Abstract
There is accumulating clinical evidence that chemotherapeutic agents induce neurological side effects, including memory deficits and mood disorders, in cancer patients who have undergone chemotherapeutic treatments. This review focuses on chemotherapy-induced neurodegeneration and hippocampal dysfunctions and related mechanisms as measured by in vivo and in vitro approaches. These investigations are helpful in determining how best to further explore the causal mechanisms of chemotherapy-induced neurological side effects and in providing direction for the future development of novel optimized chemotherapeutic agents.
Keywords: neural regeneration, reviews, neurogenesis, behavioral dysfunction, chemotherapy, hippocampal dysfunction, memory deficit, mood disorder, neurodegeneration, neuron, neuroplasticity, neurotoxicity, grants-supported paper, neuroregeneration
Research Highlights
(1) There is increasing clinical evidence that chemotherapeutic agents induce neurological side effects, including memory deficits and mood disorders, in cancer patients.
(2) This article reviews in vivo and in vitro studies of chemotherapy-induced neurodegeneration and hippocampal dysfunctions, and related mechanisms.
INTRODUCTION
Chemotherapy is one of the primary treatments for cancer. However, one of the most disturbing findings of recent studies of cancer survivors is the apparent prevalence of chemotherapy-associated adverse neurological effects, including vascular complications, seizures, mood disorders, cognitive dysfunctions, and peripheral neuropathies[1,2,3]. Epidemiological studies have revealed chemotherapy-induced cognitive impairments and psychological distress in diverse types of cancer patients with peripheral tumors, including breast cancer, colorectal cancer, and lymphoma, as well as brain tumors such as glioma, glioblastoma, and primary central nervous system lymphoma[4,5,6]. In addition, chemotherapy triggers changes in ion channels on dorsal root ganglia and dorsal horn neurons that generate secondary changes resulting in neuropathic pains[2]. There is clear evidence of the neurotoxicity of at least some forms of chemotherapy, although factors related to the use of specific diagnostic techniques and cancer treatments may also contribute to the side effects that have been identified[1,3].
Among various neurological side effects, cognitive impairment and mental depression can occur in a subset of cancer survivors, although these are generally subtle. A number of factors may either protect against cognitive impairments or place individuals at an increased risk of impaired cognitive function. These factors include the concomitant effects of the cancer and its treatment (e.g., medications, fatigue, depression, or anxiety), indirect and direct effects of chemotherapy (e.g., chemotherapy-induced anemia or menopause), factors particular to the individual patient (e.g., age, intelligence, educational level, or menopausal status)[7]. Thus, a number of factors complicate assessments of correlations between the side effects of chemotherapy and brain dysfunctions in patients, particularly memory deficits and mental depression. Despite intensive efforts to manage the neurological side effects of chemotherapy in patients, and the development of chemoprotective agents, there is no therapy generally accepted to not produce neurological side effects[8].
It is well established that the cytostatic effects of chemotherapeutic agents inhibit the process of cancer cell division. Chemotherapy may induce related changes, particularly in neurogenesis and synaptic plasticity, which are closely linked to the hippocampus. The hippocampus is a major structure of the limbic system that has been studied extensively in individuals with difficulties in learning and memory and the regulation of emotions, which can be manifested in symptoms such as depression[9]. However, further studies on experimental animals are needed to verify these correlations. Furthermore, epidemiological studies of cancer survivors exhibiting cognitive impairments are required to clarify the side effects of chemotherapeutic agents, and thus allow the development of new therapeutic agents without such side effects.
