Abstract
Human Wharton's jelly mesenchymal stem cells were isolated from fetal umbilical cord. Cells were cultured in serum-free neural stem cell-conditioned medium or neural stem cell-conditioned medium supplemented with Dkk-1, a Wnt/β catenin pathway antagonist, and LeftyA, a Nodal signaling pathway antagonist to induce differentiation into retinal progenitor cells. Inverted microscopy showed that after induction, the spindle-shaped or fibroblast-like Wharton's jelly mesenchymal stem cells changed into bulbous cells with numerous processes. Immunofluorescent cytochemical ing and reverse-transcription PCR showed positive expression of retinal progenitor cell markers, Pax6 and Rx, as well as weakly down-regulated nestin expression. These results demonstrate that Wharton's jelly mesenchymal stem cells are capable of differentiating into retinal progenitor cells in vitro.
Keywords: neural regeneration, stem cells, Wharton's jelly, mesenchymal stem cells, microenvironment induction, reagent induction, retinal progenitor cells, nerve cells, retinal disease, grants-supported paper, neuroregeneration
Research Highlights
(1) This study used neural stem cell-conditioned medium, using the telencephalon of fetal rats of embryonic day 14, to induce neural differentiation from Wharton's jelly mesenchymal stem cells. As the neural stem cells were in the development phase there were no glial cells present in the cultures, thus benefiting the induction of Wharton's jelly mesenchymal stem cells.
(2) After microenvironment induction, the cells were further cultured in neural stem cell-conditioned medium supplemented with Dkk-1, a Wnt/β catenin pathway antagonist, and LeftyA, a Nodal signaling pathway antagonist.
(3) Results showed that induced Wharton's jelly mesenchymal stem cells differentiated into bulbous cells with numerous processes and expressed the retinal progenitor cell markers Rx and Pax6.
(4) Results demonstrated that Wharton's jelly mesenchymal stem cells can be induced to differentiate into retinal progenitor cells and may be used as seed cells for the clinical treatment of jury-induced visual diseases.
INTRODUCTION
Damage or loss of photoreceptor cells is one of main culprits of visual impairment in many retinal degenerative diseases. Pharmacological treatment and surgical intervention are traditionally used to treat these retinal diseases, but they are not curative[1,2]. With the development of stem cell technology, stem cell-based therapies for retinal degenerative diseases have been recently proposed[3,4]. Stem cell-based therapies represent a newly emerging therapeutic approach by which vascular and neuronal degenerative diseases may be treated. Since most of the diseases that lead to visual loss are a result of abnormal vasculature and/or neuronal degeneration, the use of stem cells to stabilize or prevent visual loss may hold great promise. However, this new therapeutic strategy faces many difficulties, and the optimal stem cell source remains to be identified. For example, embryonic stem cells suffer from a series of constraints, including ethical concerns, limited availability, potential for teratoma formation upon transplantation, and immune rejection[5], while adult stem cells, such as mesenchymal stem cells (MSCs) derived from bone marrow, suffer from age-associated decreases in number, proliferative capacity, and differentiating potential[6,7]. Hence, an alternative source of stem cells for cell replacement therapy is urgently needed. Wharton's jelly MSCs derived from the umbilical cord have been proposed as a promising source for cell replacement therapy.
Wharton's jelly of the umbilical cord is an embryonic mucous connective tissue lying between the amniotic epithelium and the umbilical vessels, which was first described by Thomas Wharton in 1656[8,9]. Meyer et al[10] studied the network of glycoprotein microfibrils and collagen fibrils in Wharton's jelly three and a half centuries later. Wharton's jelly is composed of myofibroblast-like stromal cells, collagen fibers and proteoglycans. The interlaced collagen fibers and small, woven bundles are arranged to form a continuous soft skeleton that encases the umbilical vessels[11]. Wharton's jelly itself has very little collagen, another indicator of the primitive state of this tissue. In Wharton's jelly, the most abundant glycosaminoglycan is hyaluronic acid[12], which forms a hydrated gel around the fibroblasts and collagen fibrils and maintains the tissue architecture of the umbilical cord by protecting it from pressure[13]. In 1994, only two publications reported on umbilical cord and MSCs (including blood derived), whereas in 2009 that number increased to 349 publications[14]. Prior to the 21st century, umbilical cord research mainly focused on the structure and the composition of the extracellular matrix and stromal cells[15].
In recent years, in parallel to the enormous effort to explore novel and alternative sources of stem cells, Wharton's jelly MSCs have emerged as a promising candidate reservoir of fetal cells that could be readily used as multi-potential stem cells[16]. Wharton's jelly MSCs have faster proliferation and greater ex vivo expansion capabilities than bone marrow-derived MSCs[17]. This may be due to the expression of telomerase by Wharton's jelly MSCs[17]. Karahuseyinoglu et al[18] were able to expand Wharton's jelly MSCs more than 300-fold over seven passages. Other groups[19] have expanded Wharton's jelly MSCs to more than 1 015 times the original number of cells. In contrast to MSCs derived from bone marrow, Wharton's jelly MSCs also have a significantly higher frequency of colony-forming units[20]. Wharton's jelly MSCs can be harvested by non-invasive means, and maintained in vitro as undifferentiated cells for more than 10 passages[16]. In addition, freeze-thawed and freshly isolated Wharton's jelly MSCs present similar cell viabilities and protein expressions[17]. As multi-potential stromal cells, Wharton's jelly MSCs adhere to plastic surfaces and express MSC surface markers, such as CD105[18,21,22,23], CD44[18,19,21,23,24], CD90[19,21,22,23,24,25] and CD73[18,19,21,26]. They do not express hematopoietic stem cell markers, such as CD45[18,19,21,22,23,24,25,26], CD34[18,19,21,22,23,24,25,26], CD14[18,19], and human leukocyte antigen-DR[19,21,23,27], which are also absent in MSCs derived from bone marrow[28]. Wharton's jelly MSCs were also express low levels of some transcriptional factors that are mainly expressed in embryonic stem cells, such as Oct-4 and Nanog, both at the mRNA and protein levels, and were found to express a low amount of Wnt-signaling pathway molecules[29]. More importantly, graft-versus-host disease markers, such as CD80, CD86 and CD40, are not detectable or weakly expressed in Wharton's jelly MSCs[30], indicating that transplantation should be possible without immunosuppression. Like neural stem cells (NSCs) and MSCs, Wharton's jelly MSCs appear to migrate to areas of tumor growth[31,32,33]. Additionally, human Wharton's jelly MSCs do not appear to form teratomas when transplanted[34], unlike embryonic stem cells, which sometimes form tumors after transplantation[35,36], Thus, it has been postulated that Wharton's jelly MSCs could be used for cell transplantation therapies and represent a more eligible source of MSCs[37]. It has been increasingly recognized that Wharton's jelly MSCs may differentiate into several cell lineages from all three germ layers, including chondrocytes[20,38], osteoblasts[20,38,39], adipocytes[38,39,40], cardiomyocytes[20,38], adenocytes[41], hepatocytes[42], gliocytes[17], and neurocytes[20,43,44,45]. However, the capacity of Wharton's jelly MSCs to differentiate into retinal progenitor cells remains undetermined.
