Abstract
Exogenous neuropeptide Y has antiepileptic effects; however, the underlying mechanism and optimal administration method for neuropeptide Y are still unresolved. Previous studies have used intracerebroventricular injection of neuropeptide Y into animal models of epilepsy. In this study, a recombinant adeno-associated virus expression vector carrying the neuropeptide Y gene was injected into the lateral ventricle of rats, while the ipsilateral hippocampus was injected with kainic acid to establish the epileptic model. After transfection of neuropeptide Y gene, mossy fiber sprouting in the hippocampal CA3 region of epileptic rats was significantly suppressed, hippocampal synaptophysin (p38) mRNA and protein expression were inhibited, and epileptic seizures were reduced. These experimental findings indicate that a recombinant adeno-associated virus expression vector carrying the neuropeptide Y gene reduces mossy fiber sprouting and inhibits abnormal synaptophysin expression, thereby suppressing post-epileptic synaptic reconstruction.
Keywords: neural regeneration, gene therapy, neural plasticity, neurodegeneration, recombinant adeno-associated virus vector, neuropeptide Y, epilepsy, kainic acid, synaptic remodeling, mossy fiber sprouting, hippocampus, synaptophysin, neuroregeneration
Research Highlights
(1) Increasing evidence indicates that exogenous neuropeptide Y can suppress epileptic seizures. However, the most effective administration route of exogenous neuropeptide Y remains unclear.
(2) Adeno-associated virus was used as a vector to explore the effect of neuropeptide Y gene transfection on hippocampal mossy fibers, which undergo post-epileptic synaptic reconstruction.
(3) Neuropeptide Y gene transfection was found to inhibit mossy fiber sprouting.
INTRODUCTION
Temporal lobe epilepsy is the major type of drug-refractory epilepsy, involving neuronal circuits and fibers. Synaptic remodeling is one of the most common pathological changes after epileptic seizures. Ectopic synaptic reconstruction in the hippocampus is considered to be closely related with temporal lobe epilepsy[1,2]. Mossy fiber sprouting is the most prominent manifestation in synaptic remodeling, and it is a very complex process involving interactions between nerve cells and the epileptic seizure. However, the underlying mechanisms are not yet completely clear. Mossy fiber sprouting may trigger synaptic connections or synaptic remodeling in hippocampal CA3 pyramidal cells, which could lead to the formation of excitatory synaptic circuits, thereby increasing epileptic susceptibility[3,4,5]. Synaptophysin (p38) is a vesicle adhesion protein that contributes to synaptic structure and function. It is selectively localized in presynaptic vesicles and is involved in Ca2+-dependent neurotransmitter release and synaptic vesicle trafficking. Synaptophysin expression can be used to accurately assess dynamic changes at the synapse, including synaptogenesis and synaptic remodeling[6,7]. Moreover, synaptophysin is also a sensitive marker of hippocampal plasticity[8].
An effective drug treatment for temporal lobe epilepsy is a major challenge facing neurologists. The currently available anti-epileptic drugs mainly target neurotransmitter receptors and ion channels[9]. After long-term anti-epileptic drug treatment, 60–70% of patients remain sensitive to treatment, while the rest develop drug-refractory epilepsy, and ultimately, epileptic foci must be surgically resected[10,11]. Neuropeptide Y is considered to be closely related to epilepsy; it reduces neuronal excitability and has antiepileptic effects[12,13]. Different administration routes and treatment dosages of neuropeptide Y may lead to variations in therapeutic results[14]. Recently, a new delivery method, the use of a recombinant adeno-associated virus vector carrying the neuropeptide Y gene, has attracted widespread attention[15]. Gene therapy is used to treat epileptic patients through target gene transfection, thus avoiding surgical resection of nerve tissue[16]. As a gene transfer vector, adeno-associated virus can effectively control the expression of a single or several genes through transcriptional regulatory regions, which helps to regulate target gene expression. Moreover, the transfected viral vector is non-pathogenic and harmless to normal tissues[17]. This study aimed to observe the effects of adeno-associated virus-mediated neuropeptide Y expression on synaptic remodeling in rats with temporal lobe epilepsy, in a broader attempt to provide experimental support for its use in gene therapy.
RESULTS
Quantitative analysis of experimental animals
One hundred and sixty Wistar rats were used in this study, including 48 in the control group and 112 in the experimental group. A rat model of epilepsy was established by intracerebroventricular injection of kainic acid; 16 animals did not undergo successful surgery or modeling, while 96 were successful. The successful model rats were randomly divided into model and neuropeptide Y groups, respectively receiving intracerebroventricular injection of recombinant adeno-associated virus without neuropeptide Y or recombinant adeno-associated virus carrying neuropeptide Y prior to epileptic seizure. Ultimately, 144 rats were involved in the final analysis, with 48 rats in each group. From each group, 24 rats were used for detection of seizure severity using hippocampal Timm staining and 24 were used for synaptophysin PCR and western blot assay.
