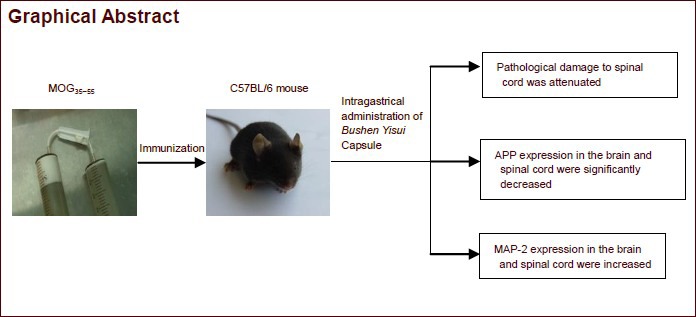
Keywords: neural regeneration, traditional Chinese medicine, multiple sclerosis, experimental autoimmune encephalomyelitis, Bushen Yisui Capsule, amyloid precursor protein, microtubule-associated protein 2, grants-supported paper, neuroregeneration
Abstract
A preliminary clinical study by our group demonstrated Bushen Yisui Capsule (formerly called Erhuang Formula) in combination with conventional therapy is an effective prescription for the treatment of multiple sclerosis. However, its effect on axonal injury during early multiple sclerosis remains unclear. In this study, a MOG35-55-immunized C57BL/6 mouse model of experimental autoimmune encephalomyelitis was intragastrically administered Bushen Yisui Capsule. The results showed that Bushen Yisui Capsule effectively improved clinical symptoms and neurological function of experimental autoimmune encephalomyelitis. In addition, amyloid precursor protein expression was down-regulated and microtubule-associated protein 2 was up-regulated. Experimental findings indicate that the disease-preventive mechanism of Bushen Yisui Capsule in experimental autoimmune encephalomyelitis was mediated by amelioration of axonal damage and promotion of regeneration. But the effects of the high-dose Bushen Yisui Capsule group was not better than that of the medium-dose and low-dose Bushen Yisui Capsule group in preventing neurological dysfunction.
INTRODUCTION
Multiple sclerosis is an inflammatory neurodegenerative disease mediated by an autoimmune response in the central nervous system[1]. Unlike other neurodegenerative diseases, relapsing-remitting multiple sclerosis is characterized by multiple temporal onset periods, the amount of lesion, the variability of lesion sites in the central nervous system and duration of disease course often lead to abnormalities in movement, sensation, and vision, with one or several symptoms such as limb weakness, vision loss, sensory disturbances or ataxia. Disease reoccurrence may exacerbate the disability, ultimately causing paralysis and blindness[2]. The high recurrence rate and high morbidity of multiple sclerosis has become one treatment target in the field of neuroscience research. Pathological features include immune inflammation-induced demyelination and axonal damage, which is the leading cause of the progressive aggravation of neurological dysfunction in multiple sclerosis patients[3]. The majority of patients are young, aged 20–40 years old and the gender ratio of males to females is 1:2. Thus, the disease seriously endangers the productivity and quality of life of young adults[4,5].
Because of the complexity of multiple sclerosis etiology and pathogenesis, there is no effective and safe treatment to date[6]. Immunotherapy (including hormones and immunosuppressants) controls inflammatory responses in multiple sclerosis patients during the acute exacerbation period[7]. When in combination with neuroprotective drugs, immunotherapy could minimize the disability rate thus, this is a potential treatment strategy of multiple sclerosis[8]. Meta-analysis of 16 clinical trials indicated that traditional Chinese medicine treatment might be advantageous in improving neurological damage and promoting neural function recovery in multiple sclerosis patients[9]. However, its mechanism of action remains unknown.
Growing evidence has shown that in multiple sclerosis, neurological damage is highly involved in axonal loss and injury, which can be detected during the early stages of the disease[10]. Amyloid precursor protein (APP) is a transmembrane glycoprotein in normal neurons, produced by the Golgi apparatus and transferred via axoplasmic transport channels[11,12]. APP is abundant in neurons and astrocytes; immune electron microscopy revealed that APP is mainly distributed in polycystic structures, nuclear membrane, Golgi apparatus and rough endoplasmic reticulum in normal brain tissue. After lesions occur, increasing APP activity is confined to swelling neurites, malnutritional axons and perinuclear bodies[13]. Microtubule-associated protein 2 (MAP-2) is a neuronal cytoskeletal protein, which is related to nerve growth and repair[14,15]. MAP-2 is mainly visible in neuronal cell bodies and dendrites and reflects neuronal survival and structural integrity. Its degradation may cause intracellular transport disorders of microtubules in neurons, thus affecting neuronal development, structural stability, protrusion formation and synaptic plasticity[16,17]. Therefore, APP and MAP-2 are recognized as molecular markers of axonal injury and regeneration. Myelin and axon damage in experimental autoimmune encephalomyelitis (EAE) are mediated by immune responses against antigens in myelin and oligodendrocytes. The EAE model develops similar pathological changes observed in human multiple sclerosis, so it is the preferred animal model of multiple sclerosis[18,19].
Bushen Yisui Capsule (formerly called Erhuang Formula) is an effective drug for multiple sclerosis, invented by Professor Fan from Beijing Tiantan Hospital affiliated to Capital Medical University in China, and has obtained approval in hospital use from Beijing Municipal Food and Drug Administration (Lin 10003). Preliminary studies[20,21,22] by our research group suggested that Bushen Yisui Capsule in combination with conventional therapy could effectively improve neurological symptoms and reduce hormone-induced side effects in multiple sclerosis patients during the acute exacerbation period. For patients in remission, the administration of Bushen Yisui Capsule for more than 6 months reduced the recurrence rate, protected neurites and delayed disease progression[20,21,22]. Furthermore, Bushen Yisui Capsule exerted regulatory effects on glial fibrillary acidic protein, oligodendrocyte transcription factor 2 and serum myelin basic protein[22,23] in the brain and spinal cord of EAE animals. Therefore, this drug reduced the severity of EAE, inhibited inflammation and demyelination, and was conducive to promoting nerve regeneration. This study aims to observe the influence of Bushen Yisui Capsule on APP and MAP-2 protein in the brain and spinal cord of EAE mice, in a broader attempt to explore the potential mechanism of reducing axonal injury and promoting repair.