In the present study, we investigated the capacity of Wharton's jelly MSCs to differentiate into retinal progenitor cells in vitro using NSC-conditioned medium supplemented with Dkk-1 and LeftyA, and verified positive expression of Pax6, Rx and nestin following induction by reverse transcription (RT)-PCR and immunofluorescence. These results provide a basis for the potential use of Wharton's jelly MSCs as a source in stem cell-based therapy for retinal degeneration diseases.
RESULTS
Morphology and phenotype of cells cultured in vitro
Human Wharton's jelly MSCs: Over the first 5–6 days of culture, the freshly isolated Wharton's jelly MSCs (passage 0) from human umbilical cord principally displayed two distinct morphological features, containing filopodia or a long slender extended cytoplasm (Figure 1A). During week 2, they typically appeared as slender cells with a narrow cytoplasm and few lamellipodia. When cells reached 80% confluency after 9–10 days of primary culture, they were found to have principally formed bipolar spindle-like shapes with parallel or whirlpool-like arrangements (Figure 1B).
Figure 1.
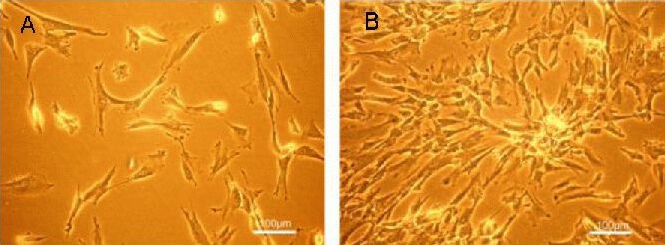
Morphological appearance of Wharton's jelly mesenchymal stem cells from human umbilical cord under an inverted microscope (scale bars: 100 μm).
Freshly isolated cells from umbilical cord displayed fibroblast or spindle-like appearance (A). Upon reaching 80% confluency, the primary cells principally formed bipolar spindle-like cells with parallel or whirlpool-like arrangements (B).
Telencephalic NSCs from embryonic rats: Freshly-isolated embryonic day 14 (E14)-NSCs, which were used to produce NSC-conditioned medium, displayed a monolayer of monopole tadpole-like cells on day 2 after seeding (Figure 2A), and labeled positively for the NSC markers, vimentin and nestin by immunofluorescence (Figure 2B, C). Quantitative analysis revealed that these markers were highly expressed in the NSC cultures after 48 hours (Figure 2D).
Figure 2.
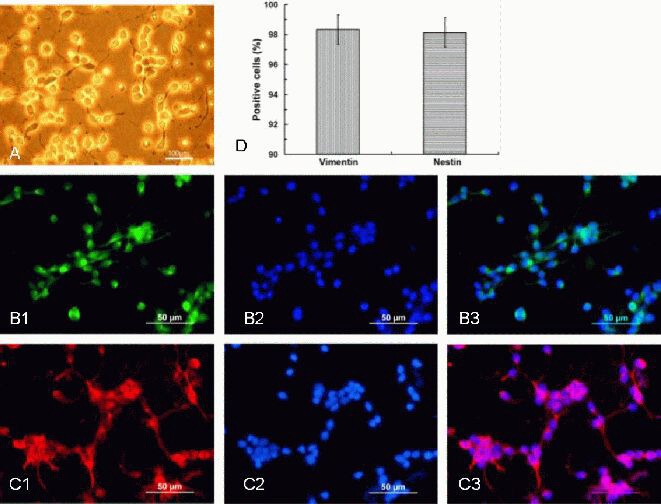
Characteristics of the E14 rat neural stem cells (scale bars in A: 100 μm: B, C: 50 μm).
(A) On day 2 after seeding, the E14 neural stem cells appeared as a monolayer of monopole tadpole-like appearance cells.
(B) Positive staining for vimentin was observed by immunofluorescence (green; B1); DAPI staining was used for nuclei visualization (B2); merged image of B1and B2 is shown (B3).
(C) Positive staining for nestin was observed by immunofluorescence (red; C1); DAPI staining was used for nuclei visualization (C2); merged image of C1 and C2 is shown (C3).
(D) The average percentage of positive cells labeled with specific antibodies, including nestin and vimentin, in the neural stem cells cultured for 48 hours.
Data are expressed as mean ± SD. Each experiment was performed six times. The percentage of positive cells (nestin or vimentin positive cells/DAPI labeled cells) was counted in campus visualis using low power magnification.
E14: Embryonic day 14; DAPI: 4′,6-diamidino-2-phenylindole.
Morphological changes in Wharton's jelly MSCs after induction
Neuronal differentiation of Wharton's jelly MSCs was induced by a multistep protocol[44]. Before the induction period, passage 3 Wharton's jelly MSCs displayed a fibroblast-like or slender morphology (Figure 3A). After initiation of induction with NSC-conditioned medium, a small portion of cells became thinner, and the majority had another elongation of several cytoplasmic extensions during days 3–10 (Figure 3B). Cells treated with NSC-conditioned medium supplemented with 100 ng/mL human recombinant Dkk-1 and LeftyA had longer and thinner extensions and showed evident polygonal morphology between days 11–28 (Figure 3C). Beyond day 29, some cells changed into bulbous shapes with the thin extensions touching each other, and one or two longer extensions emanated from the cell body up to day 37, which was perhaps indicative of the delicate structural organization of axons (Figure 3D).
Figure 3.
