Keuls test). sham: Sham surgery.
DISCUSSION
Brain tissue structural impairment as a result of TBI can be divided into primary injury and secondary injury. Primary injury occurs directly after the insult; it is caused by a mechanical strike that immediately starts to cause damage to the axons and neurons, glial cells and vessels. Primary injury may trigger a cascade of biochemical reactions within several minutes, such as cerebral edema, local ischemia and hypoxia, inflammation and massive release of excitatory amino acids. Accordingly, these reactions lead to cell injury, death, apoptosis, even disability for several days or several weeks, and sometimes coma. These changes are referred to as secondary injury[17,18,19].
Primary injury cannot be prevented in time because it occurs only a few seconds after the insult; however, secondary injury can be prevented in some cases and as such it has acquired increasing attention.
HBOT helps alleviate secondary brain injury following TBI. In this study, cognitive functioning in injured rats was assessed using a water maze task. The results from this study suggest that cognitive functioning in these rats began to decline after TBI and was significantly improved after 1 week of HBOT. This is consistent with previous findings[20]. However, the HBOT mechanism in the treatment of TBI is poorly understood.
Existing studies have summarized the following pathways as possible mechanisms: (1) Hyperbaric oxygen increases blood oxygen content, reduces intracranial pressure, ameliorates cerebral edema, blocks hypoxia-brain edema vicious cycle, and protects brain tissue around the lesion against secondary damage[21,22,23]. (2) Hyperbaric oxygen inhibits the apoptosis of nerve cells around the injury and hippocampal neurons, and reduces the loss of hippocampal neurons through up-regulating Bcl-2 and Bcl-xl expression and down-regulating Bax expression[24,25]. (3) Hyperbaric oxygen increases the brain metabolic rate and blood oxygen content, protects mitochondrial function, reduces lactate levels in the cerebrospinal fluid and increases ATP content, thereby facilitating aerobic metabolism in traumatic brain tissue[26,27]. (4) HBOT inhibits the generation of free radicals after TBI, thereby reducing lipid peroxidation; it also reduces proinflammatory factor generation and promotes anti-inflammatory factor production, thereby reducing inflammatory reactions in brain tissue and assisting to reduce TBI secondary injury[6,28]. The present study predominantly explored the mechanism of HBOT involved in the improvement of cognitive function. It is known that the optimal time window for the first time for HBOT is 6 hours after TBI[17], and that repeated therapy can significantly reduce brain injury and promote the recovery of neurological function in patients with brain injury. Thus we chose to begin HBOT treatment exactly 6 hours after the brain injury.
1H-MRS can assess the severity of central nervous system damage qualitatively and quantitatively. This damage is associated with N-acetylaspartate/Cr and choline/creatine ratios[29,30,31]. For example, Du and colleagues[32] found a correlation between N-acetylaspartate/creatine, N-acetylaspartate/choline ratios and Glasgow Coma Scale scores after severe brain injury; patients had lower N-acetylaspartate/creatine and N-acetylaspartate/choline ratios, and a higher choline/creatine ratio. Additionally, Carpentier et al[33] showed that in severe brain injury patients with negative results demonstrated on brainstem MRI examination, the N-acetylaspartate/creatine ratio was lower than the normal group; the brainstem N-acetylaspartate/creatine ratio was < 1.5 and the N-acetylaspartate/(choline + creatine + N-acetylaspartate) ratio was < 0.40 when the Glasgow Coma Scale score was 1–2 points. In patients with a GCS score of 3 points, the brainstem choline/creatine ratio > N-acetylaspartate/creatine ratio or choline/(choline + creatine + N-acetylaspartate) ratio was > 0.40. Cognitive impairment is strongly linked with hippocampal N-acetylaspartate, creatine and other metabolites, showing a significantly decreased N-acetylaspartate/creatine ratio[34,35]. The N-acetylaspartate peak is highest in a normal brain spectrum and its content is the most sensitive to changes of brain states; thus making it a well-accepted neuronal marker. N-acetylaspartate level in the brain directly reflects the functional state of neurons[36]. The present study showed that N-acetylaspartate/creatine and N-acetylaspartate/choline ratios at the ipsilateral hippocampal CA3 of TBI rats were reduced compared with the sham group at 8 hours to 2 weeks after treatment; in particular the reduction was apparent at 8 hours. This evidence suggests ipsilateral hippocampal neuronal dysfunction or damage after brain injury. The hippocampus is the main brain center for mammals’ learning and memory function, and brain damage causes hippocampal CA3 neuronal death[37]. In this study, Nissl staining results showed that, at 2 weeks after brain injury, hippocampal CA3 neurons were sparse and disorderly arranged, a large number of pyknotic and necrotic neurons exhibited atrophy, and Nissl bodies decreased or disappeared. Results from the Morris water maze task showed that cognitive functioning was significantly reduced at different time points after brain injury, suggesting that cognitive impairment is closely associated with ipsilateral hippocampal neuronal necrosis, structural changes and metabolic abnormalities.