Neuropeptide Y transfection reduced episodes of epileptic seizures
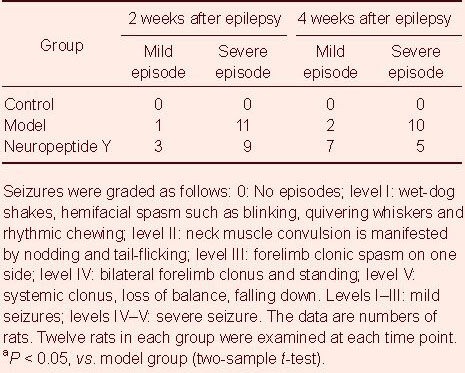
After rats were injected with kainic acid for 2 weeks, they began to exhibit epileptic seizures in both the model and neuropeptide Y groups to a similar extent. At 4 weeks after the beginning of seizures, the severity of the epileptic episodes was significantly lower in the neuropeptide Y group compared with the model group (P < 0.05); mild seizures increased, while severe seizures decreased in number. No seizures occurred in the control group (Table 1).
Table 1.
Effect of neuropeptide Y transfection on epileptic episodes in rats
Neuropeptide Y transfection reduced mossy fiber sprouting in the hippocampal CA3 region of epileptic rats
The terminals of dentate gyrus granule cell mossy fibers were abundant in Zn2+ and were stained as brownish yellow or brown particles by Timm staining[18]. After 2 weeks of kainic acid injection, Timm particles were distributed from the granule cell layer to the stratum oriens in the hippocampal CA3 region in both the model and neuropeptide Y groups. Some of these particles showed a continuous distribution. At 4 weeks, Timm particles in the hippocampal CA3 region increased, and were continuously distributed in the stratum oriens in the model group. In contrast, particles were discontinuously distributed and reduced in the neuropeptide Y group. Very few or no Timm particles were observed in the hippocampal CA3 region in the control group (Figure 1).
Figure 1.
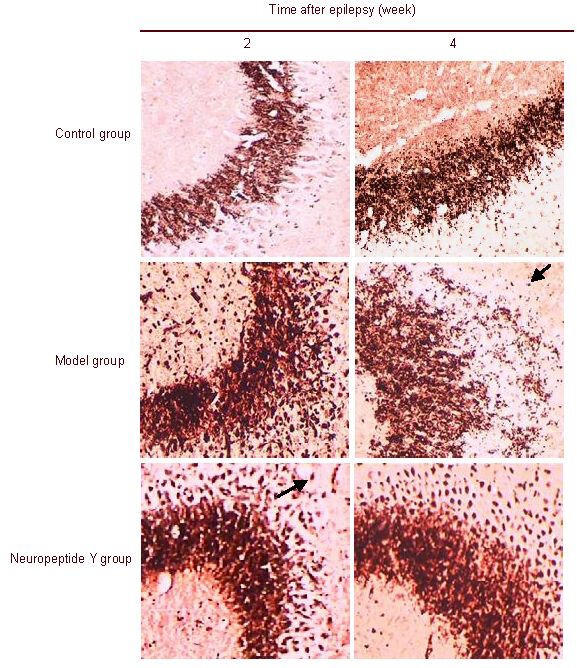
Mossy fiber sprouting in the rat hippocampal CA3 region (Timm staining, × 100).
In the control group, none or very few Timm particles were present in the hippocampal CA3 region at 2 and 4 weeks. In the model and neuropeptide Y groups, Timm particles (arrow) were found to localize from the granule cell layer to the stratum oriens in the hippocampal CA3 region. Some of these particles showed a continuous distribution in the hippocampal CA3 region at 2 weeks after the epileptic seizures. At 4 weeks, Timm particles (arrow) in the hippocampal CA3 region increased and were continuously distributed in the stratum oriens in the model group, while particles were reduced in size and were discontinuously distributed in the neuropeptide Y group.
At 2 weeks after kainic acid-induced epilepsy, the model and neuropeptide Y groups had similar levels of mossy fiber sprouting in the hippocampal CA3 region. At 4 weeks, neuropeptide Y-transfected rats showed a significantly lower level of mossy fiber sprouting compared with model rats (P < 0.05; Table 2).
Table 2.
Effect of neuropeptide Y transfection on mossy fiber sprouting in the hippocampal CA3 region in epileptic rats

Neuropeptide Y transfection reduced hippocampal synaptophysin (p38) mRNA expression in epileptic rats
Real-time PCR assay showed that, after 2 and 4 weeks of kainic acid-induced epilepsy, hippocampal synaptophysin (p38) mRNA expression increased in epileptic rats compared with the control group. After rats were transfected with neuropeptide Y, synaptophysin (p38) mRNA expression significantly diminished (P < 0.05), and mossy fiber sprouting simultaneously appeared (Table 3).