RESULTS
Quantitative analysis of experimental animals
One hundred and twenty C57BL/6 mice were randomly divided into six groups: normal group, model group, hormone group, Bushen Yisui Capsule high-, medium- and low-dose groups. Except for the normal group, active immunity, chronic, non-remitting EAE models were established in the other five groups by immunizing mice with MOG35-55. In the Bushen Yisui Capsule high-, medium- and low-dose groups, mice were given Bushen Yisui Capsule suspension by lavage after immunization. In the hormone group, mice received prednisone acetate orally at day 9 after immunization. Four mice died after immunization (model group, one mouse died of multiple sclerosis or injection at day 15 and one mouse died of gavage mistake at day 33; Bushen Yisui Capsule low-dose group, one mouse died of illness at day 13 and one mouse died of gavage mistake at day 38). Finally, there were 20 mice in each of the normal group, hormone group, Bushen Yisui Capsule high-, and medium-dose group, and 18 mice in each of the model group and Bushen Yisui Capsule low-dose group. Ten mice in each group were analyzed at day 20 after immunization, while the remaining mice were analyzed at day 40.
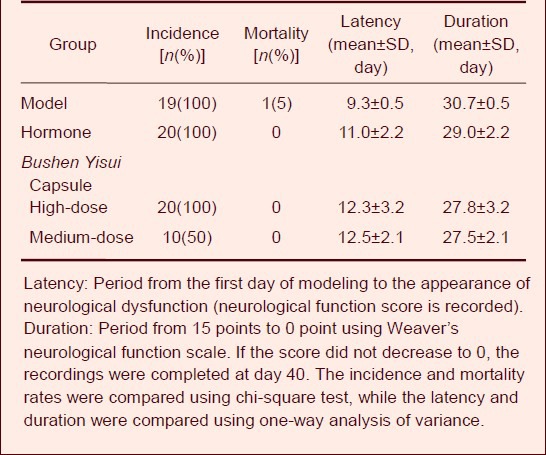
Effect of Bushen Yisui Capsule on the disease incidence, mortality, latency and duration of EAE mice
The EAE manifestations were visible at day 8 after immunization, including depression, decreased body weight, tail weakness, dragging, hind limb weakness, even severe hind limb paralysis and death. The incidence was 100% in the model, hormone, and Bushen Yisui Capsule high-dose groups, 78% in the Bushen Yisui Capsule low-dose group, and 50% in the medium-dose group. The mortality rate was 5% in the model and low-dose groups, while no death was observed in the hormone, Bushen Yisui Capsule high- and medium-dose groups. The treated mice showed longer latency and shorter disease duration compared with the model mice, but the difference was not statistically significant (P > 0.05; Table 1).
Table 1.
Effect of Bushen Yisui Capsule on the disease incidence, mortality, latency and duration of experimental autoimmune encephalomyelitis mice
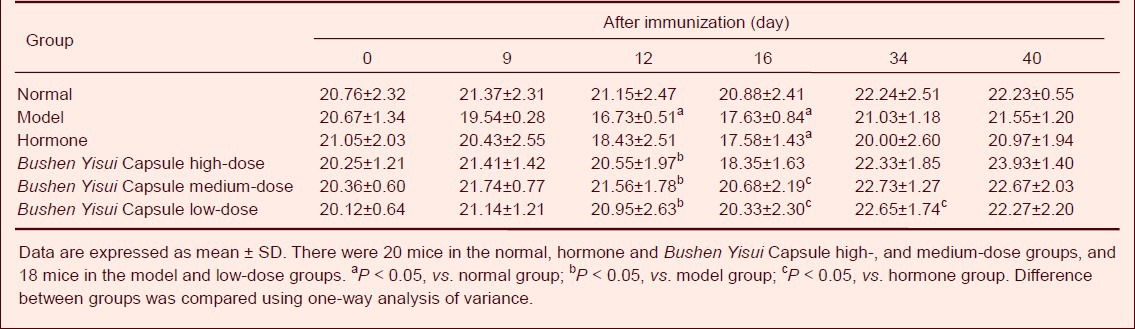
Bushen Yisui Capsule increased body weight of EAE mice
Before immunization, the body weight of mice in each group was similar (P > 0.05; Table 2). At day 9 after immunization, body weight in the model and hormone groups began to decline. At day 12, body weight in the model group was significantly lower than that in the normal group (P < 0.05). The body weight in the model group was significantly decreased compared with the Bushen Yisui Capsule groups (P < 0.05). At day 16, the body weight in the hormone group was significantly decreased compared with the Bushen Yisui Capsule medium- and low-dose groups (P < 0.05). At day 34, the Bushen Yisui Capsule low-dose group had a higher body weight than the hormone group (P < 0.05). At day 40, mice in the other five groups gradually recovered body weight to baseline levels in the normal group (Table 2).
Table 2.
Effect of Bushen Yisui Capsule on body weight (g) of experimental autoimmune encephalomyelitis mice
Bushen Yisui Capsule improved neurological functions of EAE mice
At day 9 after immunization, neurological dysfunction was visible in mice in the model, hormone and Bushen Yisui Capsule high-dose groups. Weaver's neurological function score was increased in the above three groups, while the score was 0 in the Bushen Yisui Capsule medium- and low-dose groups (Table 3).
Table 3.
Effect of Bushen Yisui Capsule on neurological function scores of experimental autoimmune encephalomyelitis mice
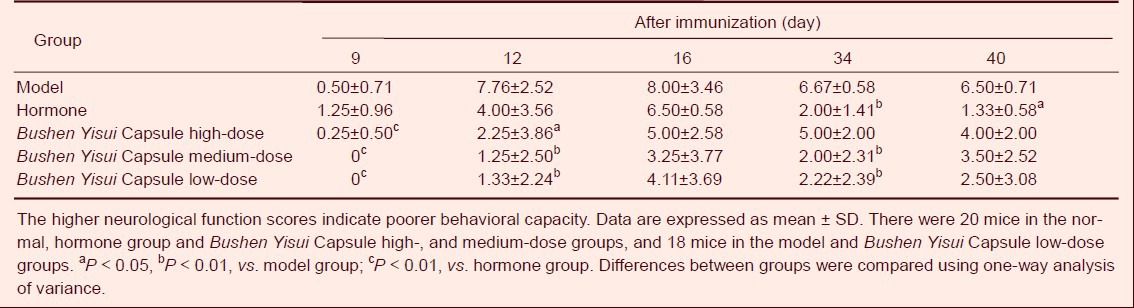
Bushen Yisui Capsule groups showed significantly lower scores than the hormone group (P < 0.01). At day 12, neurological function scores in each group were increased, and Bushen Yisui Capsule groups showed significantly lower scores than the model group (P < 0.05 in Bushen Yisui Capsule high-dose group or P < 0.01 in Bushen Yisui Capsule medium- and low-dose groups). At day 16, neurological function scores in each group reached a peak, but the scores were similar in the hormone, Bushen Yisui Capsule and model groups. At day 34, neurological function scores in each group began to decrease, and the hormone, Bushen Yisui Capsule medium- and low-dose groups showed significantly lower scores than the model group (P < 0.01). Until day 40, there was no apparent change to the scores in each group.