1H-MRS can measure trace metabolites noninvasively in the brain and assess prognosis and recovery following brain injury[38,39]. Vagnozzi et al[31] found that N-acetylaspartate/creatine and N-acetylaspartate/choline ratios were decreased in patients with mild brain injury, and gradually returned to normal as the disease conditions improved. Semenova et al[40] examined the N-acetylaspartate level to detect the prognosis of children with severe brain injury after cell therapy. In this study, the hippocampal N-acetylaspartate/creatine and N-acetylaspartate/choline ratios were detected using 1H-MRS at different time points after HBOT. The results indicated that the N-acetylaspartate/creatine ratio was significantly increased after 1 and 2 weeks of treatment in the HBOT group compared with the TBI group. Also, the N-acetylaspartate/choline ratio was significantly increased at 2 weeks and hippocampal neurons were repaired. Meanwhile, cognitive function of rats in the HBOT group was better than the TBI group. Nissl staining revealed that the number of hippocampal CA3 neurons was increased significantly at 2 weeks after treatment, the neurons were tightly arranged and the Nissl bodies were increased compared with the TBI group. However the number of Nissl bodies was still lower than in the sham group. These findings indicate that HBOT can promote the repair of hippocampal CA3 neurons and alter hippocampus metabolism, thereby promoting cognitive function recovery in rats with traumatic brain injury.
Choline levels reflect the total choline content in the brain, including phosphocholine, phosphatidyl choline and glycerol phosphate[41]. The majority of choline is present in glial cells. It is involved in cell membrane phospholipid metabolism and reflects membrane synthesis; thus an increase in the choline peak is an indicator of glial cell proliferation[42]. Epileptic patients with complex partial seizures have been found to demonstrate obvious cognitive impairment and a decreased hippocampal N-acetylaspartate/(choline + creatine) ratio[43]. The elevated choline level indicates hippocampal neuronal necrosis and glial cell proliferation[43]. Glial fibrillary acidic protein is a specific cytoskeletal protein in astrocytes and is expressed in mature astrocytes, so it is recognized as an astrocyte-specific molecular marker[44]. In this study, glial fibrillary acidic protein immunofluorescence staining showed that 2 weeks after brain injury, hippocampal N-acetylaspartate/creatine and N-acetylaspartate/choline ratios decreased, while glial fibrillary acidic protein expression was significantly up-regulated. The number of glial fibrillary acidic protein positive cells and protrusions were significantly increased and the absorbance value was also significantly higher than in the sham group. At this time point (2 weeks) cognitive functioning in rats in the TBI group was significantly lower than in the sham-operated rats. These findings indicate that cognitive impairment was closely related to glial cell proliferation in the hippocampal CA3 region and changes in hippocampal metabolism. After injury to the central nervous system, astrocyte proliferation has a “double-edged sword” effect[45,46]. On the one hand, reactive hyperplasia of astrocytes provides nutrition for nerve tissue, promotes nerve tissue repair, improves neurological function and cognitive function, and contributes to the clearance of myelin and neuronal debris at the injury. On the other hand, excessive activation and proliferation of astrocytes has toxic effects on the nerve tissue, and hinders myelin or axonal regeneration, thereby interfering with neuronal loop functions. Sandhir and colleagues[47] found that glial fibrillary acidic protein expression was up-regulated in the hippocampus after brain injury and peaked at 1 week in aged and adult rats. At 3, 7, 14 days after injury, its expression was higher than that in adult rats and longer than that in aged rats. Activated astrocytes are a potential cause for cognitive function deterioration in aged rats with brain injury. Preliminary studies of our research group demonstrated that, at 1–4 weeks after brain injury, a large number of activated glial cells was observed in the cerebral cortex around the injury site. Rehabilitation training has been demonstrated to reduce the activation of astrocytes and accordingly promote neurological functional recovery after TBI in rats[48]. The present study showed that the number of astrocytes and protrusions in the hippocampal CA3 region was significantly reduced after 2 weeks of HBOT. After treatment, the N-acetyla-spartate/creatine and N-acetylaspartate/choline ratios were increased and cognitive function gradually recovered. This evidence suggests that activated astrocytes affect cognitive function restoration, and the potential mechanisms are mediated by metabolic change in the hippocampus. Further studies are needed to explore their interaction.