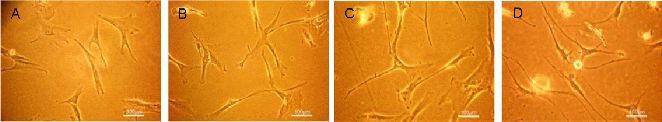
Morphological changes in Wharton's jelly mesenchymal stem cells during the induction period under an inverted microscope (scale bars: 100 μm).
(A) Wharton's jelly mesenchymal stem cells of passage 3 showed a fibroblast-like or slender morphology prior to conditioned culturing. (B) Cells became thinner, and the majority had one longer and thinner extension after induction for 3–10 days with neural stem cell-conditioned medium. (C) Cells showed evidently polygonal shapes after induction for 11–28 days with neural stem cell-conditioned medium and 100 ng/mL Dkk-1 and Lefty A. (D) At around 29–37 days, cells changed into a bulbous shape with some of the thin extensions touching each other.
Expression of retinal progenitor cell markers Pax6 and Rx in induced Wharton's jelly MSCs
To further confirm the induction effect of NSC-conditioned medium and NSC-conditioned medium supplemented with human recombinant Dkk-1 and LeftyA on Wharton's jelly MSCs, we detected the expression of the retinal progenitor markers, Pax6 and Rx, by immunofluorescence and RT-PCR in Wharton's jelly MSCs after 37 days of induction.
Immunofluorescence staining with specific antibodies revealed that Pax6 and Rx were expressed in induced Wharton's jelly MSCs (Figure 4A2, B2), whereas they were rarely observed in control cells (Figure 4A1, B1). The percentage of cells expressing Pax6 and Rx after the induction was 27.83 and 29.24, respectively (Figure 4C). Nestin, one of the markers for NSCs, was strongly expressed in the cytoplasm of the control cells (Figure 4D1), but weakly expressed in the induced cells (Figure 4D2).
Figure 4.
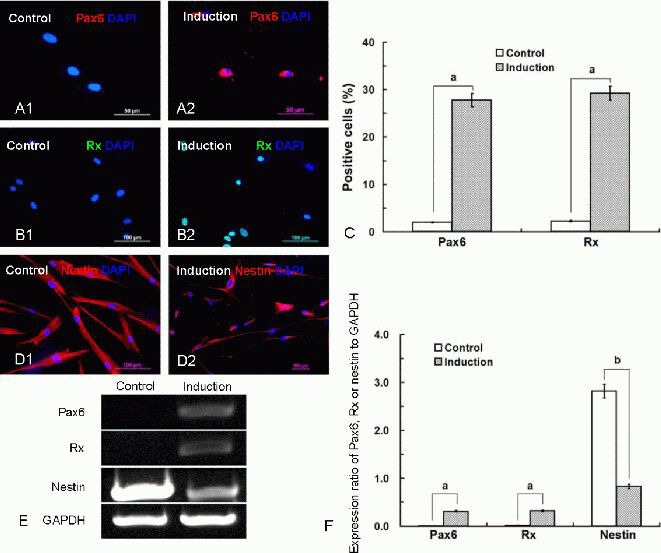
Characteristics of Wharton's jelly mesenchymal stem cells after induction.
(A, B) After induction, immunofluorescence staining with specific antibodies revealed that retinal progenitor markers, including Pax6 and Rx, were expressed in the induced Wharton's jelly mesenchymal stem cells (A2, Pax6, red; B2, Rx, green), whereas these markers were rarely detected in control cells (A1; B1; scale bars in A: 50 μm, in B: 100 μm).
(C) Histograms showing the percentage of Wharton's jelly mesenchymal stem cells expressing Pax6 and Rx after the induction; data are expressed as mean ± SD. Each experiment was performed six times. aP < 0.01, vs. control group (t-test). The percentage of positive cells (Pax6 or Rx positive cells/4′,6-diamidino-2-phenylindole (DAPI) labeled cells) was counted in campus visualis using low power magnification.
(D) Nestin was strongly expressed in the cytoplasm of the control cells (D1), but weakly expressed in the induced cells (D2).
(E) Reverse transcription-PCR analysis revealed the expression of human Pax6, Rx and nestin genes after induction, while Pax6 and Rx genes were not detected in the control cells.
(F) The gene expression (absorbance) ratios of Pax6, Rx, or nestin relative to GAPDH as detected by reverse transcription-PCR are shown. Data are expressed as mean ± SD. The experiments were conducted for six times. aP < 0.01, bP < 0.05 (t-test).
RT-PCR analysis revealed positive expression of human Pax6, Rx and nestin genes after induction, while Pax6 and Rx genes were not detected in the control cells (Figure 4E, F). Quantitative analysis relative to GAPDH revealed that the gene expression ratios of the neural retinal progenitor cell markers, Pax6 and Rx, were highly expressed in the induced cells (P < 0.01; Figure 4F), and the expression ratio of nestin was weakly down-regulated (P < 0.05; Figure 4F) compared with control cells.
DISCUSSION
In the present study, we provide evidence that Wharton's jelly MSCs can be induced by NSC-conditioned medium to differentiate into retinal progenitor cells in vitro, which was verified by a positive expression of Pax6 and Rx in the induced cells. The differentiating capacity of Wharton's jelly MSCs into retinal progenitor cells in vitro indicates a potential use of these cells as a source for stem cell-based therapies to treat retinal degenerative diseases.
The ideal donor cells for the treatment of neurological diseases should be easily available, capable of rapid expansion in culture, immunologically compatible, capable of long-term survival and integration in the host tissue, and amenable to stable transfection and long-term expression of exogenous genes[46]. It has been reported that Wharton's jelly MSCs may differentiate along several cell lineages from all three germ layers[20,41,42,43,44]. Additionally, Wharton's jelly MSCs do not appear to form teratomas after transplantation[47]. In vivo transplantation of these cells has been demonstrated to prevent progressive deterioration with brain injury. Apart from the differentiation capacities into classical mesenchymal lineages, the differentiation potency of Wharton's jelly MSCs into neural lineage cells has attracted extensive attention. In the present study, Wharton's jelly MSCs could be harvested by non-invasive means, easily expanded in vitro, and harbor the potential to differentiate into neuronal lineage cells, which could provide an excellent resource for cell transplantation therapy.