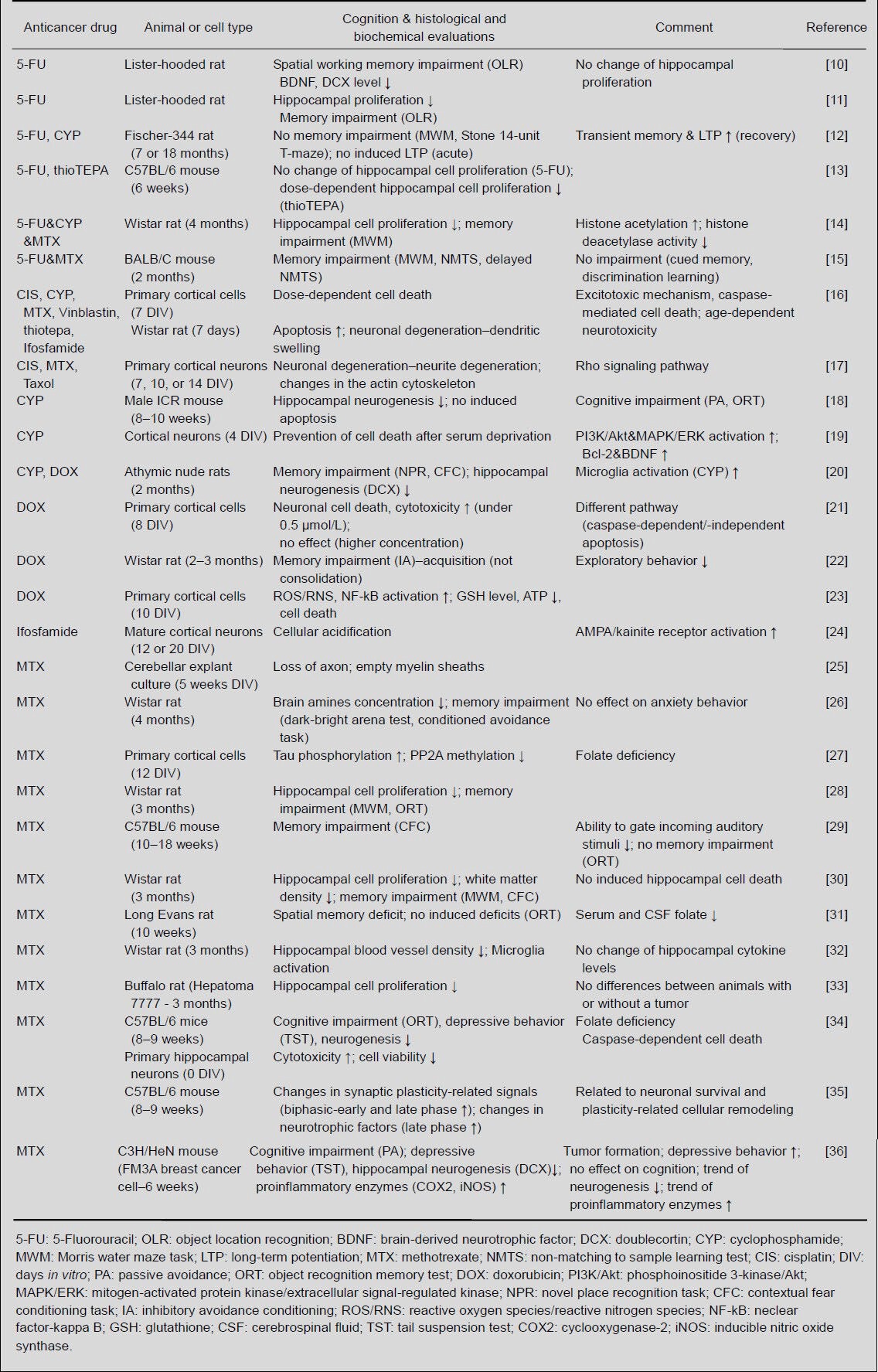
Recently, various groups using experimental in vivo and in vitro models have reported that chemotherapy induces neurodegeneration and hippocampal dysfunctions, such as memory deficits (Table 1). This review provides an overview of in vivo and in vitro studies investigating the neurological effects of chemotherapeutic agents on the hippocampus and its functions, including cognition and depression, as well as the neurotoxicity of these agents.
Table 1.