In summary, our findings show that the number of hippocampal nerve cells and the rate of metabolism vary after brain injury. HBOT alleviates injury at the site of a lesion and promotes cognitive function recovery in TBI rats. The potential mechanism depends on the promotion of hippocampal CA3 neuronal repair and the inhibition of astrocyte activation, as well as being related to increases in hippocampal N-acetylaspartate/creatine and N-acetylaspartate/choline ratios. Meanwhile, as a non-invasive detection method for in vivo metabolites, 1H-MRS technique provides important information for brain injury diagnosis, treatment and prognosis evaluation.
Some limitations of the study warrant mention. First, the sample in our experiment was relatively small, which may have affected the results. Second, our two researchers had previous experience working with HBOT; this may have had an effect on the treatment outcomes. Future studies are planned to analyze the therapeutic effect of HBOT on different degrees of brain injury in patients of different ages.
MATERIALS AND METHODS
Design
A randomized controlled animal experiment.
Time and setting
Experiments were performed at the Experimental Animal Center of Nantong University, China and Department of Rehabilitation Medicine, Affiliated Hospital of Nantong University, China from May 2012 to January 2013.
Materials
Thirty-three healthy adult male Sprague-Dawley rats, of clean grade, weighing 300–350 g, were provided by the Experimental Animal Center of Nantong University, China (license number SYXK (Su) 2012-0031). Experimental disposals were in accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals, issued by the Ministry Science and Technology of China[49].
Methods
Establishing TBI models
The TBI models were established in rats using Feeney's weight-drop method with slight modifications[50]. In brief, rats were anesthetized with an intraperitoneal injection of 1% sodium pentobarbital (40 mg/kg) and their heads were fixed in a stereotaxic apparatus (WPI, Sarasota, Florida, USA) after shaving parietal hair. A median sagittal incision was made on the scalp under routine disinfection, exposing the bregma. Then a bone window (4.0 mm diameter) was cut at 3.0 mm posterior to the bregma and 3.0 mm right lateral to the sagittal suture. A 25 g weight was allowed to fall from 30 cm height onto the right brain, to produce a moderate impact injury at 3.5 mm diameter and 3.0 mm compression depth, 750 g·cm. The model was defined successful when it caused a hematoma in the cortex immediately after injury. The dura mater was exposed in the sham-operated rats, however, they received no impact.
HBOT interventions
Rats subjected to the HBOT were placed in a homemade cage and transferred to a single hyperbaric oxygen chamber (Shanghai 701 Institute Yangyuan Medical HBO Chamber Factory, Shanghai, China). HBOT was given at 6 hours after injury[17]; the oxygen pressure was first increased for 15 minutes to 0.2 MPa, then maintained stationary for 60 minutes, and finally decreased for 15 minutes to atmospheric pressure. During the therapy, pure oxygen ventilation was given for 10 minutes to avoid CO2 accumulation. After the pressure was decreased, rats were taken out of the chamber. The therapy was performed once per day for 2 weeks.
Assessment of cognitive function after HBOT
Morris water maze equipment was provided by Shanghai Softmaze Information Technology Co., Ltd., Shanghai, China. All rats were allowed to swim freely for 2 minutes 1 day prior to the modeling, to adapt the environment. At 2 and 9 days after treatment, a 5-day spatial navigation test was performed to detect average escape latency and to assess spatial learning and memory ability, three times per day at an interval of 5 minutes. After the test was complete, rats were allowed to stay on the platform for 20 seconds. Rats entered the water pool from the fourth quadrant and the platform was located at the first quadrant. During the whole experiment, the platform was maintained in the same quadrant. The rat swimming route and the time taken to find the platform were recorded; this was the escape latency. The time limit for each test was 120 seconds. The rats that failed to find the platform within 120 seconds had their time recorded as 120 seconds. The latency was calculated at 3 days, 1 week and 2 weeks after treatment.