Bushen Yisui Capsule improved pathological morphology of spinal cord in EAE mice
Hematoxylin-eosin staining showed that spinal cord of normal mice had intact structures, with clearly visible cytoplasm and nuclei of nerve cells, and scattered glial cells. At day 20 after immunization (acute phase), a large number of lymphocytes infiltrated the spinal cord of the model group mice, and were present around blood vessels and formed typical sleeve-like changes. In the hormone and Bushen Yisui Capsule medium-dose groups, pathological changes were ameliorated to varying degrees; mild or moderate inflammatory cell infiltration was evident, and sleeve-like changes were significantly reduced. At day 40 after immunization (remission phase), a large number of spinal cord neurons exhibited pyknosis in the model group. In the hormone and Bushen Yisui Capsule medium-dose groups, pathological changes were significantly reduced, showing a small amount of neuronal pyknosis (Figure 1).
Figure 1.
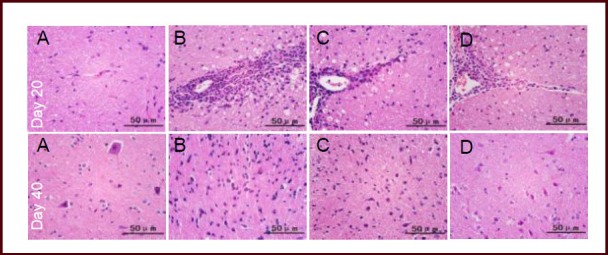
Effect of Bushen Yisui Capsule on the pathological morphology of spinal cords in experimental autoimmune encephalomyelitis mice (hematoxylin-eosin staining).
In the normal group (A), the spinal cord had intact structure and no infiltrating inflammatory cells. In the model group (B), a large number of lymphocytes had infiltrated and formed typical sleeve-like changes at day 20 after immunization. In the hormone (C) and Bushen Yisui Capsule medium-dose (D) groups, pathological changes were ameliorated to varying degrees. At day 40 after immunization, a large number of neurons exhibited pyknosis and pathological changes were reduced. Scale bars: 50 μm.
Bushen Yisui Capsule reduced APP expression in the brain and spinal cord of EAE mice
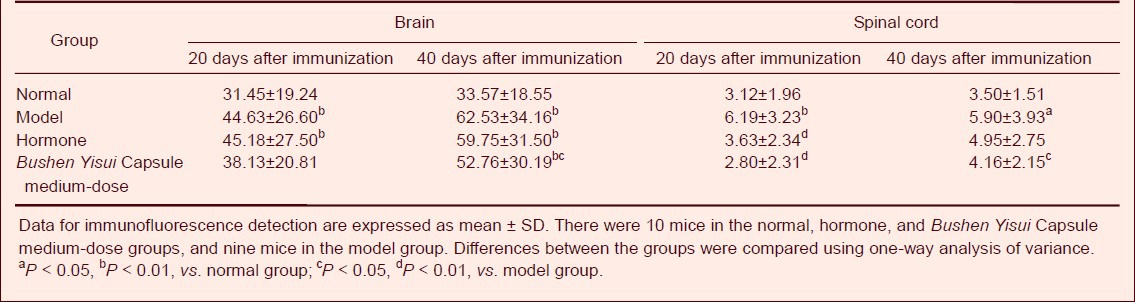
At day 20 after immunization (acute phase), APP expression in the brain and spinal cord of EAE mice was significantly increased compared with the normal group (P < 0.05, P < 0.01, respectively), while the expression level in the Bushen Yisui Capsule medium-dose group was significantly reduced compared with the model group (P < 0.01). At day 40 after immunization (remission phase), APP expression in the brain and spinal cord of EAE mice was higher than the normal group (P < 0.01, P < 0.05, respectively), while the expression level in the Bushen Yisui Capsule medium-dose group was significantly reduced compared with model group (P < 0.05). The APP expression level in the Bushen Yisui Capsule medium-dose group was similar to that in the hormone group (P > 0.05; Figures 2–3, Table 4).
Figure 2.
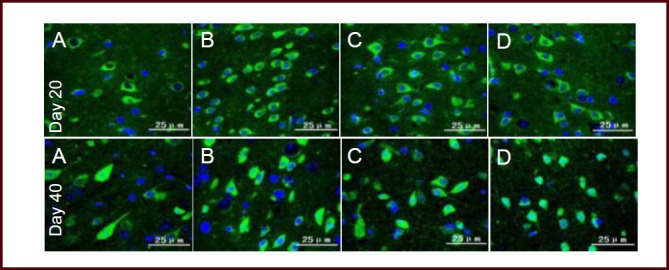
Bushen Yisui Capsule reduced amyloid precursor protein (APP) expression in the brain of experimental autoimmune encephalomyelitis mice (immunofluorescence staining, fluorescence microscopy).
At day 20 after immunization, APP expression in the brains of model group (B) mice was significantly increased compared with the normal group (A), while expression levels in the Bushen Yisui Capsule medium-dose (D) and hormone (C) groups was significantly reduced compared with the model group. Change in expression level at day 40 was similar to that at day 20. Fluorescent staining is fluorescein isothiocyanate (FITC) (green) and cell nuclei were stained blue by 4′,6-diamidino-2-phenylindole (DAPI). Scale bars: 25 μm.
Figure 3.
Bushen Yisui Capsule reduced amyloid precursor protein (APP) expression in spinal cord of experimental autoimmune encephalomyelitis mice (immunofluorescence staining, fluorescence microscopy).