Table 3.
Effect of neuropeptide Y transfection on hippocampal synaptophysin (p38) mRNA expression (absorbance ratio of p38 mRNA to GAPDH mRNA) in epileptic rats
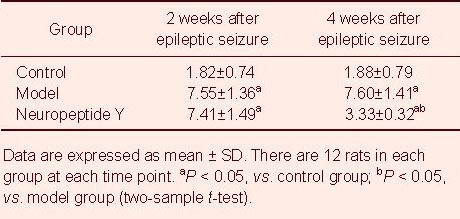
Neuropeptide Y transfection reduced hippocampal p38 expression in epileptic rats
Western blot assay results showed that, at 2 and 4 weeks after kainic acid-induced seizures, p38 expression in the hippocampus of control rats was maintained at a relatively stable level. Hippocampal p38 expression increased in epileptic rats compared with the control group (P < 0.05). Two weeks after neuropeptide Y transfection, hippocampal p38 expression was similar to that in the model group (P > 0.05). At 4 weeks, hippocampal p38 expression was significantly lower than in the model group (P < 0.05; Figure 2).
Figure 2.

Effect of transfected neuropeptide Y on hippocampal synaptophysin (p38) expression in epileptic rats.
(A) Western blot assay for hippocampal synaptophysin (p38) expression. (B) Quantitative results of synaptophysin (p38) expression. p38 protein was expressed as the integrated absorbance ratio of p38 band to GAPDH band. Data are expressed as mean ± SD. There are 12 rats in each group at each time point. aP < 0.05, vs. control group; bP < 0.05, vs. model group (two-sample t-test).
DISCUSSION
Synaptophysin is currently used as an indicator in neural plasticity research, and its expression may reflect synaptic plasticity[19]. Synaptophysin immunoreactivity in the hippocampal CA3 region was significantly enhanced in the acute epileptic phase, which is consistent with the Timm particle distribution, while synaptophysin expression was upregulated during the chronic phase of epilepsy, which is consistent with mossy fiber sprouting[20,21]. Karson et al[22] observed synaptophysin expression at the majority of axo-somatic, axo-dendritic and axoaxonic contacts under immunofluorescence confocal microscopy. An increasing amount of research has focused on whether drug treatment can inhibit synaptic remodeling after epilepsy by modulating synaptophysin expression. After 2 weeks of kainic acid-induced epilepsy in rats, mossy fiber and synaptophysin were highly expressed in the hippocampal CA3 region, and was maintained until 4 weeks (chronic phase). These findings are in agreement with previous studies[20,21,22]. Our present experimental observations indicate that neuropeptide Y gene transfection reduces abnormal expression of synaptophysin, thereby inhibiting synaptic reconstruction.
Neuropeptide Y is a 36-amino acid peptide that is widely distributed in the central and peripheral nervous systems, especially in the hippocampus[23,24,25].
In mossy fibers, neuropeptide Y is transported to the nerve endings, where it is released. It then binds presynaptic Y2 autoreceptors to inhibit glutamate release from the nerve endings and inhibit N-type Ca2+ channels. As a result, intracellular Ca2+ levels are reduced, and intracellular Ca2+ homeostasis is maintained[26,27]. Exogenous neuropeptide Y has antiepileptic effects; however, the mechanism of action remains unclear. Previous studies focused on the role of neuropeptide Y in the hippocampus, and found that neuropeptide Y inhibits hippocampal excitability by increasing GABA inhibition or reducing excitation by glutamate. Neuropeptide Y provides feedback to the presynaptic terminals via the autoreceptors to suppress glutamate secretion[27,28]. Baraban et al[29] showed that neuropeptide Y gene knockout mice had a shorter latency of kainic acid-induced seizures and required fewer dose of kainic acid. Furthermore, mortality significantly increased after kainic acid injection compared with the control group. Intracerebroventricular administration of neuropeptide Y prior to kainic acid injection may significantly decrease mortality. Reibel et al[30] demonstrated that neuropeptide Y, injected into the hippocampus for 7 consecutive days, delayed epileptic seizures induced by kindling, while anti-neuropeptide Y immunoglobulin G injection aggravated seizure severity. To the best of our knowledge, prior to the present study, there was no data on the influence of exogenous neuropeptide Y on synaptic remodeling, including mossy fiber sprouting or synaptophysin expression. In addition, no research had evaluated methods for endogenous neuropeptide Y expression. Direct intraventricular injection in animals is the only commonly used method at present.