1H-MRS and data analysis
Rats were scanned using 1H-MRS with small animal-dedicated coils using a Signa 3.0 T MR scanner (Signa Excite HD, GE Healthcare, Fairfield, CT, USA) at 8 hours, 24 hours, 48 hours, 1 week, and 2 weeks after treatment. The scalp was scanned from the bregma through 10 layers, at 1.8 mm thickness and 0.2 mm gap. Bilateral hippocampi were selected as regions of interest in the coronal T2FSE. Scanning parameters (two-dimensional multi-voxel spectroscopy): PRESS sequence, repetition time = 5 000 ms, echo time = 115.9 ms, frequency = 90/256, field of view = 5.0 cm, number of excitations = 4.0, region of interest = 2.4 mm2, voxel thickness = 7 mm, voxel size = 2.4 mm × 2.4 mm × 7.0 mm, duration = 5 minutes and 28 seconds. The N-acetylaspartate, creatine and choline contents were recorded. N-acetylaspartate/choline and N-acetylaspartate/creatine ratios were calculated.
Chemical shift images obtained using two-dimensional multi-voxel spectroscopy were scanned and processed using commercial software, Functool 2. The N-acetylaspartate/choline and N-acetylaspartate/creatine ratios were calculated with the relative quantitative values of the hippocampal CA3 metabolites.
Nissl staining for the morphology of rat hippocampal CA3 nerve cells
At 2 weeks after treatment, rats were anesthetized with 1% sodium pentobarbital (40 mg/kg) and the heart was rapidly perfused with 0.9% physiology 150 mL and fixed in 4% paraformaldehyde 250 mL. Subsequently, the rats were killed, the brains were removed and fixed in 4% paraformaldehyde for 6 hours, dehydrated in sucrose gradient, precipitated and serially sectioned into coronal frozen sections (Leica Microsystems, Heidelberg, Germany) at 15 μm thickness. Each section out of every two sections was stained with cresyl violet for 20 minutes, rinsed with distilled water for 5 minutes, and differentiated in 75% and 95% ethanol for 1 minute. The differentiation was controlled under endoscopy and was terminated when the background was clean and Nissl bodies were clearly visible. After differentiation, sections were rapidly immersed in ethanol for dehydration, xylene transparency and mounting. Nissl bodies in the cytoplasm of hippocampal CA3 nerve cells were observed under optical microscope (Leica).
Immunofluorescence staining of glial fibrillary acidic protein expression in the rat hippocampal CA3 region
Sections adjacent to the stained sections were rinsed, blocked and incubated with 8 % normal goat serum for 1 hour. After the serum was discarded, sections were incubated with mouse anti-rat glial fibrillary acidic protein monoclonal antibody (Millipore Corporation, Billerica, MA, USA) at 4°C overnight and Alexa Fluor 595-labeled goat anti-mouse antibody (Invitrogen, Carlsbad, CA, USA; 1: 1 000) at room temperature for 1 hour. Then sections were mounted with 50% glycerol. Between each step, sections were rinsed with 0.01 mol/L PBS (pH 7.4) three times for 15 minutes each. The controls were incubated with normal goat serum (1:50) instead of primary antibody and the result was negative. The glial fibrillary acidic protein-positive cells in the hippocampal CA3 region were observed under fluorescence microscope (200 × magnification; Leica) and image analysis was performed using JEDA 801 Series morphological analysis software (Jiangsu JEDA Science-Technology Development Co., Ltd., Nanjing, Jiangsu Province, China). Six sections in each rat were randomly selected to calculate the mean absorbance value in eight fields of view (2 cm × 2 cm).
Statistical analysis
Data are expressed as mean ± SD and were analyzed using SPSS 17.0 software (SPSS, Chicago, IL, USA). Differences between the groups were compared with one-way analysis of variance and the Student-Newman-Keuls test. A value of P < 0.05 was considered a statistically significant difference.