The degeneration of neurons and photoreceptors in the retina that occurs in a number of disorders is a common cause of blindness, for which there are few therapies currently available. With the increasing number of reports on the successful in vitro induction of neural differentiation of Wharton's jelly MSCs, it has been increasingly recognized that these cells could possibly be used in new approaches to repair the retina in a wide variety of retinal degenerative disorders. Fu et al[48] transdifferentiated Wharton's jelly MSCs into neurons in vitro using neuronal-conditioned medium derived from the culture supernatants of day 7 postnatal Sprague-Dawley rat brains. A three-step method (neural induction, neural commitment, and neural differentiation) could also successfully induce in vitro neural differentiation of Wharton's jelly MSCs[49]. The process of in vitro differentiation into a somatic cell type always simulates its development in vivo, as does retinal differentiation. In this study, based on the fact that neurogenesis occurs at the stages from E12 to E18 in the developing central nervous system of the rats[50,51], we used NSC-conditioned medium derived from the culture supernatants of E14 Sprague-Dawley rat brains to treat Wharton's jelly MSCs for the first step of the procedure, and then transdifferentiated these cells into retinal progenitor cells in vitro using NSC-conditioned medium supplemented with nodal signaling inhibitors, Dkk-1 and LeftyA, which inhibit Wnt signaling.
It has been reported that some anterior neural tissues could be produced by the induction of Wnt singnalling or antagonism of bone morphogenic protein in human embryonic stem cells[52,53]. Thus, the combination of NSC-conditioned medium with Dkk-1 and LeftyA is likely to promote the induction of various regions of the anterior central nervous system, including the retina. Although the specific molecular signals required for eye field specification are not completely defined in any model system, there are several protocols reported for the differentiation of retinal progenitor cells using supplementation with Dkk-1 and LeftyA. For example, Ikeda et al[54] reported that mouse embryonic stem cells could be directed to a Pax6+/Rx+ retinal progenitor identity at reasonable efficiency (26%) using a combination of Dkk-1 and LeftyA. Osakada et al[55] successfully established a defined culture method that induces mouse and human embryonic stem cells to develop into both photoreceptors and retinal pigment epithelium cells. Hirami et al[56] also succeeded in producing induced pluripotent stem cells of mouse and human origin using a similar strategy. Therefore, we treated Wharton's jelly MSCs with a combination of Dkk-1 and LeftyA for 4 weeks, and directed these cell into a retinal progenitor identity. It is possible that this result further strengthens the belief that information obtained from developmental model systems can be applied to the design of conditions to direct Wharton's jelly MSCs to specific fates.
Nestin, an intermediate filament protein expressed in mitotically active areas of the developing and adult central nervous system, is not a specific marker for NSCs, as it is expressed by differentiated astrocytes and neuronal progenitors and is also up-regulated in glial cells after central nervous system injury[57]. However, in the embryo, unlike most of the neural progenitors in the central nervous system, Rx+ neural retinal precursors do not express nestin[58]. Similarly, Ikeda et al[54] found that most Rx+ neural retinal precursors from embryonic stem cells were negative for nestin. Studies have shown that Wharton's jelly MSCs express nestin as a mesenchymal marker[44,45], and we found strong expression in control group cells, but weak expression in the induced cells. A possible reason for the discrepancies among these findings relates to differences in the cell types that were used, although the tendencies are similar.
In summary, Wharton's jelly MSCs could be induced into retinal progenitors and the induced cells are similar to retinal progenitors derived from human fetal stages, suggesting that Wharton's jelly MSCs are a promising source of stem cells for the production of retinal progenitors for retinal replacement cell therapies. However, further studies are required to develop a high-throughput method for quality control. Additionally, the current differentiation procedures are not sophisticated enough to guarantee efficiency and safety. For example, photoreceptor cells differentiated from stem cells are not high-performance in obtaining high cell numbers. Although Reh[59] efficiently generated retinal progenitor cells and photoreceptor precursors, the final photoreceptor cells seemed very scare[60]. The method of Osakada's group[55] produced a higher percentage of rod photoreceptors, but in vitro induction requires more than 4 months for developing; thus, it would be beneficial to establish a new system to generate photoreceptor cells with higher efficiency in a shorter time.
MATERIALS AND METHODS
Design
A non-randomized in vitro concurrent controlled study.
Time and setting
The experiment was performed at the Research Center for Translational Medicine, Shanghai East Hospital Affiliated to Tongji University, China from June 2011 to July 2012.
Materials
Animals
Sprague-Dawley rats of gestation day 14 were purchased from the Experimental Animal Center of School of Basic Medical Sciences, Shanghai Tongji University, China (SYXK(Hu)2009-0022). All animal experiments were performed in compliance with the Guidance Suggestions for the Care and Use of Laboratory Animals, issued by the Ministry of Science and Technology of China[61].
Human umbilical cords
Human umbilical cords were collected from healthy full-term infants delivered by caesarean section in Shanghai East Hospital Affiliated to Tongji University, China.
Methods
Isolation and culture of Wharton's jelly MSCs from human umbilical cord
Human umbilical cords were collected from full-term infants delivered by caesarean section. After disinfection in 75% ethanol for 30 seconds, the umbilical cords were stored at 4°C in sterile 0.9% saline or Hanks’ balanced salt solution for transportation to the laboratory. For processing, the cord was rinsed in sterile PBS supplemented with 4 μg/mL amphotericin B, 20 μg/mL gentamicin, 100 U/mL penicillin, and 100 μg/mL streptomycin and placed in a sterile petri dish. Following removal of umbilical cord arteries and veins in Dulbecco's modified Eagle's medium (DMEM; Gibco, Grand Island, NY, USA) by blunt dissection, the remaining umbilical cord tissue, including the Wharton's jelly component, was diced into cubes of about 0.5 cm3. The cubed explants were transferred to culture dishes containing medium composed of DMEM-Ham's F-12 (1:1 v/v; Gibco) supplemented with 10% fetal bovine serum (Gibco) and 2 mmol/L L-glutamine (Gibco). The culture dishes were placed in an incubator with saturated humidity, at 37°C and atmosphere of 5% (v/v) CO2. After 4 or 5 days of undisturbed growth, the tissue explants were removed from the dishes to allow for migration of cells from the explants. Medium was changed every 48 hours. When the cells reached 80–90% confluency, they were detached by incubating with 0.1% trypsin + 1.0 mmol/L ethylenediamine tetraacetic acid in PBS (trypsin/ethylenediamine tetraacetic acid; Invitrogen, Carlsbad, CA, USA) for 3 minutes. Cells that had detached in this time were transferred to new flasks at a ratio of 1:3 for subculturing (designated as passage 1). The MSCs were then used directly for cultures or stored in liquid nitrogen for use at a later time.
