Abstract
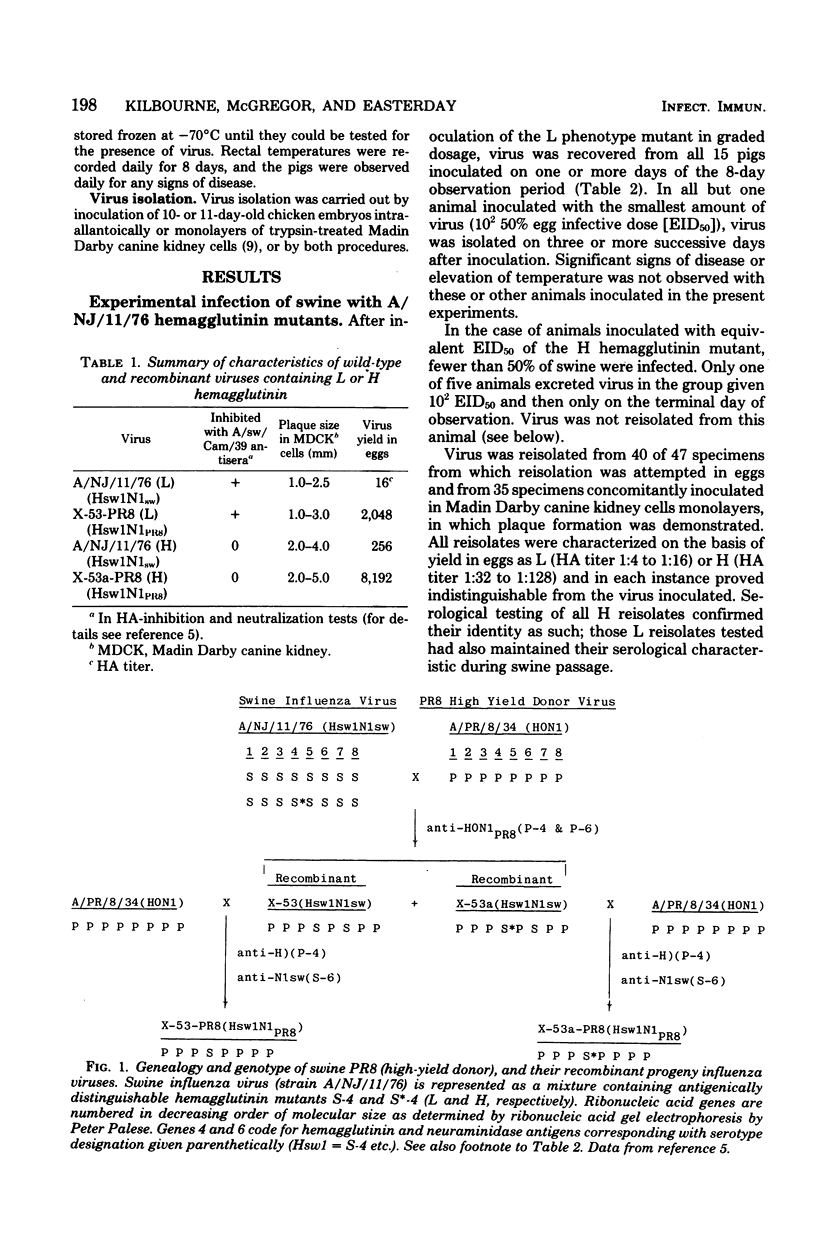
In two mutant clones (L and H) of A/NJ/11/76 (Hsw 1N1) influenza viruses which differ slightly antigenically and markedly in replication characteristics in chicken embryos and Madin Darby canine kidney cells, these pleiotropic differences are mediated by mutation in the hemagglutinin gene (E. D. Kilbourne, Proc. Natl. Acad. Sci. U.S.A. 75:6258--6262, 1978). Experimental infection of swine with either the mutant L and H clones or recombinant viruses differing genetically only with respect to the presence of L or H hemagglutinin demonstrated greater infectivity for the natural host of viruses bearing the L hemagglutinin. Introduction of the L but not the H hemagglutinin gene into the human influenza virus A/PR/8/34 rendered it infective for swine. Both L and H variants were isolated from pigs naturally infected with contemporary swine influenza viruses when selective conditions for the suppression of the more prevalent L mutant were employed. The L and H mutants of swine influenza virus are yet another example of viral dimorphism in nature and probably are not mere artifacts of laboratory selection. In any event, the frequent apparent allelic appearance of the two forms suggests frequent mutation and/or reversion involving a point mutation in the hemagglutinin gene. The present studies demonstrate the importance of a single gene in the pathogenesis of an influenza viral infection in its natural host.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almond J. W. A single gene determines the host range of influenza virus. Nature. 1977 Dec 15;270(5638):617–618. doi: 10.1038/270617a0. [DOI] [PubMed] [Google Scholar]
- CHOPPIN P. W., EGGERS H. J. Heterogeneity of Coxsackie B4 virus: two kinds of particles which differ in antibody sensitivity, growth rate, and plaque size. Virology. 1962 Nov;18:470–476. doi: 10.1016/0042-6822(62)90037-5. [DOI] [PubMed] [Google Scholar]
- CHOPPIN P. W., TAMM I. Two kinds of particles with contrasting properties in influenza A virus strains from the 1957 pandemic. Virology. 1959 Aug;8:539–542. doi: 10.1016/0042-6822(59)90059-5. [DOI] [PubMed] [Google Scholar]
- Kendal A. P., Noble G. R., Dowdle W. R. Swine influenza viruses isolated in 1976 from man and pig contain two coexisting subpopulations with antigenically distinguishable hemagglutinins. Virology. 1977 Oct 1;82(1):111–121. doi: 10.1016/0042-6822(77)90037-x. [DOI] [PubMed] [Google Scholar]
- Kilbourne E. D. Genetic dimorphism in influenza viruses: characterization of stably associated hemagglutinin mutants differing in antigenicity and biological properties. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6258–6262. doi: 10.1073/pnas.75.12.6258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laver W. G., Gerhard W., Webster R. G., Frankel M. E., Air G. M. Antigenic drift in type A influenza virus: peptide mapping and antigenic analysis of A/PR/8/34 (HON1) variants selected with monoclonal antibodies. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1425–1429. doi: 10.1073/pnas.76.3.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarowitz S. G., Choppin P. W. Enhancement of the infectivity of influenza A and B viruses by proteolytic cleavage of the hemagglutinin polypeptide. Virology. 1975 Dec;68(2):440–454. doi: 10.1016/0042-6822(75)90285-8. [DOI] [PubMed] [Google Scholar]
- Palese P., Schulman J. L. Mapping of the influenza virus genome: identification of the hemagglutinin and the neuraminidase genes. Proc Natl Acad Sci U S A. 1976 Jun;73(6):2142–2146. doi: 10.1073/pnas.73.6.2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slobodă E., Buimovici-Klein E., Klein R. Value of the fluctuation test for establishing the origin of agar-resistant L particles emerging in S subclones of ECHO virus 19. Arch Gesamte Virusforsch. 1969;28(1):1–6. doi: 10.1007/BF01250839. [DOI] [PubMed] [Google Scholar]