As researchers clarify the pathogenesis of epilepsy, gene therapy for drug-refractory epilepsy has become a major concern of molecular neurobiologists. Recombinant adeno-associated virus has many advantages as a gene transfer vector, such as negligible neurological toxicity, low immunogenicity and stable expression of the target gene[31,32,33]. Recombinant adeno-associated virus 2/1 serotype vectors have been used to significantly inhibit kainic acid-induced epileptic seizures in rats, reduce synaptic reconstruction, and modulate learning and memory functions[34,35]. In addition, enhanced green fluorescent protein, serving as a reporter gene for recombinant adeno-associated virus 2/1-mediated expression, is clearly visible by fluorescence microscopy, and fluorescence-labeled neuropeptide Y can be directly observed in the hippocampal hilar area, and the CA1 and CA3 regions[36,37]. In our previous studies, we used fluorescence-labeled neuropeptide Y, and we found that neuropeptide Y, transfected using adeno-associated virus, was expressed in the CA3 region, as determined by fluorescence microscopy.
In this study, rats were given an intraventricular injection of neuropeptide Y adeno-associated virus, and then treated with kainic acid to induce epileptic seizures. There was no significant difference in seizure severity compared with the control group at 2 weeks. The neuropeptide Y group only exhibited mild seizures at 4 weeks. We also observed mossy fiber sprouting and synaptophysin protein expression in the hippocampal CA3 region. After 4 weeks of neuropeptide Y injection, mossy fiber sprouting significantly diminished, and synaptophysin protein expression was significantly reduced. The mechanism underlying the effect of neuropeptide Y on synaptic remodeling remains unclear. Some researchers have suggested that neuropeptide Y gene expression inhibits the release of the excitatory neurotransmitter glutamate from presynaptic terminals and alters synapse-related events triggered by glutamate. Synaptic remodeling, mossy fiber sprouting and synaptophysin secretion also undergo a series of changes[38]. Our experimental findings show that recombinant adeno-associated virus 2/neuropeptide Y/enhanced green fluorescent protein can suppress mossy fiber sprouting and reduce synaptophysin protein expression. Further investigation of the mechanisms underlying the effects of neuropeptide Y on synaptic remodeling are warranted.
In summary, the neuropeptide Y gene, transfected using adeno-associated virus, has clear antiepileptic effects. Additional research is required to optimize this treatment strategy for refractory epilepsy. The safety and potential adverse reactions of this method also require further study.
MATERIALS AND METHODS
Design
A comparative animal experiment focusing on neural pathophysiology.
Time and setting
Experiments were performed from August 2010 to August 2012 in the Clinical Research Center, Hebei General Hospital, China.
Materials
Animals
One hundred and sixty healthy, clean, adult, male Wistar rats, aged 12–20 weeks, weighing 250–279 g, were purchased from the Experimental Animal Center of Hebei Medical University, China (license No. SCXK (Ji) 2008-1-003, certificate No. 909106). Rats were raised in individual cages in the Clinical Research Center, Hebei General Hospital, China at 20–26°C, allowing ad libitum access to food and water. Experimental procedures were performed in accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals, formulated by the Ministry of Science and Technology of China[39].
Recombinant virus
Recombinant 2/1 type adeno-associated virus vector (VGTC Gene Technology Co., Ltd., Beijing, China) was used for endogenous neuropeptide Y therapeutic gene and enhanced green fluorescent protein reporter gene transfection, at a gene titer of 5 × 1011 vector genomes.
Drug
Kainic acid of 99% purity was purchased from Sigma, USA (Shanghai Sigma-Aldrich (Shanghai) Trading Co., Ltd., Shanghai, China). Chemical structural formula is 2-carboxy-3-carboxymethy1-4-isopropenylpyrrolidine and molecular formula is C10H15NO4.
Methods
Establishment of the kainic acid-induced epilepsy model and viral transfection
Rats were intraperitoneally anesthetized with 10% chloral hydrate (3.5 mg/kg) and fixed in a stereotaxic apparatus (Shenzhen RWD Life Science Co., Ltd., Shenzhen, Guangdong Province, China). After the scalp hair was cut and the skin was disinfected with povidone iodine, a midline longitudinal incision was made on the scalp. According to the rat brain stereotaxic atlas by George Paxinos and Charles Watson[40], 10 μL recombinant adeno-associated virus 2/neuropeptide Y/enhanced green fluorescent protein was injected into the lateral ventricle (X = −1.0 mm, Y = 1.5 mm, Z = −3.8 mm) of rats in the neuropeptide Y group using a microsyringe (Shanghai Guangzheng Medical Equipment Co., Ltd., Shanghai, China). The titer was 5 × 1011 μg/mL, injection time was 20 minutes and injection speed was 0.5 μL/min. Model and control groups received 10 μL saline via intracerebroventricular injection. Ten minutes later, the neuropeptide Y and model groups were injected with 2 μL kainic acid (0.4 μg/μL) into the ipsilateral hippocampal CA3 region (X = 5.3 mm, Y = 4.0 mm, Z = −6.0 mm), while the control group was injected with 2 μL recombinant adeno-associated virus without neuropeptide Y. Epilepsy was assessed with the Racine 6-level evaluation criteria immediately after modeling. Three successive episodes of epileptic seizure above level I were regarded as successful modeling[32,33,34,35,36,37,38,41].