Preparation of NSC-conditioned medium
NSC-conditioned medium was used here as a microenvironment for Wharton's jelly MSC culture. Sprague-Dawley rats of gestation day 14 were sacrificed by overdose injection of ketamine/xylazin anesthetics. Fetuses at E14 were removed and placed in PBS. Telencephali were removed and transferred into sterile Hanks’ balanced salt solution, and the meninges surrounding the brains were removed under dissecting microscope (SMZ-800; Nikon, Tokyo, Japan). The desired tissues were cut into small pieces, and enzymatically dissociated using collagenase type I (Sigma, St. Louis, MO, USA). After dilution in PBS, the cell suspensions were centrifuged at 500 × g for 5 minutes. The centrifuged cells were then quickly resuspended in fresh culture medium composed of 33% Neurobasal medium (Gibco), 66% DMEM/F-12, 1% B27 (Gibco), 20 μg/L fibroblast growth factor (Peprotech, Rocky Hill, NJ, USA), and 2 mmol/L L-glutamine, and plated onto dishes (2 × 105 cells/cm2) pre-coated with poly-L-lysine (Sigma). Half of the medium was changed each day of incubation, and the removed NSC-conditioned medium was filtered for use in the subsequent induction procedure. After 48 hours of culture, the cells were harvested and seeded onto coverslips precoated with poly-L-lysine for immunocytochemical analysis.
Immunocytochemical staining for the phenotype of NSCs
The telencephalic cells were fixed by incubating with 4% paraformaldehyde at room temperature for 5 minutes and then washed three times with 0.01 mol/L PBS. The cells were permeabilized by incubating with 0.1% Triton X-100 for 10 minutes and washed with PBS. Cells were then treated with a solution composed of 5% bovine serum albumin and 5% normal goat serum for 30 minutes and incubated overnight at 4°C with primary antibodies for mouse anti-rat nestin and vimentin (1:100 dilution; Santa Cruz Biotechnology, Santa Cruz, CA, USA). Excess primary antibody was removed by washing with PBS five times, and the cells were then incubated with secondary antibodies for fluorescein-conjugated goat anti-mouse IgG and rhodamine-conjugated goat anti- mouse IgG (Santa Cruz Biotechnology) at 37°C for 1 hour. After washing with PBS, 4′,6-diamidoino-2-phenylindole stain was applied for nuclei visualization. The cells were finally overlaid with glycerol and visualized, photographed by laser scanning microscopy (Nikon).
Induced differentiation of Wharton's jelly MSCs
To induce transdifferentiation of Wharton's jelly MSCs into retinal progenitor cells, passage 3 cells were incubated in DMEM-Ham's F-12 medium supplemented with 10% fetal bovine serum at a density of 1–2 × 103 cells /cm2 for 1 day, following which one-half medium was changed to NSC-conditioned medium for the next 2 days. Thereafter, the cells were cultured in NSC-conditioned medium alone for 1 week, and the medium was changed every 2 days. Finally, cells were cultured in NSC-conditioned medium supplemented with 100 ng/mL human recombinant Dkk-1 (an antagonist of the Wnt/β catenin pathway; R&D Systems, Minneapolis, MN, USA) and LeftyA (an inhibitor of Nodal signaling; R&D Systems), with media changes every 2 days for up to 4 weeks. As a control, cells were cultured in DMEM-Ham's F-12 medium containing only 10% fetal bovine serum. Morphological appearance of human umbilical cord Wharton's jelly MSCs were observed under inverted microscope (IX71-22PH; Olympus, Tokyo, Japan).
Immunofluorescent cytochemical staining for nestin, Pax6, and Rx protein expression in the induced Wharton's jelly MSCs
The induced Wharton's jelly MSCs were collected and fixed in 4% paraformaldehyde at room temperature for 20 minutes. After blocking with 0.1% Triton X-100 for 10 minutes, cells were treated with solution composed of 5% bovine serum albumin and 5% normal goat serum for 30 minutes and incubated overnight at 4°C with primary monoclonal antibodies for rabbit anti-human nestin (1:200; Santa Cruz Biotechnology), rabbit anti-human Pax6 (1:200; Santa Cruz Biotechnology), or rabbit anti- human Rx (1:100; Santa Cruz Biotechnology). Excess primary antibody was removed by five washes with PBS and the cells were then incubated with secondary antibodies for rhodamine-conjugated goat anti-rabbit IgG (1:100; Santa Cruz Biotechnology) and fluorescein-conjugated goat anti-rabbit IgG (1:100; Santa Cruz Biotechnology) at 37°C for 1 hour. After washing with PBS, 4′,6-diamidoino-2-phenylindole was used for nuclei visualization. Fluorescence was excited and detected using laser scanning microscopy. Each experiment was repeated twice. The percentage of positive cells was analyzed by Image-Pro Plus 6.0 software (DXM1200C; Nikon).
RT-PCR for nestin, Pax6, and Rx mRNA expression in the induced Wharton's jelly MSCs
Total RNA was extracted from induced Wharton's jelly MSCs using TRIzol reagent (Invitrogen). The RNA was reverse transcribed into cDNA using the Quantscript RT kit (Tiangen Biotech, Beijing, China). PCR was performed using ExTaq polymerase (TaKaRa, Shiga, Japan) according to the manufacturer's protocol. The primer sequences are as follows:
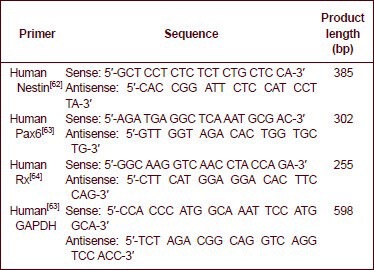
All primers were synthesized by Sangon Biotech (Shanghai) Co., Ltd. For each sample, 5 μg of total RNA was reverse transcribed to synthesize the first-strand cDNA at 65°C for 10 minutes in a 33 μL reaction volume. PCR amplification was performed using a step program of 1 minute at 95°C; 40 cycles of 1 minute at 50°C, followed by 1 minute at 72°C; and a 15-minute final extension at 72°C. The PCR products were fractionated by electrophoresis on 1.5% agarose gels and viewed by ethidium bromide staining. Gel Imaging Analytic System (GIS-ZF-258; Tanon, Shanghai, China) was used for quantification, and the absorbance ratio of target gene to GAPDH was calculated.