At day 20 after immunization, APP expression in the model group (B) of mice was significantly increased compared with the normal group (A), while expression levels in the Bushen Yisui Capsule medium-dose (D) and hormone (C) groups was significantly reduced compared with the model group. Change in expression level at day 40 was similar to that at day 20. Fluorescent staining was fluorescein isothiocyanate (FITC) (green) and cell nuclei were stained blue by 4′,6-diamidino-2-phenylindole (DAPI). Scale bars: 25 μm.
Table 4.
Effect of Bushen Yisui Capsule on the number of amyloid precursor protein positive cells (n/20 × magnification) in the brain and spinal cord of experimental autoimmune encephalomyelitis mice
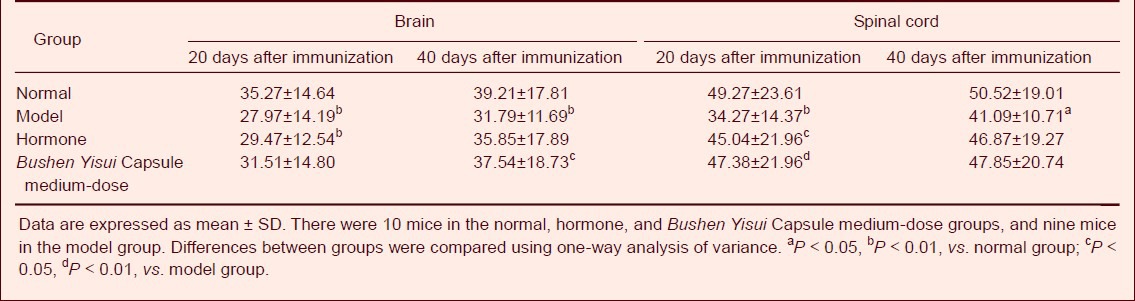
Bushen Yisui Capsule reduced MAP-2 expression in the brain and spinal cord of EAE mice
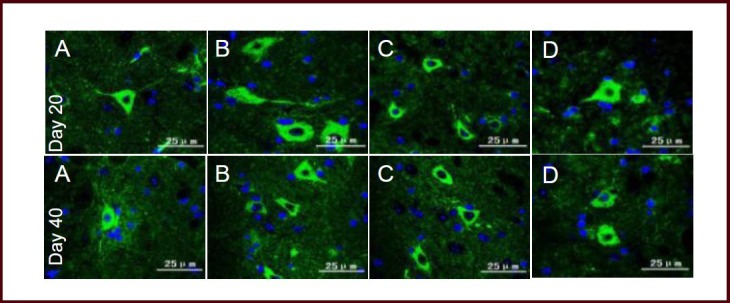
At day 20 after immunization (acute phase), MAP-2 protein expression in the brain and spinal cord of EAE mice was significantly decreased compared with the normal group (P < 0.01), while the expression level in the Bushen Yisui Capsule medium-dose and hormone groups was significantly increased compared with the model group (P < 0.01, P < 0.05). At day 40 after immunization (remission phase), MAP-2 protein expression in the model group was lower than the normal group (P < 0.01), while the expression level in the Bushen Yisui Capsule medium-dose group was significantly increased compared with the model group (P < 0.05). The MAP-2 expression level in the Bushen Yisui Capsule medium-dose and hormone groups was unchanged (P > 0.05; Figures 4–5, Table 5).
Figure 4.
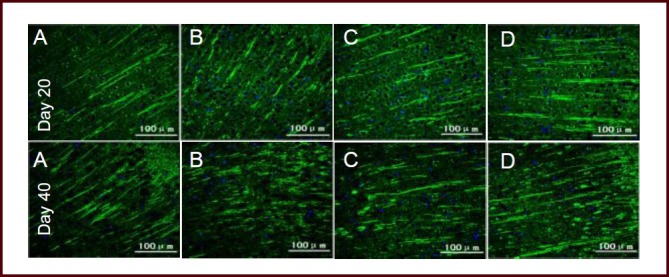
Bushen Yisui Capsule reduced microtubule-associated protein 2 (MAP-2) expression in the brain of experimental autoimmune encephalomyelitis mice (immunofluorescence staining, fluorescence microscope).
At day 20 after immunization, MAP-2 expression in the model group (B) of mice was significantly decreased compared with the normal group (A), while the expression level in the Bushen Yisui Capsule medium-dose (D) and hormone (C) groups was significantly increased compared with the model group. Change in expression level at day 40 was similar to that at day 20. Fluorescent staining was fluorescein isothiocyanate (FITC) (green) and cell nuclei were stained blue by 4′,6-diamidino-2-phenylindole (DAPI). Scale bars: 100 μm.
Figure 5.
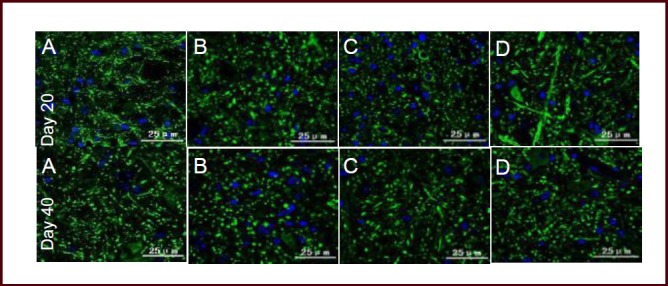
Bushen Yisui Capsule reduced microtubule-associated protein 2 (MAP-2) expression in spinal cord of experimental autoimmune encephalomyelitis mice (immunofluorescence staining, fluorescence microscopy). At day 20 after immunization, MAP-2 expression in the model group (B) of mice was significantly increased compared with the normal group (A), while expression levels in the Bushen Yisui Capsule medium-dose (D) and hormone (C) groups was significantly decreased compared with the model group. Change in expression level at day 40 was similar to that at day 20. Fluorescent staining was fluorescein isothiocyanate (FITC) (green) and cell nuclei were stained blue by 4′,6-diamidino-2-phenylindole (DAPI). Scale bars: 25 μm.
Table 5.