Research background: The proliferation of glial scar formation is closely linked with spatial learning and memory defects in neonatal rats after hypoxic-ischemic brain damage. Hyperbaric oxygen therapy can reduce hippocampal neuronal loss and improve cognitive function in rats.
Research frontiers: Hydrogen magnetic resonance spectroscopy imaging techniques have been used for observations of brain tissue metabolism and cognitive function changes in multiple sclerosis, Parkinson's disease and other diseases. The role of hydrogen ion magnetic resonance spectroscopy in dynamic observation of hyperbaric oxygen therapy on brain injury is rarely reported.
Clinical significance: This study was the first to observe changes of hippocampus metabolites in rats with traumatic brain injury at different courses of hyperbaric oxygen therapy using hydrogen ion magnetic resonance spectroscopy. This was a broad attempt to provide evidence for understanding the effect of hyperbaric oxygen therapy in traumatic brain injury.
Academic terminology: Magnetic resonance spectroscopy – the only non-invasive technique for the determination of certain chemical compositions in tissue in vivo; it is the product of magnetic resonance imaging and magnetic resonance spectroscopy, and it provides another functional analysis and diagnostic method in addition to magnetic resonance imaging.
Peer review: This study observed the correlation between changes of hippocampal CA3 neurons and expression of glial fibrillary acidic protein in rats after hyperbaric oxygen therapy. In addition, the potential mechanism underlying hyperbaric oxygen therapy on improving cognitive functions was analyzed. These experimental findings provide a reliable theoretical basis for choosing an optimal hyperbaric oxygen therapy scheme for the clinical treatment of brain injury.
Acknowledgments
We would like to thank Zhang ZJ and Dong YL from Department of Anatomy, Medical School of Nantong University, China for their excellent technical assistance.
Footnotes
Conflicts of interest: None declared.
Ethical approval: The study gained full ethical approval from the Animal Ethics Committee of Nantong College in China.
(Reviewed by James K, Frenchman B, Peng ZR, Sun XJ)
(Edited by Wang J, Yang Y, Li CH, Song LP, Liu WJ, Zhao M)
REFERENCES
- [1].Flanagan SR, Cantor JB, Ashman TA. Traumatic brain injury: future assessment tools and treatment prospects. Neuropsychiatr Dis Treat. 2008;4(5):877–892. doi: 10.2147/ndt.s1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Broomhall LG, Clark CR, McFarlane AC, et al. Early stage assessment and course of acute stress disorder after mild traumatic brain injury. J Nerv Ment Dis. 2009;197(3):178–181. doi: 10.1097/NMD.0b013e318199fe7f. [DOI] [PubMed] [Google Scholar]
- [3].Bales JW, Wagner AK, Kline AE, et al. Persistent cognitive dysfunction after traumatic brain injury: A dopamine hypothesis. Neurosci Biobehav Rev. 2009;33(7):981–1003. doi: 10.1016/j.neubiorev.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Fujimoto ST, Longhi L, Saatman KE, et al. Motor and cognitive function evaluation following experimental traumatic brain injury. Neurosci Biobehav Rev. 2004;28(4):365–378. doi: 10.1016/j.neubiorev.2004.06.002. [DOI] [PubMed] [Google Scholar]
- [5].Thom SR. Hyperbaric oxygen: its mechanisms and efficacy. Plast Reconstr Surg. 2011;127(1):131S–141S. doi: 10.1097/PRS.0b013e3181fbe2bf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lin KC, Niu KC, Tsai KJ, et al. Attenuating inflammation but stimulating both angiogenesis and neurogenesis using hyperbaric oxygen in rats with traumatic brain injury. J Trauma Acute Care Surg. 2012;72(3):650–659. doi: 10.1097/TA.0b013e31823c575f. [DOI] [PubMed] [Google Scholar]