Statistical analysis
Statistical analyses were performed using SPSS software (version 12.0; SPSS, Chicago, IL, USA), and all data were presented as mean ± SD. Intergroup comparisons were performed by t-test analysis. P < 0.05 was considered statistically significant.
Acknowledgments:
We thank the Stem Cell Research Institute of Shanghai East Hospital Affiliated to Tongji University, China for providing research facilities for this study and would like to thank Liu ZX and Zhang JF, Research Center for Translational Medicine, Shanghai East Hospital Affiliated to Tongji University, China for excellent assistance of laboratory procedures.
Footnotes
Funding: This study was supported by 2010 Com-advanced School Young Diaph Support Project of Heilongjiang Province, China, No. 1155G60.
Conflicts of interest: None declared.
Ethical approval: This study was approved by the Clinical Ethics Committee of Shanghai East Hospital Affiliated to Tongji University, China and written informed consent was obtained from each newborn's parents.
(Reviewed by Wallace M, Frenchman B, Teng Y, Zhong YS)
(Edited by Wang LM, Su LL, Li CH, Song LP, Liu WJ, Zhao M)
REFERENCES
- [1].Sohocki MM, Daiger SP, Bowne SJ, et al. Prevalence of mutations causing retinitis pigmentosa and other inherited retinopathies. Hum Mutat. 2001;17(1):42–51. doi: 10.1002/1098-1004(2001)17:1<42::AID-HUMU5>3.0.CO;2-K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Degenring RF, Cordes A, Schrage NF. Autologous translocation of the retinal pigment epithelium and choroid in the treatment of neovascular age-related macular degeneration. Acta Ophthalmol. 2011;89(7):654–659. doi: 10.1111/j.1755-3768.2010.01867.x. [DOI] [PubMed] [Google Scholar]
- [3].Enzmann V, Yolcu E, Kaplan HJ, et al. Stem cells as tools in regenerative therapy for retinal degeneration. Arch Ophthalmol. 2009;127(4):563–571. doi: 10.1001/archophthalmol.2009.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Lund RD, Kwan AS, Keegan DJ, et al. Cell transplantation as a treatment for retinal disease. Prog Retin Eye Res. 2001;20(4):415–449. doi: 10.1016/s1350-9462(01)00003-9. [DOI] [PubMed] [Google Scholar]
- [5].Blum B, Benvenisty N. The tumorigenicity of human embryonic stem cells. Adv Cancer Res. 2008;100:133–158. doi: 10.1016/S0065-230X(08)00005-5. [DOI] [PubMed] [Google Scholar]
- [6].Stolzing A, Jones E, McGonagle D, et al. Age-related changes in human bone marrow-derived mesenchymal stem cells: consequences for cell therapies. Mech Ageing Dev. 2008;129(3):163–173. doi: 10.1016/j.mad.2007.12.002. [DOI] [PubMed] [Google Scholar]
- [7].Rao MS, Mattson MP. Stem cells and aging: expanding the possibilities. Mech Ageing Dev. 2001;122(7):713–734. doi: 10.1016/s0047-6374(01)00224-x. [DOI] [PubMed] [Google Scholar]
- [8].Nekanti U, Dastidar S, Venugopal P, et al. Increased proliferation and analysis of differential gene expression in human Wharton's jelly-derived mesenchymal stromal cells under hypoxia. Int J Biol Sci. 2010;6(5):499–512. doi: 10.7150/ijbs.6.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wharton TW. Oxford: Oxford University Press; 1996. Adenographia. [Google Scholar]
- [10].Meyer FA, Laver-Rudich Z, Tanenbaum R. Evidence for a mechanical coupling of glycoprotein microfibrils with collagen fibrils in Wharton's jelly. Biochim Biophys Acta. 1983;755(3):376–387. doi: 10.1016/0304-4165(83)90241-6. [DOI] [PubMed] [Google Scholar]
- [11].Vizza E, Correr S, Goranova V, et al. The collagen skeleton of the human umbilical cord at term. A scanning electron microscopy study after 2N-NaOH maceration. Reprod Fertil Dev. 1996;8(5):885–894. doi: 10.1071/rd9960885. [DOI] [PubMed] [Google Scholar]
- [12].Malkowski A, Sobolewski K, Jaworski S, et al. FGF binding by extracellular matrix components of Wharton's jelly. Acta Biochim Pol. 2007;54(2):357–363. [PubMed] [Google Scholar]
- [13].Sakamoto T, Ono H, Saito Y. Electron microscopic histochemical studies on the localization of hyaluronic acid in Wharton's jelly of the human umbilical cord. Nihon Sanka Fujinka Gakkai Zasshi. 1996;48(7):501–507. [PubMed] [Google Scholar]
- [14].Moretti P, Hatlapatka T, Marten D, et al. Mesenchymal stromal cells derived from human umbilical cord tissues: primitive cells with potential for clinical and tissue engineering applications. Adv Biochem Eng Biotechnol. 2010;123:29–54. doi: 10.1007/10_2009_15. [DOI] [PubMed] [Google Scholar]
- [15].Wang L, Ott L, Seshareddy K, et al. Musculoskeletal tissue engineering with human umbilical cord mesenchymal stromal cells. Regen Med. 2011;6(1):95–109. doi: 10.2217/rme.10.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Can A, Karahuseyinoglu S. Concise review: human umbilical cord stroma with regard to the source of fetus-derived stem cells. Stem Cells. 2007;25(11):2886–2895. doi: 10.1634/stemcells.2007-0417. [DOI] [PubMed] [Google Scholar]
- [17].Mitchell KE, Weiss ML, Mitchell BM, et al. Matrix cells from Wharton's jelly form neurons and glia. Stem Cells. 2003;21(1):50–60. doi: 10.1634/stemcells.21-1-50. [DOI] [PubMed] [Google Scholar]
- [18].Karahuseyinoglu S, Cinar O, Kilic E, et al. Biology of stem cells in human umbilical cord stroma: in situ and in vitro surveys. Stem Cells. 2007;25(2):319–331. doi: 10.1634/stemcells.2006-0286. [DOI] [PubMed] [Google Scholar]