Effect of Bushen Yisui Capsule on the number of microtubule-associated protein 2 positive cells (× 102 μm2/40 × magnification) in the brain and spinal cord of experimental autoimmune encephalomyelitis mice
DISCUSSION
Neuroprotective effect of Bushen Yisui Capsule in EAE mice
In this study, EAE mice exhibited disease onset symptoms at day 8 after immunization, and the onset peaked at day 16, consistent with the body weight loss and neurological function scores. The mortality rate was highest in the model group. Bushen Yisui Capsule and prednisone administration improved clinical symptoms and neurological dysfunction in EAE mice. In Bushen Yisui Capsule treatment groups, body weight loss was less and neurological function scores were lower compared to the model and hormone groups prior to or at the peak of disease. The medium-dose Bushen Yisui Capsule given the mice was equivalent to human dose of medication. The high-dose Bushen Yisui Capsule was equivalent to twice of human dose, speculating that poor absorption of animals towards high dose, thus affecting the therapeutic effects. This was the cause that the low and medium doses were more effective than the high dose. EAE incidence was lowest in the Bushen Yisui Capsule medium-dose group and no deaths were observed. Therefore, we suggest the medium-dose treatment for further study.
Hematoxylin-eosin staining revealed that a large number of lymphocytes infiltrated into the mouse spinal cord, and were present in the blood vessels and produced typical sleeve-like changes in the model group.
This indicated that nerve tissue was attacked by pro-inflammatory molecules causing inflammatory responses in the model mice. Bushen Yisui Capsule treatment significantly inhibited pathological changes as only mild or moderate inflammatory cell infiltration was observed and sleeve-like changes were significantly reduced. These findings indicated the effect of intervention inflammation in Bushen Yisui Capsule.
Effect of Bushen Yisui Capsule on APP expression in the brain and spinal cord of EAE mice
It was recently reported that APP is deposited in the brain, spinal cord[25] and cerebrospinal fluid[26] of multiple sclerosis patients, as well as in the brain and spinal cord of EAE animals[27,28,29,30]. In this study, the number of APP positive cells in the brain and spinal cord of EAE mice was significantly higher than the normal group at day 20 after onset (acute phase), and the number continued increasing up to day 40 (remission phase). This can be explained by the attack of immuno-inflammatory factors on the brain and spinal cord, which produced axonal degeneration, shown as axonal swelling, rupture and disintegration[31,32,33]. APP is a fast reactive protein, and accumulates in the axonal transport damaged area. After Bushen Yisui Capsule administration, APP content in the mouse brain and spinal cord was significantly decreased. This evidence shows that Bushen Yisui Capsule ameliorated axonal injury, and that its effect was related to a reduction of inflammatory responses. A preliminary study found that Bushen Yisui Capsule had a regulatory role on lymphocyte subsets CD4+, CD8+, CD4+/CD8+ ratio[34,35,36], Th1/Th2 ratio[37] in peripheral blood, brain and spinal cord, as well as peripheral blood natural killer cells[34,36,38] in EAE animals.
Effect of Bushen Yisui Capsule on MAP-2 expression in the brain and spinal cord of EAE mice
Because MAP-2 is extremely sensitive to cerebral ischemia, MAP-2 expression is rarely observed at the ischemic center, and is selectively increased in intact neurons and at the ischemic penumbra, which corresponds to neuronal recovery[39]. At present, existing studies focused on the role of MAP-2 in cerebral ischemic diseases, while little evidence is available in multiple sclerosis and EAE. A previous study found that MAP-2 expression in rat brain and spinal cord was decreased during the EAE remission phase[40]. In this study, MAP-2 expression levels in the brain and spinal cord were significantly downregulated in the model group at day 20 after onset (acute phase), compared with the normal group. This suggests that the cell cytoskeleton was degraded causing neuronal death. At day 40 (remission phase), MAP-2 expression levels were increased slightly, possibly due to self-repair functions, however, this recovery mechanism was insufficient to repair damaged nerve tissue. After Bushen Yisui Capsule administration, MAP-2 content in the mouse brain and spinal cord was significantly increased, indicating that this drug promotes MAP-2 synthesis and neuronal restoration.
In summary, Bushen Yisui Capsule has neuroprotective effects on EAE mice, can improve clinical symptoms of EAE animals, lower neurological function scores, reduce the morbidity and mortality rate, prolong the latency, reduce disease severity, shorten the duration and promote their recovery, as well as reduce myelin and axon injury.
MATERIALS AND METHODS
Design
A randomized controlled animal experiment.
Time and setting
Experiments were performed at Encephalopathy Laboratory, School of Traditional Chinese Medicine, Capital Medical University, China from April to November 2012.
Materials
Animals
Female C57BL/6 mice, aged 6–8 weeks, weighing 18–22 g, of specific pathogen free grade, were provided by Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China) with license No. SCXK (Jing) 2006-0009. Animals were housed in the Experimental Animal Center of Capital Medical University, China (license No. SYXK (Jing) 2010-0020), at 20–25°C, 20–25% humidity, under 8-hour light per day. The disposal of experimental animals was in accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals, issued by the Ministry of Science and Technology of China[41].
Drugs
Bushen Yisui Capsule was provided by Beijing ASIA-EAST Biopharmaceutical Co., Ltd. (Drug Approval No. Lin 10003), and consisted of rehmannia dried rhizome, prepared rhizome of rehmannia, Bulbus Fritillariae Thunbergii, Herba Leonuri, Hirudo and Scorpio. Prior to its use, the capsule shells were removed and the powder was dissolved in saline to prepare a suspension.
Prednisone tablets (5 mg) were provided by Tianjin Pacific Pharmaceutical Co., Ltd., Tianjin, China (Drug Approval No. H33021207). Prior to their use, prednisone tablets were dissolved in physiological saline to prepare a suspension.
Methods
Preparation of active, chronic, non-remitting EAE models
Active, chronic non-remitting EAE models were established in C57BL/6 mice using MOG35-55 immunization[42]. The mice were subcutaneously injected with the following reagents into four points in the back: 0.2 mL antigen (Beijing Scilight Biotechnology Co., Ltd., Beijing, China) containing 50 μg MOG35-55 peptide (amino acid sequence: MEVGWYRSPFSRVVHLYR NGK) and 100 μL complete Freund's adjuvant (Sigma, St. Louis, MO, USA) containing 2 mg/mL inactivated mycobacterium tuberculosis H37Ra (Difco Laboratories, Franklin Lakes, NJ, USA). Each mouse was intraperitoneally injected with pertussis toxin (Sigma) 25 mg/kg on the day of immunization and the day after immunization, to induce EAE. Mice in the normal group were injected with an equal volume (0.2 mL) of saline. The success of the model is determined upon observation of clinical symptoms and signs, such as tail dystonia, gait instability, hind limb paralysis, complete paralysis and death. Neurological function scores were then evaluated[43,44], and mice in the acute phase were recorded as a mean of eight points. The brain and spinal cord were analyzed for inflammatory cell infiltration and sleeve-like changes by hematoxylin-eosin staining under a light microscope.