Rat behavioral observation
At 2 and 4 weeks after seizure, the rat’s behavior and the severity of seizures were continuously monitored using a camera. Epileptic seizure severity was assessed using a modification of the Racine 6-level evaluation criteria[42]: 0: no episodes; level I: wet-dog shakes, hemifacial spasm such as blinking, quivering whiskers and rhythmic chewing; level II: neck muscle convulsion is manifested by nodding and tail-flicking; level III: forelimb clonic spasm of one side; level IV: bilateral forelimb clonus and standing; level V: systemic clonus, loss of balance, falling down. Levels I–III: mild seizures; levels IV–V: severe seizures.
Timm staining for semi-quantitative observation of mossy fiber sprouting in the rat hippocampal CA3 region
At 2 and 4 weeks after seizure, rats were anesthetized with 10% chloral hydrate and killed. Brains were rapidly removed and frozen to prepare hippocampal consecutive coronal frozen sections, at 25 μm thickness. Frozen sections were incubated with Timm medium (consisting of 50% gum arabic 60 mL, citrate buffer 10 mL, 5.67% hydroquinone 30 mL, and 71% nitric acid silver solution 0.5 mL; Department of Pathology, Hebei General Hospital, China) at room temperature for 50 minutes. Subsequently, sections were washed with distilled water, dehydrated with a graded ethanol series, cleared with xylene, and mounted. Mossy fiber sprouting in the hippocampal CA3 region was semi-quantitatively evaluated with Timm staining under the light microscope[43]: 0: no Timm staining; 1: occasionally observed Timm stained particles, in a discontinuous distribution; 2: Timm stained particles discontinuously distributed; 3: Timm stained particles nearly continuously distributed; 4: Timm stained particles continuously distributed; 5: Timm stained particles forming a dense laminar layer.
Fluorescence real-time quantitative PCR detection of synaptophysin (p38) mRNA expression in the rat hippocampus
At 2 and 4 weeks after seizure, rats were killed under deep anesthesia and the brains were quickly harvested. Total RNA was extracted from hippocampal tissue using Trizol (CoWin Biotech Co., Ltd. (CWBIO), Beijing, China). Total RNA content, purity and integrity were measured using a UV spectrophotometer (type 756, Certified Genetool Inc., Pleasanton, CA, USA) and agarose gel electrophoresis (DYY-6B steady-voltage, steady-flow electrophoresis apparatus, Beijing Liuyi Instrument Factory, Beijing, China). 2 μL RNA for each sample was reverse transcribed into cDNA, and 1 μL cDNA was used for PCR amplification. The primer sequences were as follows: synaptophysin (p38) upstream primer sequence: 5′-TTT GCC TTC CTC TAC TCC AT-3′; downstream primer sequence: 5′-GCC CTT TGT TGT TCT CTC G-3′; amplification length: 79 bp; GAPDH upstream sequence: 5′-ACC ACA GTC CAT GCC ATC AC-3′; GAPDH downstream sequence: 5′-TCC ACC ACC CTG TTG CTG-3′; amplification length: 452 bp. SyberGreen fluorescent quantitative PCR reaction parameters were as follows: 96°C pre-denaturation for 4 minutes; 40 cycles of 94°C denaturation for 30 seconds, 58°C annealing for 30 seconds, 72°C extension for 30 seconds; 72°C fluorescence signal collection for 30 seconds. After amplification, the results were analyzed on an ABI 7300 Real-Time PCR System (PE Company, Norwalk, USA), with GAPDH as an internal reference gene to permit comparison with sample No. 1 in the control group. Finally, the relative quantitative value of target gene expression was obtained.
Western blot analysis of p38 protein expression in the rat hippocampus
At 2 and 4 weeks after seizure, rats were killed under deep anesthesia and brains were quickly removed. The hippocampus was dissected out and mixed with protein lysing solution (Beijing Solarbio, Beijing, China) at a 1:9 ratio to prepare a 10% protein homogenate, and the supernatant was collected after centrifugation. 10 μL supernatant was extracted for protein quantification using the BCA method[42]. Solution containing 50 μg protein was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The separated proteins were transferred to polyvinylidene fluoride membranes and incubated with mouse anti-rat p38 monoclonal antibody (1:300; Santa Cruz Biotechnology, Santa Cruz, CA, USA) and mouse anti-rat GAPDH monoclonal antibody (1:300; Santa Cruz Biotechnology) at 4°C overnight. Then, samples were incubated with horseradish peroxidase-conjugated rabbit anti-mouse IgG (1:5 000; Santa Cruz Biotechnology) at 37°C for 1 hour and developed using chemiluminescence solution[44]. X-ray film was exposed to the blot and then analyzed using a UVP gel scanning system (Ultra-Violet Products Ltd., Cambridge, UK). p38 protein expression levels were expressed as the integrated absorbance ratio of p38 band to GAPDH band[44].