- [7].Rockswold SB, Rockswold GL, Zaun DA, et al. A prospective, randomized clinical trial to compare the effect of hyperbaric to normobaric hyperoxia on cerebral metabolism, intracranial pressure, and oxygen toxicity in severe traumatic brain injury. J Neurosurg. 2010;112(5):1080–1094. doi: 10.3171/2009.7.JNS09363. [DOI] [PubMed] [Google Scholar]
- [8].Palzur E, Zaaroor M, Vlodavsky E, et al. Neuroprotective effect of hyperbaric oxygen therapy in brain injury is mediated by preservation of mitochondrial membrane properties. Brain Res. 2008;1221:126–133. doi: 10.1016/j.brainres.2008.04.078. [DOI] [PubMed] [Google Scholar]
- [9].Pang HP, Wu GY. Progress in the research of functional magnetic resonance imaging in traumatic brain injury. Fangshexue Shijian. 2010;12(25):12–15. [Google Scholar]
- [10].Jansen JF, Backes WH, Nicolay K, et al. 1H MR spectroscopy of the brain: absolute quantification of metabolites. Radiology. 2006;240(2):318–332. doi: 10.1148/radiol.2402050314. [DOI] [PubMed] [Google Scholar]
- [11].Kirov II, Tal A, Babb JS, et al. Serial proton MR spectroscopy of gray and white matter in relapsing-remitting MS. Neurology. 2013;80(1):39–46. doi: 10.1212/WNL.0b013e31827b1a8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Giorgio A, De Stefano N. Cognition in multiple sclerosis: relevance of lesions, brain atrophy and proton MR spectroscopy. Neurol Sci. 2010;31(Suppl 2):S245–248. doi: 10.1007/s10072-010-0370-x. [DOI] [PubMed] [Google Scholar]
- [13].Metarugcheep P, Hanchaiphiboolkul S, Viriyavejakul A, et al. The usage of proton magnetic resonance spectroscopy in Parkinson's disease. J Med Assoc Thai. 2012;95(7):949–952. [PubMed] [Google Scholar]
- [14].Nie K, Zhang Y, Huang B, et al. Marked N-acetylaspartate and choline metabolite changes in Parkinson's disease patients with mild cognitive impairment. Parkinsonism Relat Disord. 2013;19(3):329–334. doi: 10.1016/j.parkreldis.2012.11.012. [DOI] [PubMed] [Google Scholar]
- [15].Churchill S, Weaver LK, Deru K, et al. A prospective trial of hyperbaric oxygen for chronic sequelae after brain injury (HYBOBI) Undersea Hyperb Med. 2013;40(2):165–193. [PubMed] [Google Scholar]
- [16].Huang Z, Liu J, Cheung PY, et al. Long-term cognitive impairment and myelination deficiency in rat model of perinatal hypoxic-ischemic brain injury. Brain Res. 2009;1301:100–109. doi: 10.1016/j.brainres.2009.09.006. [DOI] [PubMed] [Google Scholar]
- [17].Wang GH, Zhang XG, Jiang ZL, et al. Neuroprotective effects of hyperbaric oxygen treatment on traumatic brain injury in the rat. J Neurotrauma. 2010;27(9):1733–1743. doi: 10.1089/neu.2009.1175. [DOI] [PubMed] [Google Scholar]
- [18].Shlosberg D, Benifla M, Kaufer D, et al. Blood-brain barrier breakdown as a therapeutic target in traumatic brain injury. Nat Rev Neurol. 2010;6(7):393–403. doi: 10.1038/nrneurol.2010.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Werner C, Engelhard K. Pathophysiology of traumatic brain injury. Br J Anaesth. 2007;99(1):4–9. doi: 10.1093/bja/aem131. [DOI] [PubMed] [Google Scholar]
- [20].Wolf G, Cifu D, Baugh L, et al. The effect of hyperbaric oxygen on symptoms after mild traumatic brain injury. J Neurotrauma. 2012;29(17):2606–2612. doi: 10.1089/neu.2012.2549. [DOI] [PubMed] [Google Scholar]
- [21].Vlodavsky E, Palzur E, Soustiel JF. Hyperbaric oxygen therapy reduces neuroinflammation and expression of matrix metalloproteinase-9 in the rat model of traumatic brain injury. Neuropathol Appl Neurobiol. 2006;32(1):40–50. doi: 10.1111/j.1365-2990.2005.00698.x. [DOI] [PubMed] [Google Scholar]