Drug treatment
On day 1 after immunization, mice in high-, medium- and low-dose groups were given Bushen Yisui Capsule suspension at 6.47, 3.34 and 1.62 g/kg orally, once per day, for 40 days. The normal group and model group were administered with normal saline (0.2 mL). In the hormone group, mice were given normal saline (0.2 mL) for 8 days, and then changed to prednisone 5 mg/kg orally, once per day, for 40 days, after the detection of neurological function was complete. Mice were observed and weighed daily and neurological function scores were recorded. On day 40 after immunization, body weight, neurological score, incidence, mortality, latency and duration were analyzed.
Neurological function score
Weaver's 15-point neurological function scale was used to assess neurological function: tail activity is divided into three levels and limb activity is divided into four levels; the final scores are obtained by accumulating points. Tail: 0 point, asymptomatic; 1 point, tail tension reduces or tail distant paralysis; 2 points, tail complete paralysis; limbs: 0 point, asymptomatic; 1 point, gait instability; 2 points, limb hemiparesis, limbs drag when walking; 3 points, complete paralysis of limbs, limb valgus when walking. The tail and limb paralysis was evaluated and the total scores were obtained by accumulating scores. Death was recorded as 15 points[46,47] (supplementary Figure 1 online).
Specimen harvesting and pathological detection of spinal cord
On days 20 and 40 after immunization, specimens were harvested. In brief, mice were immediately anesthetized with 10% chloral hydrate and the brain and spinal cord were removed. Then specimens were fixed in paraformaldehyde, dehydrated, embedded in paraffin, and cut into serial slices using a microtome. Slices were stained with hematoxylin-eosin and pathological changes were observed under an optical microscope (Nikon, Tokyo, Japan).
Immunofluorescence detection of APP and MAP-2 expression in mouse brain and spinal cord
The brain and spinal cord were formalin-fixed, conventionally dehydrated, embedded in paraffin, and cut into serial sections. The sections were dewaxed, rinsed with PBS three times for 3 minutes, and boiled with citric acid at 95°C for 20 minutes. Then sections were cooled to 30°C, rinsed with PBS three times for 3 minutes, and blocked with 10% goat serum at 37°C for 60 minutes. Subsequently, sections were incubated with rabbit anti-mouse APP antibody (1:50; Abcam, Cambridge, UK) and rabbit anti-mouse MAP-2 antibody (1:50; Boaosen, Beijing, China) at 4°C for 48 hours. After 37°C rewarming for 60 minutes and three PBS washes for 10 minutes, sections were incubated with fluorescein isothiocyanate (FITC)-labeled goat anti-rabbit IgG (1:70) and Alexa Fluor488 FITC-labeled goat anti-rabbit IgG (1:200; Jackson Company, Jackson, MS, USA) at 37°C for 60 minutes. After incubation, sections were rinsed with PBS three times for 10 minutes and with distilled water twice for 5 minutes, then mounted with 4′,6-diamidino-2-phenylindole (DAPI)-Fluoromount-G, and stored at 4°C.
Finally, sections were observed under a Leica DM4000B fluorescence microscope (Leica, Solms, Germany). The image analysis was performed using Leica Qwin analysis software (Leica). Eighteen fields of view were randomly selected from each brain and spinal cord section and the number of APP-positive cells (20 × magnification) and the area of MAP-2 positive cells (40 × magnification) were measured.
Statistical analysis
Data were expressed as mean ± SD. For statistical analysis, quantitative data were subjected to normality test. One-way analysis of variance was applied for the normal distribution. Difference between groups was compared using chi-square test. All statistical analysis was performed using SPSS 11.5 software (SPSS, Chicago, IL, USA).
Research background: Multiple sclerosis is pathologically characterized by immune inflammation-induced demyelination and axonal injury. In particular, axonal injury is the contributing factor for progressive aggravation of neurological dysfunction in patients with multiple sclerosis.
Research frontiers: Preliminary study results of our research group suggested that based on conventional therapy, Bushen Yisui Capsule given to multiple sclerosis patients during the acute exacerbation period could effectively improve neurological symptoms and reduce side effects caused by hormone treatment. During remission period, Bushen Yisui Capsule administration for more than 6 months reduced disease recurrence and delayed disease progression.
Clinical significance: Bushen Yisui Capsule is a potential treatment for multiple sclerosis and related neurodegenerative diseases, and is expected to be used in the clinic.
Academic terminology: Experimental autoimmune encephalomyelitis is mediated by specifically sensitized CD4+ T cells, and is an autoimmune disease characterized by mononuclear cell infiltration and myelin loss around small blood vessels in the central nervous system, and is widely recognized as an animal model for human multiple sclerosis.
Peer review: This study aims to evaluate the effect of Bushen Yisui Capsule on improving the clinical symptoms of experimental autoimmune encephalomyelitis disease and the potential mechanisms from the perspective of axonal injury and regeneration. Experimental findings indicate that Bushen Yisui Capsule reduce myelin and axonal damage, and promoted neuronal regeneration.
Footnotes
Funding: This study was supported by the National Natural Science Foundation of China, No. 81072765, 81173237, 81273742; Scientific Research Key Project of Beijing Municipal Commission of Education, No. KZ201310025023; and Special Fund for Capital Traditional Chinese Medicine and Nursing Research, No. 12ZYH01.
Conflicts of interest: None declared.
Ethical approval: The study was approved by the Experimental Animal Ethics Committee of Capital Medical University in China.
Supplementary information: Supplementary data associated with this article can be found, in the online version, by visiting www.nrronline.org.