Statistical analysis
Data were analyzed using SPSS 13.0 software (SPSS, Chicago, IL, USA) and expressed as mean ± SD.
Completely randomized one-way analysis of variance was applied for comparisons among groups, and two groups were compared using two-sample t-test. A P value less than 0.05 was considered statistically significant.
Acknowledgments
We would like to thank Professor Yubin Hao, Associate Professor Chao Wang and other staff from the Clinical Research Center of Hebei Province in China for providing great guidance and selfless help.
Footnotes
Fan Zhang, Studying for doctorate, Associate professor, Associate chief physician.
Conflicts of interest: None declared.
Ethical approval: The experimental procedures conformed with the Animal Ethical Requirements, formulated by the Animal Ethics Committee of Hebei General Hospital in China.
(Reviewed by Patel B, Norman C, Chen JF, Ye M)
(Edited by Yu J, Yang Y, Li CH, Song LP)
REFERENCES
- [1].Cavazos JE, Cross DJ. The role of synaptic reorganization in mesial temporal lobe epilepsy. Epilepsy Behav. 2006;8(3):483–493. doi: 10.1016/j.yebeh.2006.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Beck H, Goussakov IV, Lie A, et al. Synaptic plasticity in the human dentate gyrus. J Neurosci. 2000;20(18):7080–7086. doi: 10.1523/JNEUROSCI.20-18-07080.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Babb TL, Ying Z, Mikuni N, et al. Brain plasticity and cellular mechanisms of epileptogenesis in human and experimental cortical dysplasia. Epilepsia. 2000;41(Suppl 6):S76–81. doi: 10.1111/j.1528-1157.2000.tb01561.x. [DOI] [PubMed] [Google Scholar]
- [4].Cavazos JE, Zhang P, Qazi R, et al. Ultrastructural features of sprouted mossy fiber synapses in kindled and kainic acid-treated rats. J Comp Neurol. 2003;458(3):272–292. doi: 10.1002/cne.10581. [DOI] [PubMed] [Google Scholar]
- [5].Buckmaster PS, Zhang GF, Yamawaki R. Axon sprouting in a model of temporal lobe epilepsy creates a predominantly excitatory feedback circuit. J Neurosci. 2002;22(15):6650–6658. doi: 10.1523/JNEUROSCI.22-15-06650.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Scharfman HE, Sollas AL, Berger RE, et al. Electrophysiological evidence of monosynaptic excitatory transmission between granule cells after seizure-induced mossy fiber sprouting. J Neurophysiol. 2003;90(4):2536–2547. doi: 10.1152/jn.00251.2003. [DOI] [PubMed] [Google Scholar]
- [7].Tarsa L, Goda Y. Synaptophysin regulates activity-dependent synapse formation in cultured hippocampal neurons. Proc Natl Acad Sci U S A. 2002;99(2):1012–1016. doi: 10.1073/pnas.022575999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hanaya R, Boehm N, Nehlig A. Dissociation of the immunoreactivity of synaptophysin and GAP-43 during the acute and latent phases of the lithium-pilocarpine model in the immature and adult rat. Exp Neurol. 2007;204(2):720–732. doi: 10.1016/j.expneurol.2007.01.002. [DOI] [PubMed] [Google Scholar]
- [9].Rogawski MA, Löscher W. The neurobiology of antiepileptic drugs. Nat Rev Neurosci. 2004;5(7):553–564. doi: 10.1038/nrn1430. [DOI] [PubMed] [Google Scholar]
- [10].Foldvary N, Bingaman WE, Wyllie E. Surgical treatment of epilepsy. Neurol Clin. 2001;19(2):491–515. doi: 10.1016/s0733-8619(05)70028-1. [DOI] [PubMed] [Google Scholar]
- [11].Duncan JS, Sander JW, Sisodiya SM, et al. Adult epilepsy. Lancet. 2006;367(9516):1087–1100. doi: 10.1016/S0140-6736(06)68477-8. [DOI] [PubMed] [Google Scholar]
- [12].Vezzani A, Sperk G, Colmers WF. Neuropeptide Y: emerging evidence for a functional role in seizure modulation. Trends Neurosci. 1999;22(1):25–30. doi: 10.1016/s0166-2236(98)01284-3. [DOI] [PubMed] [Google Scholar]
- [13].DePrato Primeaux S, Holmes PV, Martin RJ, et al. Experimentally induced attenuation of neuropeptide-Y gene expression in transgenic mice increases mortality rate following seizures. Neurosci Lett. 2000;287(1):61–64. doi: 10.1016/s0304-3940(00)01137-x. [DOI] [PubMed] [Google Scholar]