Summary of in vivo and in vitro studies evaluating the effects of various chemotherapeutic agents on behavioral dysfunctions and neurobiology
ROLES OF HIPPOCAMPAL NEUROGENESIS AND PLASTICITY IN HIPPOCAMPAL FUNCTIONS
In the adult brain, progenitor/stem cells give rise to new neural cells in two active germinal zones called the subgranular zone in the dentate gyrus, which generates new granular cells in the adult hippocampus, and the forebrain subventricular zone, which gives rise to granular cells in the olfactory bulb[37,38]. The hippocampus is a limbic structure that plays a critical role in the formation and recovery of certain types of memory and the regulation of emotion, such as in depression. New neurons in the adult hippocampus are derived from neural progenitor/stem cells located at the border between the hilus and granular cell layer of the dentate gyrus, a region called the subgranular zone, during the process of hippocampal neurogenesis[9]. Factors such as age, genetics, excitatory input, ionizing radiation exposure, physiological stimuli, and environmental conditions[39,40,41,42,43,44,45,46,47,48] regulate the rate of cell proliferation in the hippocampus, which in turn influences behavior[49]. Mental depression and cognitive impairment may be due not only to changes in neurotransmitter concentrations and receptor activity levels but also to the impairment of brain plasticity, tissue remodeling, and alterations in adult hippocampal neurogenesis[49,50,51,52,53]. Manipulations that decrease neurogenesis, such as radiation exposure, impair an animal’s performance in hippocampal-dependent learning and memory tasks. Conversely, factors that increase neurogenesis, such as exercise, and improve hippocampal performance[40,54]. In addition, animal models of depression have also been reported to result in a decline in adult hippocampal neurogenesis, through either the proliferation of hippocampal progenitors or short-term survival of daughter cells[55]. Thus, reduced rates of hippocampal neurogenesis lead to cognitive impairments and/or depression-like effects in experimental models[40,47]. Therefore, neurotoxic agents affecting hippocampal neurogenesis may be useful tools to clarify the relationship between hippocampal neurogenesis and hippocampal dysfunction.
Neuronal synaptic plasticity in the adult brain, which is closely linked with neurogenesis, is manifested at the cellular level by modifications in dendritic growth, axonal sprouting, synaptic remodeling, and the creation of new synapses[56]. Hippocampal principal synapses are especially plastic, as they have been shown to be strengthened or weakened by activity[57]. The ability of a synapse to change the efficacy of its synaptic transmission, a process known as synaptic plasticity, is crucial to brain functions, and therefore, hippocampal plasticity plays an important role in hippocampal functions. In the mammalian brain, two of the best-characterized forms of synaptic transmission in the hippocampus are long-term potentiation and long-term depression, which have been identified as cellular substrates that are clearly related to learning and memory[58].
Several studies have shown that neurotoxic agents and neurodegenerative diseases induce changes in synaptic plasticity and neurogenesis in the adult hippocampus[59,60]. Therefore, further research on changes in plasticity-related signals under various conditions should provide a greater understanding of the specific relationships between plasticity and brain functions.
Other limbic structures, including the amygdala, hypothalamus, and fornix, are also involved in cognition and emotional regulation. The amygdala, mammillary body, and hypothalamus play pivotal roles in memory and mental depression[61]. In addition to the limbic system, the striatum and cortex are also thought to be associated with some types of memory[62]. The frontal and temporal cortices of the forebrain, the basal nuclei, and pituitary gland have been implicated in mental depression[63]. The structure and function of the hippocampus, which is closely associated with both cognition and emotional regulation, have been studied under various conditions. Therefore, this review will focus mainly on the detrimental effects of chemotherapy in the hippocampus of experimental animals.
CHEMOTHERAPY-INDUCED NEUROTOXICITY IN VIVO
Chemotherapy is one of the primary treatments for cancer, and has been applied successfully to extend patients’ life spans. However, there is increasing clinical interest in its adverse neurotoxic symptoms, including leukoencephalopathy, seizures, cerebral infarctions, and cognitive impairments[64]. There is now a growing body of evidence that cancer therapy-associated cognitive dysfunction occurs in many adults undergoing treatment for cancer and cannot simply be attributed to stress, fatigue, or depression[1]. Therefore, behavioral evaluations of hippocampal dysfunction induced by chemotherapy in experimental models are necessary to gain further insights into the genuine phenomena of chemotherapy-induced cognitive impairments and depression.
Several studies using animal models have shown that various chemotherapeutic agents impair performance in one or more cognitive tests (Table 1). However, the outcomes of tests of cognitive impairment after treatment with chemotherapeutic agents are highly dependent on the protocols used[12,22] and the specific learning tasks applied[15,29]. Furthermore, some chemotherapy agents that can easily cross the blood-brain barrier (e.g., 5-fluorouracil and methotrexate) directly or indirectly have detrimental effects on brain functions such as memory and depression[10,34]. However, several antineoplastic agents that do not cross blood-brain barrier (e.g., cyclophosphamide) also affect cognitive functions[18]. Hence, we do not yet mechanistically understand the effects of chemotherapy on cognition and emotional regulation.