- [22].Hayakawa T, Kanai N, Kuroda R, et al. Response of cereborspinal fluid pressure to hyperbaric oxygenation. J Neurol Neurosurg Psychiatry. 1971;34(5):580–586. doi: 10.1136/jnnp.34.5.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Fu M, Zhao H, Zhang LD, et al. Effects of hyperbaric oxygen therapy on the brain trauma injury in rabbits with magnetic resonance imaging. Zhongguo hanghaiyixue yu Gaoqiya Yixue Zazhi. 2011;18(2):82–85. [Google Scholar]
- [24].Vlodavsky E, Palzur E, Feinsod M, et al. Evaluation of the apoptosis-related proteins of the BCL-2 family in the traumatic penumbra area of the rat model of cerebral contusion, treated by hyperbaric oxygen therapy: a quantitative immunohistochemical study. Acta Neuropathol. 2005;110(2):120–126. doi: 10.1007/s00401-004-0946-8. [DOI] [PubMed] [Google Scholar]
- [25].Liu Z, Jiao QF, You C, et al. Effect of hyperbaric oxygen on cytochrome C, Bcl-2 and Bax expression after experimental traumatic brain injury in rats. Chin J Traumatol. 2006;9(3):168–174. [PubMed] [Google Scholar]
- [26].Daugherty WP, Levasseur JE, Sun D, et al. Effects of hyperbaric oxygen therapy on cerebral oxygenation and mitochondrial function following moderate lateral fluid-percussion injury in rats. J Neurosurg. 2004;101(3):499–504. doi: 10.3171/jns.2004.101.3.0499. [DOI] [PubMed] [Google Scholar]
- [27].Gossett WA, Rockswold GL, Rockswold SB, et al. The safe treatment, monitoring and management of severe traumatic brain injury patients in a monoplace chamber. Undersea Hyperb Med. 2010;37(1):35–48. [PubMed] [Google Scholar]
- [28].Zhang XG, Wang GH, Li YC, et al. The influences of hyperbaric oxygen on the oxidative stress variables and pro-/anti-inflammatory cytokines in rats after traumatic brain injury. Chin J Appl Physiol. 2010;26(3):373–375. [PubMed] [Google Scholar]
- [29].Barkovich AJ, Baranski K, Vigneron D, et al. Proton MR spectroscopy for the evaluation of brain injury in asphyxiated, term neonates. AJNR Am J Neuroradiol. 1999;20(8):1399–1405. [PMC free article] [PubMed] [Google Scholar]
- [30].Xu S, Zhuo J, Racz J, et al. Early microstructural and metabolic changes following controlled cortical impact injury in rat: a magnetic resonance imaging and spectroscopy study. J Neurotrauma. 2011;28(10):2091–2102. doi: 10.1089/neu.2010.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Vagnozzi R, Signoretti S, Cristofori L, et al. Assessment of metabolic brain damage and recovery following mild traumatic brain injury: a multicentre, proton magnetic resonance spectroscopic study in concussed patients. Brain. 2010;133(11):3232–3242. doi: 10.1093/brain/awq200. [DOI] [PubMed] [Google Scholar]
- [32].Du Y, Li Y, Lan Q. 1H-Magnetic resonance spectroscopy correlates with injury severity and can predict coma duration in patients following severe traumatic brain injury. Neurol India. 2011;59(5):679–684. doi: 10.4103/0028-3886.86540. [DOI] [PubMed] [Google Scholar]
- [33].Carpentier A, Galanaud D, Puybasset L, et al. Early morphologic and spectroscopic magnetic resonance in severe traumatic brain injuries can detect “invisible brain stem damage” and predict “vegetative states”. J Neurotrauma. 2006;23(5):674–685. doi: 10.1089/neu.2006.23.674. [DOI] [PubMed] [Google Scholar]
- [34].Zhou H, Lu WJ, Teng GJ, et al. The study of cognitive function and proton magnetic resonance spectroscopy on hippocampus in patients with type 2 diabetes mellitus. Linchuang Yixue Yingxiang Jishu. 2009;25(8):1367–1370. [Google Scholar]
- [35].Sun YA, He XB, Miao CC, et al. Magnetic resonance spectroscopy in evaluating hippocampus changes of patients with mild cognitive impairment. Zhongguo Zuzhi Gongcheng Yanjiu yu Linchuang Kangfu. 2009;13(26):5098–5103. [Google Scholar]