- [14].Vezzani A, Civenni G, Rizzi M, et al. Enhanced neuropeptide Y release in the hippocampus is associated with chronic seizure susceptibility in kainic acid treated rats. Brain Res. 1994;660(1):138–143. doi: 10.1016/0006-8993(94)90847-8. [DOI] [PubMed] [Google Scholar]
- [15].Noe’ F, Nissinen J, Pitkänen A, et al. Gene therapy in epilepsy: the focus on NPY. Peptides. 2007;28(2):377–383. doi: 10.1016/j.peptides.2006.07.025. [DOI] [PubMed] [Google Scholar]
- [16].Perucca E. What clinical trial designs have been used to test antiepileptic drugs and do we need to change them? Epileptic Disord. 2012;14(2):124–131. doi: 10.1684/epd.2012.0511. [DOI] [PubMed] [Google Scholar]
- [17].Lentz TB, Gray SJ, Samulski RJ. Viral vectors for gene delivery to the central nervous system. Neurobiol Dis. 2012;48(2):179–188. doi: 10.1016/j.nbd.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Seress L, Gallyas F. The use of a sodium tungstate developer markedly improves the electron microscopic localization of zinc by the Timm method. J Neurosci Methods. 2000;100(1-2):33–39. doi: 10.1016/s0165-0270(00)00227-2. [DOI] [PubMed] [Google Scholar]
- [19].Takaki M, Ujike H, Kodama M, et al. Increased expression of synaptophysin and stathmin mRNAs after methamphetamine administration in rat brain. Neuroreport. 2001;12(5):1055–1060. doi: 10.1097/00001756-200104170-00038. [DOI] [PubMed] [Google Scholar]
- [20].Li S, Reinprecht I, Fahnestock M, et al. Activity-dependent changes in synaptophysin immunoreactivity in hippocampus, piriform cortex, and entorhinal cortex of the rat. Neuroscience. 2002;115(4):1221–1229. doi: 10.1016/s0306-4522(02)00485-2. [DOI] [PubMed] [Google Scholar]
- [21].Elmér E, Kokaia M, Kokaia Z, et al. Delayed kindling development after rapidly recurring seizures: relation to mossy fiber sprouting and neurotrophin, GAP-43 and dynorphin gene expression. Brain Res. 1996;712(1):19–34. doi: 10.1016/0006-8993(95)01424-1. [DOI] [PubMed] [Google Scholar]
- [22].Karson MA, Tang AH, Milner TA, et al. Synaptic cross talk between perisomatic-targeting interneuron classes expressing cholecystokinin and parvalbumin in hippocampus. J Neurosci. 2009;29(13):4140–4154. doi: 10.1523/JNEUROSCI.5264-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Mathern GW, Babb TL, Pretorius JK, et al. Reactive synaptogenesis and neuron densities for neuropeptide Y, somatostatin, and glutamate decarboxylase immunoreactivity in the epileptogenic human fascia dentata. J Neurosci. 1995;15(5 Pt 2):3990–4004. doi: 10.1523/JNEUROSCI.15-05-03990.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Tu B, Timofeeva O, Jiao Y, et al. Spontaneous release of neuropeptide Y tonically inhibits recurrent mossy fiber synaptic transmission in epileptic brain. J Neurosci. 2005;25(7):1718–1729. doi: 10.1523/JNEUROSCI.4835-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Vezzani A, Schwarzer C, Lothman EW, et al. Functional changes in somatostatin and neuropeptide Y containing neurons in the rat hippocampus in chronic models of limbic seizures. Epilepsy Res. 1996;26(1):267–279. doi: 10.1016/s0920-1211(96)00059-9. [DOI] [PubMed] [Google Scholar]
- [26].Heilbronn R, Weger S. Viral vectors for gene transfer: current status of gene therapeutics. Handb Exp Pharmacol. 2010;197:143–170. doi: 10.1007/978-3-642-00477-3_5. [DOI] [PubMed] [Google Scholar]
- [27].Nadler JV, Tu B, Timofeeva O, et al. Neuropeptide Y in the recurrent mossy fiber pathway. Peptides. 2007;28(2):357–364. doi: 10.1016/j.peptides.2006.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kudin AP, Debska-Vielhaber G, Vielhaber S, et al. The mechanism of neuroprotection by topiramate in an animal model of epilepsy. Epilepsia. 2004;45(12):1478–1487. doi: 10.1111/j.0013-9580.2004.13504.x. [DOI] [PubMed] [Google Scholar]