To date, various groups have focused on the hippocampus, which has a critical role in memory processing, and they suggested that most cognitive deficits induced by chemotherapeutic agents in diverse paradigms are associated with the inhibition of hippocampal neurogenesis[11,14,20,28,30]. In addition, although the influence of chemotherapeutic agents on mood disorders has been less well studied than their effects on cognition, Yang et al[34] reported recently that methotrexate increases depression-like behaviors in mice both 1 and 7 days after treatment, which was related to reduced hippocampal neurogenesis and cell viability and increased cytotoxicity and apoptosis. However, some studies have indicated that chemotherapeutic agents induce memory impairments and mood disorders, but do not affect hippocampal cell proliferation[10,13].
Several possible pathways that may contribute to the neurotoxicity observed after chemotherapy in breast cancer patients have been elucidated, including indirect chemical toxicity and oxidative damage, direct neuronal injury, inflammation, or a type of autoimmune response[4]. Inflammatory mechanisms within the central nervous system contribute to cognitive impairments through interactions between neurons and glial cells[65]. Several agents, including cyclophosphamide and methotrexate, activate microglia in a manner that is characterized by thicker and longer processes and persistent activation[20,32], and these increased inflammatory responses may contribute to cognitive impairment. Folate is also critical to optimal cognitive function within the mature brain, neurotoxic amino acid metabolism, and neurotransmitter synthesis[31]. Folate deficiency induced by methotrexate treatment is associated with mild cognitive dysfunction, depression, and a reduction in proliferating cells in the hippocampal dentate gyrus of adult mice[66,67,68]. In addition, chemotherapy-induced memory impairments are also involved in diverse factors including reductions of brain amine concentrations and increases in histone acetylation and apoptosis[14,26,34]. Moreover, chemotherapeutic agents (e.g., methotrexate) affect synaptic plasticity-related signals, so such changes are associated with neuronal survival and plasticity-related cellular remodeling in the hippocampus[35]. Thus, these factors may affect brain function, especially in the hippocampus, and thereby possibly impair cognition and induce depression.
In addition, tumor formation itself may affect hippocampal functions, possibly through inflammatory responses[36,69,70]. In murine cancer models, tumor formation induces depression-like behavior and memory deficits[69,70], and upregulates proinflammatory enzymes, including cyclooxygenase-2 and inducible nitric oxide synthase, in the hippocampus of tumor-bearing mice[36]. In a mouse model of breast cancer, methotrexate treatment further leads to mental depression and memory deficits and inhibition of hippocampal neurogenesis[36]. However, compared with tumor-free mice, tumor-bearing mice do exhibit a slight, non-significant decrease in hippocampal neurogenesis[33,36]. In addition to cognitive impairment and mental depression, alterations in consciousness, seizures, cerebral infarctions, paralysis, peripheral neuropathy, leukoencephalopathy, and ototoxicity are common and often dose-limiting complications of chemotherapy treatment[2,64,71]. These drugs induce such neurotoxicity via diverse mechanisms, including decreased hippocampal cell proliferation and hippocampal blood vessel density, cell death and neuronal degeneration, and the activation of microglia without changes in hippocampal cytokine levels in vivo [16,32,34]. Additional studies of chemotherapy-induced neurotoxicity may assist in the development of strategies for ameliorating these serious side effects in cancer survivors.
EFFECTS OF CHEMOTHERAPY ON NEURONS IN VITRO
The prevalence of neurotoxicity induced by chemotherapy markedly increases when the blood-brain barrier is either overwhelmed or bypassed[72]. Several chemotherapeutic agents are used to treat brain tumors because they readily cross the blood-brain barrier (e.g., methotrexate, an antimetabolite and folic acid antagonist; carmustine and lomustine, alkylating agents; cisplatin, a heavy metal; ifosfamide, an alkylating prodrug)[73]. As some chemotherapeutic drugs, including the anthracycline anticancer drug doxorubicin, do not cross the blood-brain barrier[74], several modified delivery systems have recently been developed to circumvent the limited access of such drugs to the brain, to allow these compounds to be used against brain tumors[75,76].
In addition to passage across the blood-brain barrier, other factors may contribute to the neurotoxicity of chemotherapy, including genetic predispositions, nutritional deficiencies, metabolic abnormalities, accompanying medications, depression, anxiety, or fatigue[77]. While there have been many in vivo studies associated with chemotherapy-induced memory deficits, there are complications in the direct extrapolation of specific data from in vivo studies. This is because the damage seen in vivo following radiation exposure and other treatments affects not only neurons but also surrounding cells, such as glial and vascular endothelial cells[78,79]. Therefore, to exclude these secondary effects of chemotherapy-induced damage to glial or vascular endothelial cells or neurons, it is important to clarify the precise negative effects of chemotherapy on neurons in vitro.