- [19].Lund RD, Wang S, Lu B, et al. Cells isolated from umbilical cord tissue rescue photoreceptors and visual functions in a rodent model of retinal disease. Stem Cells. 2007;25(3):602–611. doi: 10.1634/stemcells.2006-0308. [DOI] [PubMed] [Google Scholar]
- [20].Lu LL, Liu YJ, Yang SG, et al. Isolation and characterization of human umbilical cord mesenchymal stem cells with hematopoiesis-supportive function and other potentials. Haematologica. 2006;91(8):1017–1026. [PubMed] [Google Scholar]
- [21].Sarugaser R, Lickorish D, Baksh D, et al. Human umbilical cord perivascular (HUCPV) cells: a source of mesenchymal progenitors. Stem Cells. 2005;23(2):220–229. doi: 10.1634/stemcells.2004-0166. [DOI] [PubMed] [Google Scholar]
- [22].Conconi MT, Burra P, Di Liddo R, et al. CD105(+) cells from Wharton's jelly show in vitro and in vivo myogenic differentiative potential. Int J Mol Med. 2006;18(6):1089–1096. [PubMed] [Google Scholar]
- [23].Lupatov AY, Karalkin PA, Suzdal’tseva YG, et al. Cytofluorometric analysis of phenotypes of human bone marrow and umbilical fibroblast-like cells. Bull Exp Biol Med. 2006;142(4):521–526. doi: 10.1007/s10517-006-0407-6. [DOI] [PubMed] [Google Scholar]
- [24].Wu KH, Zhou B, Lu SH, et al. In vitro and in vivo differentiation of human umbilical cord derived stem cells into endothelial cells. J Cell Biochem. 2007;100(3):608–616. doi: 10.1002/jcb.21078. [DOI] [PubMed] [Google Scholar]
- [25].Baksh D, Yao R, Tuan RS. Comparison of proliferative and multilineage differentiation potential of human mesenchymal stem cells derived from umbilical cord and bone marrow. Stem Cells. 2007;25(6):1384–1392. doi: 10.1634/stemcells.2006-0709. [DOI] [PubMed] [Google Scholar]
- [26].Kadivar M, Khatami S, Mortazavi Y, et al. In vitro cardiomyogenic potential of human umbilical vein-derived mesenchymal stem cells. Biochem Biophys Res Commun. 2006;340(2):639–647. doi: 10.1016/j.bbrc.2005.12.047. [DOI] [PubMed] [Google Scholar]
- [27].Kadner A, Zund G, Maurus C, et al. Human umbilical cord cells for cardiovascular tissue engineering: a comparative study. Eur J Cardiothorac Surg. 2004;25(4):635–641. doi: 10.1016/j.ejcts.2003.12.038. [DOI] [PubMed] [Google Scholar]
- [28].Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- [29].Carlin R, Davis D, Weiss M, et al. Expression of early transcription factors Oct-4, Sox-2 and Nanog by porcine umbilical cord (PUC) matrix cells. Reprod Biol Endocrinol. 2006;4:8. doi: 10.1186/1477-7827-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Weiss ML, Mitchell KE, Hix JE, et al. Transplantation of porcine umbilical cord matrix cells into the rat brain. Exp Neurol. 2003;182(2):288–299. doi: 10.1016/s0014-4886(03)00128-6. [DOI] [PubMed] [Google Scholar]
- [31].Aboody KS, Brown A, Rainov NG, et al. Neural stem cells display extensive tropism for pathology in adult brain: evidence from intracranial gliomas. Proc Natl Acad Sci USA. 2000;97(23):12846–12851. doi: 10.1073/pnas.97.23.12846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Studeny M, Marini FC, Champlin RE, et al. Bone marrow-derived mesenchymal stem cells as vehicles for interferon-beta delivery into tumors. Cancer Res. 2002;62(13):3603–3608. [PubMed] [Google Scholar]
- [33].Studeny M, Marini FC, Dembinski JL, et al. Mesenchymal stem cells: potential precursors for tumor stroma and targeted-delivery vehicles for anticancer agents. J Natl Cancer Inst. 2004;96(21):1593–1603. doi: 10.1093/jnci/djh299. [DOI] [PubMed] [Google Scholar]
- [34].Troyer DL, Weiss ML. Wharton's jelly-derived cells are a primitive stromal cell population. Stem Cells. 2008;26(3):591–599. doi: 10.1634/stemcells.2007-0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Arnhold S, Klein H, Semkova I, et al. Neurally selected embryonic stem cells induce tumor formation after long-term survival following engraftment into the subretinal space. Invest Ophthalmol Vis Sci. 2004;45(12):4251–4255. doi: 10.1167/iovs.03-1108. [DOI] [PubMed] [Google Scholar]
- [36].Wakitani S, Takaoka K, Hattori T, et al. Embryonic stem cells injected into the mouse knee joint form teratomas and subsequently destroy the joint. Rheumatology (Oxford) 2003;42(1):162–165. doi: 10.1093/rheumatology/keg024. [DOI] [PubMed] [Google Scholar]
- [37].Fong CY, Richards M, Manasi N, et al. Comparative growth behaviour and characterization of stem cells from human Wharton's jelly. Reprod Biomed Online. 2007;15(6):708–718. doi: 10.1016/s1472-6483(10)60539-1. [DOI] [PubMed] [Google Scholar]
- [38].Wang HS, Hung SC, Peng ST, et al. Mesenchymal stem cells in the Wharton's jelly of the human umbilical cord. Stem Cells. 2004;22(7):1330–1337. doi: 10.1634/stemcells.2004-0013. [DOI] [PubMed] [Google Scholar]
- [39].Wexler SA, Donaldson C, Denning-Kendall P, et al. Adult bone marrow is a rich source of human mesenchymal ‘stem’ cells but umbilical cord and mobilized adult blood are not. Br J Haematol. 2003;121(2):368–374. doi: 10.1046/j.1365-2141.2003.04284.x. [DOI] [PubMed] [Google Scholar]
- [40].Frank MH, Sayegh MH. Immunomodulatory functions of mesenchymal stem cells. Lancet. 2004;363(9419):1411–1412. doi: 10.1016/S0140-6736(04)16134-5. [DOI] [PubMed] [Google Scholar]
- [41].Kadam SS, Bhonde RR. Islet neogenesis from the constitutively nestin expressing human umbilical cord matrix derived mesenchymal stem cells. Islets. 2010;2(2):112–120. doi: 10.4161/isl.2.2.11280. [DOI] [PubMed] [Google Scholar]