- [29].Baraban SC, Hollopeter G, Erickson JC, et al. Knock-out mice reveal a critical antiepileptic role for neuropeptide Y. J Neurosci. 1997;17(23):8927–8936. doi: 10.1523/JNEUROSCI.17-23-08927.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Reibel S, Larmet Y, Carnahan J, et al. Endogenous control of hippocampal epileptogenesis: a molecular cascade involving brain-derived neurotrophic factor and neuropeptide Y. Epilepsia. 2000;41(Suppl 6):S127–133. doi: 10.1111/j.1528-1157.2000.tb01571.x. [DOI] [PubMed] [Google Scholar]
- [31].Cucchiarini M, Ren XL, Perides G, et al. Selective gene expression in brain microglia mediated via adeno-associated virus type 2 and type 5 vectors. Gene Ther. 2003;10(8):657–667. doi: 10.1038/sj.gt.3301925. [DOI] [PubMed] [Google Scholar]
- [32].Noé F, Frasca A, Balducci C, et al. Neuropeptide Y overexpression using recombinant adeno-associated viral vectors. Neurotherapeutics. 2009;6(2):300–306. doi: 10.1016/j.nurt.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Olesen MV, Christiansen SH, Gøtzsche CR, et al. Neuropeptide Y Y1 receptor hippocampal overexpression via viral vectors is associated with modest anxiolytic-like and proconvulsant effects in mice. J Neurosci Res. 2012;90(2):498–507. doi: 10.1002/jnr.22770. [DOI] [PubMed] [Google Scholar]
- [34].Sørensen AT, Kanter-Schlifke I, Carli M, et al. NPY gene transfer in the hippocampus attenuates synaptic plasticity and learning. Hippocampus. 2008;18(6):564–574. doi: 10.1002/hipo.20415. [DOI] [PubMed] [Google Scholar]
- [35].Richichi C, Lin EJ, Stefanin D, et al. Anticonvulsant and antiepileptogenic effects mediated by adeno-associated virus vector neuropeptide Y expression in the rat hippocampus. J Neurosci. 2004;24(12):3051–3059. doi: 10.1523/JNEUROSCI.4056-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Yi F, Abuhamed MM, Long LL, et al. Neuronal synaptic reconstruction in hippocampus in chronic phase of pilocarpine-treated rats. Zhonghua Yi Xue Za Zhi. 2011;91(19):1335–1339. [PubMed] [Google Scholar]
- [37].Ueta Y, Fujihara H, Serino R, et al. Transgenic expression of enhanced green fluorescent protein enables direct visualization for physiological studies of vasopressin neurons and isolated nerve terminals of the rat. Endocrinology. 2005;146(1):406–413. doi: 10.1210/en.2004-0830. [DOI] [PubMed] [Google Scholar]
- [38].Silva AP, Carvalho AP, Carvalho CM, et al. Functional interaction between neuropeptide Y receptors and modulation of calcium channels in the rat hippocampus. Neuropharmacology. 2003;44(2):282–292. doi: 10.1016/s0028-3908(02)00382-9. [DOI] [PubMed] [Google Scholar]
- [39].The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006 Sep 30; [Google Scholar]
- [40].Jamal L, Khan AN, Butt S, et al. The level and distribution of the GABA(B)R1 and GABA(B)R2 receptor subunits in the rat's inferior colliculus. Front Neural Circuits. 2012;6:92. doi: 10.3389/fncir.2012.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Li GQ, Zhou Z, Liu SY, et al. Establishing rat model of chronic temporal lobe epilepsy with the injection of kainic acid into lateral ventricle. Zhangguo Linchuang Kangfu. 2005;9(25):117–119. [Google Scholar]
- [42].Beamer E, Otahal J, Sills GJ, et al. N(w)-Propyl-l- arginine (L-NPA) reduces status epilepticus and early epileptogenic events in a mouse model of epilepsy: behavioural, EEG and immunohistochemical analyses. Eur J Neurosci. 2012;36(9):3194–3203. doi: 10.1111/j.1460-9568.2012.08234.x. [DOI] [PubMed] [Google Scholar]
- [43].Liu Z, Yang Y, Silveira DC, et al. Consequences of recurrent seizures during early brain development. Neuroscience. 1999;92(4):1443–1454. doi: 10.1016/s0306-4522(99)00064-0. [DOI] [PubMed] [Google Scholar]
- [44].Sun YM, Hao HQ, Kong WN, et al. Effect of intracerebroventricular injection of rAAV-HIF-1α on hippocampal neuronal apoptosis in a rat model of Alzheimer disease. Nan Fang Yi Ke Da Xue Xue Bao. 2010;30(12):2711–2714. [PubMed] [Google Scholar]