Despite intensive efforts to determine ways to successfully manage the neurological side effects of chemotherapy, and the development of chemoprotective agents, there is no generally accepted therapeutic protocol that precludes these untoward effects[8]. Therefore, various studies have focused on identification and characterization of the mechanisms underlying chemotherapy-induced neurotoxicity. Although few studies have suggested that pretreatment with some chemotherapeutic agents (e.g., cyclophosphamide) prevents neuronal cell death caused by serum deprivation[19], most research has shown that diverse chemotherapeutic agents have detrimental effects on neuronal cells due to the resultant changes in neuronal morphology[17,25,33]. Furthermore, it has been reported that potential toxic effects in mature neuronal cells exposed to anticancer drugs can be alleviated through inhibition of the Rho signaling pathway[17], the generation of neuronal nitric oxide synthase, and subsequent reactive oxygen species/reactive nitrogen species[23], as well as activation of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid/kainite receptors[24]. In addition, folate deficiency caused by chemotherapy induces abnormal Tau phosphorylation and amyloid precursor protein upregulation, which can contribute to the pathogenesis of Alzheimer’s disease. This indicates that folate deficiency is related to neurotoxicity, and its effects are comparable to the results of a previous in vivo study[27]. Moreover, excitotoxic mechanisms and caspase-mediated cell death contribute to the neurotoxicity of some compounds in mature neuronal cultures[21,33].
However, as mature neurons have stopped differentiating and are less sensitive than differentiating cells[80], further investigations related to chemotherapy-induced effects on immature neurons are required. In immature neurons, methotrexate induces increases in cytotoxicity and reduction of cell viability by a caspase-dependent pathway[34]. The expression of active caspase-3 and caspase-specific poly(ADP-ribose) polymerase cleavage are increased markedly in immature neurons following methotrexate treatment, and the capspase-3 inhibitor, Z-DEVD, significantly blocks the methotrexate-induced cytotoxicity, supporting the suggestion that the neurotoxicity of methotrexate in immature cells depends on the proapoptotic caspase pathway, particularly the caspase-3 related pathway. However, mature hippocampal neurons are resistant to the toxicity of methotrexate, suggesting that immature hippocampal cells are significantly more sensitive to methotrexate treatment than mature cells, and that the susceptibility of such hippocampal cells is consequentially dependent on cell differentiation[34]. Therefore, these in vitro systems may be useful in the study of the diverse neurotoxic effects of cancer chemotherapy.
CONCLUSION
Despite the abundance of data regarding chemotherapy-induced neurotoxicity in cancer patients, there is still considerable uncertainty regarding the underlying mechanisms. Moreover, many clinical studies are limited by diverse factors that complicate identification of the causes of such neurotoxic effects and resultant symptoms[7]. Thus, there have been many cellular and molecular studies of chemotherapy-induced neurotoxicity, especially of cognitive impairment and mental depression, and its underlying mechanisms using experimental in vivo and in vitro approaches, such as animal behavioral tests. Studies using animal behavioral tests have demonstrated that treatment with various chemotherapeutic agents engenders memory deficits and depression, which have been linked to hippocampal neurogenesis. Several mechanisms of chemotherapy-induced neurotoxicity have been elucidated from in vivo models, including inflammatory responses and changes in levels of brain amines, folate, and histones. However, it is difficult to determine which direct pathways are linked to chemotherapeutic-agent-induced neurotoxicity in vivo because of secondary effects of diverse factors, such as changes in glial cells and endothelial cells and various other factors. Using in vitro approaches, most anticancer drugs have been shown to induce morphological changes and various toxic effects in neurons through several pathways. Although far from complete, these investigations using in vivo and in vitro approaches are helpful in determining how best to further explore the causal mechanisms of chemotherapy-induced neurological side effects, and also in providing direction for the future development of optimized chemotherapeutic agents.
Footnotes
Miyoung Yang, D.V.M., M.S., Ph.D., Post-doctoral fellow, MSU, USA.