(Reviewed by Croxford L, Haase R, Zhu HF, Liu Y)
(Edited by Yu J, Yang Y, Li CH, Song LP, Liu WJ, Zhao M)
REFERENCES
- [1].Herz J, Zipp F, Siffrin V. Neurodegeneration in autoimmune CNS inflammation. Exp Neurol. 2010;225(1):9–17. doi: 10.1016/j.expneurol.2009.11.019. [DOI] [PubMed] [Google Scholar]
- [2].Weiner HL. New York: Crown Publishers; 2004. Curing MS: How Science is Solving the Mysteries of Multiple Sclerosis. [PubMed] [Google Scholar]
- [3].Vogt J, Paul F, Aktas O, et al. Lower motor neuron loss in multiple sclerosis and experimental autoimmune encephalomyelitis. Ann Neurol. 2009;66(3):310–322. doi: 10.1002/ana.21719. [DOI] [PubMed] [Google Scholar]
- [4].Liu GZ. Beijing: Peking University Medical Press; 2012. Multiple Sclerosis. [Google Scholar]
- [5].Zhou WB, Cui YZ, Xiao B. Epidemiological studies of multiple sclerosis. Zhongguo Shenjing Mianyi Xue he Shenjing Bing Xue Zazhi. 2005;12(6):373–375. [Google Scholar]
- [6].Ontaneda D, Hyland M, Cohen JA. Multiple sclerosis: new insights in pathogenesis and novel therapeutics. Annu Rev Med. 2012;63:389–404. doi: 10.1146/annurev-med-042910-135833. [DOI] [PubMed] [Google Scholar]
- [7].Chinese Medical Association, Chinese Society of Neurology. Expert consensus of multiple sclerosis's diagnosis and treatment in China. Zhonghua Shenjing Ke Zazhi. 2010;43(7):516–521. [Google Scholar]
- [8].Van der Walt A, Butzkueven H, Kolbe S, et al. Neuroprotection in multiple sclerosis: a therapeutic challenge for the next decade. Pharmacol Ther. 2010;126(1):82–93. doi: 10.1016/j.pharmthera.2010.01.006. [DOI] [PubMed] [Google Scholar]
- [9].Liu J, Gao Y, Kan BH, et al. Systematic review and meta-analysis of randomized controlled trials of Chinese herbal medicine in treatment of multiple sclerosis. Zhongxiyi Jiehe Xuebao. 2012;10(2):141–153. doi: 10.3736/jcim20120204. [DOI] [PubMed] [Google Scholar]
- [10].Seehusen F, Baumgärtner W. Axonal pathology and loss precede demyelination and accompany chronic lesions in a spontaneously occurring animal model of multiple sclerosis. Brain Pathol. 2010;20(3):551–559. doi: 10.1111/j.1750-3639.2009.00332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Sherriff FE, Bridges LR, Sivaloganathan S. Early detection of axonal injury after human head trauma using immunocytochemistry for beta-amyloid precursor protein. Acta Neuropathol. 1994;87(1):55–62. doi: 10.1007/BF00386254. [DOI] [PubMed] [Google Scholar]
- [12].Blumbergs PC, Scott G, Manavis J, et al. Staining of amyloid precursor protein to study axonal damage in mild head injury. Lancet. 1994;344(8929):1055–1056. doi: 10.1016/s0140-6736(94)91712-4. [DOI] [PubMed] [Google Scholar]
- [13].Tomimoto H, Akiguchi I, Wakita H, et al. Ultrastructural localization of amyloid protein precursor in the normal and post-ischemic gerbil brain. Brain Res. 1995;672(1-2):187–195. doi: 10.1016/0006-8993(94)01160-j. [DOI] [PubMed] [Google Scholar]
- [14].Nunez J. Immature and mature variants of MAP2 and tau proteins and neuronal plasticity. Trends Neurosci. 1988;11(11):477–479. doi: 10.1016/0166-2236(88)90004-5. [DOI] [PubMed] [Google Scholar]
- [15].Dawson DA, Hallenbeck JM. Acute focal ischemia-induced alterations in MAP2 immunostaining: description of temporal changes and utilization as a marker for volumetric assessment of acute brain injury. J Cereb Blood Flow Metab. 1996;16(1):170–174. doi: 10.1097/00004647-199601000-00020. [DOI] [PubMed] [Google Scholar]
- [16].Posmantur RM, Kampfl A, Liu SJ, et al. Cytoskeletal derangements of cortical neuronal processes three hours after traumatic brain injury in rats: an immunofluorescence study. J Neuropathol Exp Neurol. 1996;55(1):68–80. doi: 10.1097/00005072-199601000-00007. [DOI] [PubMed] [Google Scholar]
- [17].Morales P, Fiedler JL, Andrés S, et al. Plasticity of hippocampus following perinatal asphyxia: effects on postnatal apoptosis and neurogenesis. J Neurosci Res. 2008;86(12):2650–2662. doi: 10.1002/jnr.21715. [DOI] [PubMed] [Google Scholar]
- [18].Baker D, Gerritsen W, Rundle J, et al. Critical appraisal of animal models of multiple sclerosis. Mult Scler. 2011;17(6):647–657. doi: 10.1177/1352458511398885. [DOI] [PubMed] [Google Scholar]
- [19].Constantinescu CS, Farooqi N, O’Brien K, et al. Experimental autoimmune encephalomyelitis (EAE) as a model for multiple sclerosis (MS) Br J Pharmacol. 2011;164(4):1079–1106. doi: 10.1111/j.1476-5381.2011.01302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Fan YP, Wang P, Zhang XH, et al. Treatment of relapsing multiple sclerosis with Erhuang Formula. Beijing Zhongyiyao Daxue Xuebao. 2006;29(4):273. [Google Scholar]
- [21].Fan YP, Wang P, Zhang XH, et al. Mechanism exploration of Erhuang Formula in treating acute episode of disseminated sclerosis. Zhonghua Zhongyiyao Zazhi. 2007;22(1):25. [Google Scholar]
- [22].Zhou L, Fan YP. Clinical research on Erhuangfang for reducing axonal injury and recurrence in multiple sclerosis. Zhongguo Zhongyiyao Xinxi Zazhi. 2012;19(8):14. [Google Scholar]
- [23].Wang L, Fan YP, Liu XZ, et al. Experimental study on Erhuang Decoction in preventing and curing experimental allergic encephalomyelitis (EAE) Zhonghua Zhongyiyao Zazhi. 2005;20(8):475–477. [Google Scholar]