- [42].Campard D, Lysy PA, Najimi M, et al. Native umbilical cord matrix stem cells express hepatic markers and differentiate into hepatocyte-like cells. Gastroenterology. 2008;134(3):833–848. doi: 10.1053/j.gastro.2007.12.024. [DOI] [PubMed] [Google Scholar]
- [43].Weiss ML, Medicetty S, Bledsoe AR, et al. Human umbilical cord matrix stem cells: preliminary characterization and effect of transplantation in a rodent model of Parkinson's disease. Stem Cells. 2006;24(3):781–792. doi: 10.1634/stemcells.2005-0330. [DOI] [PubMed] [Google Scholar]
- [44].Fu YS, Cheng YC, Lin MY, et al. Conversion of human umbilical cord mesenchymal stem cells in Wharton's jelly to dopaminergic neurons in vitro: potential therapeutic application for Parkinsonism. Stem Cells. 2006;24(1):115–124. doi: 10.1634/stemcells.2005-0053. [DOI] [PubMed] [Google Scholar]
- [45].Ma L, Feng XY, Cui BL, et al. Human umbilical cord Wharton's Jelly-derived mesenchymal stem cells differentiation into nerve-like cells. Chin Med J (Engl) 2005;118(23):1987–1993. [PubMed] [Google Scholar]
- [46].Björklund A. Neurobiology. Better cells for brain repair. Nature. 1993;362(6419):414–415. doi: 10.1038/362414a0. [DOI] [PubMed] [Google Scholar]
- [47].Jomura S, Uy M, Mitchell K, et al. Potential treatment of cerebral global ischemia with Oct-4+ umbilical cord matrix cells. Stem Cells. 2007;25(1):98–106. doi: 10.1634/stemcells.2006-0055. [DOI] [PubMed] [Google Scholar]
- [48].Fu YS, Shih YT, Cheng YC, et al. Transformation of human umbilical mesenchymal cells into neurons in vitro. J Biomed Sci. 2004;11(5):652–660. doi: 10.1007/BF02256131. [DOI] [PubMed] [Google Scholar]
- [49].Ding DC, Shyu WC, Chiang MF, et al. Enhancement of neuroplasticity through upregulation of beta1-integrin in human umbilical cord-derived stromal cell implanted stroke model. Neurobiol Dis. 2007;27(3):339–353. doi: 10.1016/j.nbd.2007.06.010. [DOI] [PubMed] [Google Scholar]
- [50].López-Coviella I, Berse B, et al. Induction and maintenance of the neuronal cholinergic phenotype in the central nervous system by BMP-9. Science. 2000;289(5477):313–316. doi: 10.1126/science.289.5477.313. [DOI] [PubMed] [Google Scholar]
- [51].López-Coviella I, Berse B, Thies RS, et al. Upregulation of acetylcholine synthesis by bone morphogenetic protein 9 in a murine septal cell line. J Physiol Paris. 2002;96(1-2):53–59. doi: 10.1016/s0928-4257(01)00080-8. [DOI] [PubMed] [Google Scholar]
- [52].Itsykson P, Ilouz N, Turetsky T, et al. Derivation of neural precursors from human embryonic stem cells in the presence of noggin. Mol Cell Neurosci. 2005;30(1):24–36. doi: 10.1016/j.mcn.2005.05.004. [DOI] [PubMed] [Google Scholar]
- [53].Watanabe K, Kamiya D, Nishiyama A, et al. Directed differentiation of telencephalic precursors from embryonic stem cells. Nat Neurosci. 2005;8(3):288–296. doi: 10.1038/nn1402. [DOI] [PubMed] [Google Scholar]
- [54].Ikeda H, Osakada F, Watanabe K, et al. Generation of Rx+/Pax6+ neural retinal precursors from embryonic stem cells. Proc Natl Acad Sci USA. 2005;102(32):11331–11336. doi: 10.1073/pnas.0500010102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Osakada F, Ikeda H, Mandai M, et al. Toward the generation of rod and cone photoreceptors from mouse, monkey and human embryonic stem cells. Nat Biotechnol. 2008;26(2):215–224. doi: 10.1038/nbt1384. [DOI] [PubMed] [Google Scholar]
- [56].Hirami Y, Osakada F, Takahashi K, et al. Generation of retinal cells from mouse and human induced pluripotent stem cells. Neurosci Lett. 2009;458(3):126–131. doi: 10.1016/j.neulet.2009.04.035. [DOI] [PubMed] [Google Scholar]
- [57].Chojnacki AK, Mak GK, Weiss S. Identity crisis for adult periventricular neural stem cells: subventricular zone astrocytes, ependymal cells or both? Nat Rev Neurosci. 2009;10(2):153–163. doi: 10.1038/nrn2571. [DOI] [PubMed] [Google Scholar]
- [58].Yang J, Bian W, Gao X, et al. Nestin expression during mouse eye and lens development. Mech Dev. 2000;94(1-2):287–291. doi: 10.1016/s0925-4773(00)00301-4. [DOI] [PubMed] [Google Scholar]
- [59].Reh TA. Neurobiology: right timing for retina repair. Nature. 2006;444(7116):156–157. doi: 10.1038/444156a. [DOI] [PubMed] [Google Scholar]
- [60].Lamba DA, Karl MO, Ware CB, et al. Efficient generation of retinal progenitor cells from human embryonic stem cells. Proc Natl Acad Sci USA. 2006;103(34):12769–12774. doi: 10.1073/pnas.0601990103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006 Sep 30; [Google Scholar]
- [62].Hollborn M, Ulbricht E, Rillich K, et al. The human Müller cell line MIO-M1 expresses opsins. Mol Vis. 2011;17:2738–2750. [PMC free article] [PubMed] [Google Scholar]
- [63].Bhatia B, Jayaram H, Singhal S, et al. Differences between the neurogenic and proliferative abilities of Müller glia with stem cell characteristics and the ciliary epithelium from the adult human eye. Exp Eye Res. 2011;93(6):852–861. doi: 10.1016/j.exer.2011.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Osakada F, Jin ZB, Hirami Y, et al. In vitro differentiation of retinal cells from human pluripotent stem cells by small-molecule induction. J Cell Sci. 2009;122(Pt 17):3169–3179. doi: 10.1242/jcs.050393. [DOI] [PubMed] [Google Scholar]