Funding: This work was supported by a National Research Foundation of Korea Grant funded by the Korean Government, No. NRF-2011-0003380; 2012R1A1B44001262; and a grant from Animal Medical Institute of Chonnam National University.
Conflicts of interest: None declared.
(Reviewed by Murnane K, Raye Z, Cao JR, Fu HJ)
(Edited by Li CH, Song LP)
REFERENCES
- [1].Dietrich J, Monje M, Wefel J, et al. Clinical patterns and biological correlates of cognitive dysfunction associated with cancer therapy. Oncologist. 2008;13(12):1285–1295. doi: 10.1634/theoncologist.2008-0130. [DOI] [PubMed] [Google Scholar]
- [2].Jaggi AS, Singh N. Mechanisms in cancer-chemotherapeutic drugs-induced peripheral neuropathy. Toxicology. 2012;291(1-3):1–9. doi: 10.1016/j.tox.2011.10.019. [DOI] [PubMed] [Google Scholar]
- [3].Vardy J, Tannock I. Cognitive function after chemotherapy in adults with solid tumours. Crit Rev Oncol Hematol. 2007;63(3):183–202. doi: 10.1016/j.critrevonc.2007.06.001. [DOI] [PubMed] [Google Scholar]
- [4].Barton D, Loprinzi C. Novel approaches to preventing chemotherapy-induced cognitive dysfunction in breast cancer: the art of the possible. Clin Breast Cancer. 2002;3(Suppl 3):S121–127. doi: 10.3816/cbc.2002.s.023. [DOI] [PubMed] [Google Scholar]
- [5].Abrey LE. The impact of chemotherapy on cognitive outcomes in adults with primary brain tumors. J Neurooncol. 2012;108(2):285–290. doi: 10.1007/s11060-012-0807-6. [DOI] [PubMed] [Google Scholar]
- [6].Galica J, Rajacich D, Kane D, et al. The impact of chemotherapy-induced cognitive impairment on the psychosocial adjustment of patients with nonmetastatic colorectal cancer. Clin J Oncol Nurs. 2012;16(2):163–169. doi: 10.1188/12.CJON.163-169. [DOI] [PubMed] [Google Scholar]
- [7].Jansen C, Miaskowski C, Dodd M, et al. Potential mechanisms for chemotherapy-induced impairments in cognitive function. Oncol Nurs Forum. 2005;32(6):1151–1163. doi: 10.1188/05.ONF.1151-1163. [DOI] [PubMed] [Google Scholar]
- [8].Verstappen CC, Heimans JJ, Hoekman K, et al. Neurotoxic complications of chemotherapy in patients with cancer: clinical signs and optimal management. Drugs. 2003;63(15):1549–1563. doi: 10.2165/00003495-200363150-00003. [DOI] [PubMed] [Google Scholar]
- [9].Kempermann G, Kuhn HG, Gage FH. Genetic influence on neurogenesis in the dentate gyrus of adult mice. Proc Natl Acad Sci U S A. 1997;94(19):10409–10414. doi: 10.1073/pnas.94.19.10409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Mustafa S, Walker A, Bennett G, et al. 5-Fluorouracil chemotherapy affects spatial working memory and newborn neurons in the adult rat hippocampus. Eur J Neurosci. 2008;28(2):323–330. doi: 10.1111/j.1460-9568.2008.06325.x. [DOI] [PubMed] [Google Scholar]
- [11].ElBeltagy M, Mustafa S, Umka J, et al. Fluoxetine improves the memory deficits caused by the chemotherapy agent 5-fluorouracil. Behav Brain Res. 2010;208(1):112–117. doi: 10.1016/j.bbr.2009.11.017. [DOI] [PubMed] [Google Scholar]
- [12].Lee GD, Longo DL, Wang Y, et al. Transient improvement in cognitive function and synaptic plasticity in rats following cancer chemotherapy. Clin Cancer Res. 2006;12(1):198–205. doi: 10.1158/1078-0432.CCR-05-1286. [DOI] [PubMed] [Google Scholar]
- [13].Mignone RG, Weber ET. Potent inhibition of cell proliferation in the hippocampal dentate gyrus of mice by the chemotherapeutic drug thioTEPA. Brain Res. 2006;1111(1):26–29. doi: 10.1016/j.brainres.2006.06.093. [DOI] [PubMed] [Google Scholar]