- [36].Lucetti C, Del Dotto P, Gambaccini G, et al. Proton magnetic resonance spectroscopy (1H-MRS) of motor cortex and basal ganglia in de novo Parkinson's disease patients. Neurol Sci. 2001;22(1):69–70. doi: 10.1007/s100720170051. [DOI] [PubMed] [Google Scholar]
- [37].Lei Z, Deng P, Li J, et al. Alterations of A-type potassium channels in hippocampal neurons after traumatic brain injury. J Neurotrauma. 2012;29(2):235–245. doi: 10.1089/neu.2010.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Signoretti S, Marmarou A, Aygok GA, et al. Assessment of mitochondrial impairment in traumatic brain injury using high-resolution proton magnetic resonance spectroscopy. J Neurosurg. 2008;108(1):42–52. doi: 10.3171/JNS/2008/108/01/0042. [DOI] [PubMed] [Google Scholar]
- [39].Wang HZ, Qiu SJ, Lv XF, et al. Diffusion tensor imaging and 1H-MRS study on radiation-induced brain injury after nasopharyngeal carcinoma radiotherapy. Clin Radiol. 2012;67(4):340–345. doi: 10.1016/j.crad.2011.09.008. [DOI] [PubMed] [Google Scholar]
- [40].Semenova NA, Sidorin SV, Akhadov TA, et al. Effect of cell therapy on metabolite content in brain structures of children with consequences of severe brain injury:1H magnetic resonance spectroscopy study. Bull Exp Biol Med. 2011;151(4):532–535. doi: 10.1007/s10517-011-1374-0. [DOI] [PubMed] [Google Scholar]
- [41].Marjañska M, Emir UE, Deelchand DK, et al. Faster metabolite (1)h transverse relaxation in the elder human brain. PLoS One. 2013;8:10. doi: 10.1371/journal.pone.0077572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Nicola SD, Balestr P, Dotti MT, et al. Severe metabolite abnormalities in the white matter of patients with vacuolating megalencephalic leukoencephalopathy with subcortical cyst: a proton spectroscopic imaging study. J Neurol. 2001;248(5):403–409. doi: 10.1007/s004150170182. [DOI] [PubMed] [Google Scholar]
- [43].Ye B, Huang HP, Che CH, et al. Correlation analysis of cognitive function and changes in proton magnetic resonance spectroscopy of the hippocampus in patients with complex partial seizures. Zhonghua Shenjing Yixue Zazhi. 2010;9(2):158–161. [Google Scholar]
- [44].Li D, Li P, He Z, et al. Human intravenous immunoglobulins suppress seizure activities and inhibit the activation of GFAP-positive astrocytes in the hippocampus of picrotoxin-kindled rats. Int J Neurosci. 2012;122(4):200–208. doi: 10.3109/00207454.2011.639470. [DOI] [PubMed] [Google Scholar]
- [45].Vartak-Sharma N, Ghorpade A. Astrocyte elevated gene-1 regulates astrocyte responses to neural injury: implications for reactive astrogliosis and neurodegeneration. J Neuroinflammation. 2012;9:195. doi: 10.1186/1742-2094-9-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Barreto G, White RE, Ouyang Y, et al. Astrocytes: targets for neuroprotection in stroke. Cent Nerv Syst Agents Med Chem. 2011;11(2):164–173. doi: 10.2174/187152411796011303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Sandhir R, Onyszchuk G, Berman NE. Exacerbated glial response in the aged mouse hippocampus following controlled cortical impact injury. Exp Neurol. 2008;213(2):372–380. doi: 10.1016/j.expneurol.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Liu S, Shen GY, Wu QF, et al. The effects of rehabilitative training on neural function and the expression of glial fibrillary acidic protein and ionized calcium binding adaptor molecule-1 after traumatic brain injury. Zhonghua Wuli Yixue yu Kangfu Zazhi. 2008;34(6):415–420. [Google Scholar]
- [49].The Ministry Science and Technology of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals 2006-09-30 [Google Scholar]
- [50].Feeney DM, Boyeson MG, Linn RT, et al. Responses to cortical injury: I. Methodology and local effects of contusions in the rat. Brain Res. 1981;211(1):67–77. doi: 10.1016/0006-8993(81)90067-6. [DOI] [PubMed] [Google Scholar]