- [24].Li KN, Fan YL, Chen KL, et al. Effect of Erhuang Capsule on the acute inflammatory reaction and myelin sheath repairing in model rats of experimental allergic encephalomyelitis. Shoudu Yike Daxue Xuebao. 2011;32(1):110–115. [Google Scholar]
- [25].Gehrmann J, Banati RB, Cuzner ML, et al. Amyloid precursor protein (APP) expression in multiple sclerosis lesions. Glia. 1995;15(2):141–151. doi: 10.1002/glia.440150206. [DOI] [PubMed] [Google Scholar]
- [26].Mai W, Hu X, Lu Z, et al. Cerebrospinal fluid levels of soluble amyloid precursor protein and β-amyloid 42 in patients with multiple sclerosis, neuromyelitis optica and clinically isolated syndrome. J Int Med Res. 2011;39(6):2402–2413. doi: 10.1177/147323001103900641. [DOI] [PubMed] [Google Scholar]
- [27].Banati RB, Gehrmann J, Lannes-Vieira J, et al. Inflammatory reaction in experimental autoimmune encephalomyelitis (EAE) is accompanied by a microglial expression of the beta A4-amyloid precursor protein (APP) Glia. 1995;14(3):209–215. doi: 10.1002/glia.440140306. [DOI] [PubMed] [Google Scholar]
- [28].Beilin O, Karussis DM, Korczyn AD, et al. Increased KPI containing amyloid precursor protein in experimental autoimmune encephalomyelitis brains. Neuroreport. 2007;18(6):581–584. doi: 10.1097/WNR.0b013e328091c1e6. [DOI] [PubMed] [Google Scholar]
- [29].Garay L, Deniselle MC, Meyer M, et al. Protective effects of progesterone administration on axonal pathology in mice with experimental autoimmune encephalomyelitis. Brain Res. 2009;1283:177–185. doi: 10.1016/j.brainres.2009.04.057. [DOI] [PubMed] [Google Scholar]
- [30].Clarner T, Buschmann JP, Beyer C, et al. Glial amyloid precursor protein expression is restricted to astrocytes in an experimental toxic model of multiple sclerosis. J Mol Neurosci. 2011;43(3):268–274. doi: 10.1007/s12031-010-9419-9. [DOI] [PubMed] [Google Scholar]
- [31].Eng LF, Ghirnikar RS, Lee YL. Inflammation in EAE: role of chemokine/cytokine expression by resident and infiltrating cells. Neurochem Res. 1996;21(4):511–525. doi: 10.1007/BF02527717. [DOI] [PubMed] [Google Scholar]
- [32].Bettini M, Rosenthal K, Evavold BD. Pathogenic MOG-reactive CD8+ T cells require MOG-reactive CD4+ T cells for sustained CNS inflammation during chronic EAE. J Neuroimmunol. 2009;213(1-2):60–68. doi: 10.1016/j.jneuroim.2009.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Wu F, Cao W, Yang Y, et al. Extensive infiltration of neutrophils in the acute phase of experimental autoimmune encephalomyelitis in C57BL/6 mice. Histochem Cell Biol. 2010;133(3):313–322. doi: 10.1007/s00418-009-0673-2. [DOI] [PubMed] [Google Scholar]
- [34].Wang L, Fan YP, Gong MX, et al. Effects of Erhuang Capsule on lymphocyte subgroups and natural killer cells in rats with experimental autoimmune encephalomyelitis. Zhongyao Xinyao yu Linchuang Yaoli. 2008;19(3):165–169. [Google Scholar]
- [35].Fan YP, Song LJ, Ye M, et al. Influence of Erhuang Capsule on the immunohistochemical expression of IFN-ã, TGF-β, MMP-9 and lymphocyte subsets in central nervous system of EAE rat. Zhongguo Shiyan Fangji Xue Zazhi. 2010;16(5):142–146. [Google Scholar]
- [36].Zhao H, Wang L, Gong MX, et al. Effects of Erhuang Capsule on cell immunity of mice with experimental autoimmune encephalomyelitis. Shoudu Yike Daxue Xuebao. 2009;30(1):15–19. [Google Scholar]
- [37].Zhou L, Fan YP, Wang L, et al. Influences of Erhuangfang on cytokines and balance of Th1/Th2 in EAE rats. Shoudu Yike Daxue Xuebao. 2009;30(1):20–23. [Google Scholar]
- [38].Fan YP, Liu XZ, Wang L, et al. Effects of Erhuang Formula on NK cells and cytokines in peripheral blood of EAE rats. Beijing Zhongyiyao Daxue Xuebao. 2007;30(3):165–168. [Google Scholar]
- [39].Lin RC, Matesic DF. Immunohistochemical demonstration of neuron-specific enolase and microtubule-associated protein 2 in reactive astrocytes after injury in the adult forebrain. Neuroscience. 1994;60(1):11–16. doi: 10.1016/0306-4522(94)90199-6. [DOI] [PubMed] [Google Scholar]
- [40].Wang L, Zhao H, Fan YP, et al. Effects of kidney-tonifying therapy on GAP-43 and MAP-2 expression in brain and spinal cord tissues in rats with experimental allergic encephalomyelitis. Shoudu Yike Daxue Xuebao. 2007;28(6):748–752. [Google Scholar]
- [41].The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animais 2006-09-30 [Google Scholar]
- [42].Lu ZQ, Zhu CS, Zheng XP, et al. Comparison of various animal models of multiple sclerosis induced by different antigens on different animals. Zhongshan Daxue Xuebao: Yixue Kexue Ban. 2008;9(6):684–689. [Google Scholar]
- [43].Weaver A, Goncalves da Silva A, Nuttall RK, et al. An elevated matrix metalloproteinase (MMP) in an animal model of multiple sclerosis is protective by affecting Th1/Th2 polarization. FASEB J. 2005;19(12):1668–1670. doi: 10.1096/fj.04-2030fje. [DOI] [PubMed] [Google Scholar]
- [44].Dasilva AG, Yong VW. Expression and regulation of matrix metalloproteinase-12 in experimental autoimmune encephalomyelitis and by bone marrow derived macrophages in vitro. J Neuroimmunol. 2008;199(1-2):24–34. doi: 10.1016/j.jneuroim.2008.04.034. [DOI] [PubMed] [Google Scholar]