- [14].Briones TL, Woods J. Chemotherapy-induced cognitive impairment is associated with decreases in cell proliferation and histone modifications. BMC Neurosci. 2011;12:124. doi: 10.1186/1471-2202-12-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Winocur G, Vardy J, Binns MA, et al. The effects of the anti-cancer drugs, methotrexate and 5-fluorouracil, on cognitive function in mice. Pharmacol Biochem Behav. 2006;85(1):66–75. doi: 10.1016/j.pbb.2006.07.010. [DOI] [PubMed] [Google Scholar]
- [16].Rzeski W, Pruskil S, Macke A, et al. Anticancer agents are potent neurotoxins in vitro and in vivo. Ann Neurol. 2004;56(3):351–360. doi: 10.1002/ana.20185. [DOI] [PubMed] [Google Scholar]
- [17].James SE, Burden H, Burgess R, et al. Anti-cancer drug induced neurotoxicity and identification of Rho pathway signaling modulators as potential neuroprotectants. Neurotoxicology. 2008;29(4):605–612. doi: 10.1016/j.neuro.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Yang M, Kim JS, Song MS, et al. Cyclophosphamide impairs hippocampus-dependent learning and memory in adult mice: Possible involvement of hippocampal neurogenesis in chemotherapy-induced memory deficits. Neurobiol Learn Mem. 2010;93(4):487–494. doi: 10.1016/j.nlm.2010.01.006. [DOI] [PubMed] [Google Scholar]
- [19].Kitazawa H, Numakawa T, Adachi N, et al. Cyclophosphamide promotes cell survival via activation of intracellular signaling in cultured cortical neurons. Neurosci Lett. 2010;470(2):139–144. doi: 10.1016/j.neulet.2009.12.073. [DOI] [PubMed] [Google Scholar]
- [20].Christie LA, Acharya MM, Parihar VK, et al. Impaired cognitive function and hippocampal neurogenesis following cancer chemotherapy. Clin Cancer Res. 2012;18(7):1954–1965. doi: 10.1158/1078-0432.CCR-11-2000. [DOI] [PubMed] [Google Scholar]
- [21].Lopes MA, Meisel A, Dirnagl U, et al. Doxorubicin induces biphasic neurotoxicity to rat cortical neurons. Neurotoxicology. 2008;29(2):286–293. doi: 10.1016/j.neuro.2007.12.003. [DOI] [PubMed] [Google Scholar]
- [22].Liedke PE, Reolon GK, Kilpp B, et al. Systemic administration of doxorubicin impairs aversively motivated memory in rats. Pharmacol Biochem Behav. 2009;94(2):239–243. doi: 10.1016/j.pbb.2009.09.001. [DOI] [PubMed] [Google Scholar]
- [23].Lopes MA, Meisel A, Carvalho FD, et al. Neuronal nitric oxide synthase is a key factor in doxorubicin-induced toxicity to rat-isolated cortical neurons. Neurotox Res. 2011;19(1):14–22. doi: 10.1007/s12640-009-9135-9. [DOI] [PubMed] [Google Scholar]
- [24].Chatton JY, Idle JR, Vagbo CB, et al. Insights into the mechanisms of ifosfamide encephalopathy: drug metabolites have agonistic effects on alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)/kainate receptors and induce cellular acidification in mouse cortical neurons. J Pharmacol Exp Ther. 2001;299(3):1161–1168. [PubMed] [Google Scholar]
- [25].Gilbert MR, Harding BL, Grossman SA. Methotrexate neurotoxicity: in vitro studies using cerebellar explants from rats. Cancer Res. 1989;49(9):2502–2505. [PubMed] [Google Scholar]
- [26].Madhyastha S, Somayaji SN, Rao MS, et al. Hippocampal brain amines in methotrexate-induced learning and memory deficit. Can J Physiol Pharmacol. 2002;80(11):1076–1084. doi: 10.1139/y02-135. [DOI] [PubMed] [Google Scholar]
- [27].Yoon SY, Choi HI, Choi JE, et al. Methotrexate decreases PP2A methylation and increases tau phosphorylation in neuron. Biochem Biophys Res Commun. 2007;363(3):811–816. doi: 10.1016/j.bbrc.2007.09.060. [DOI] [PubMed] [Google Scholar]
- [28].Seigers R, Schagen SB, Beerling W, et al. Long-lasting suppression of hippocampal cell proliferation and impaired cognitive performance by methotrexate in the rat. Behav Brain Res. 2008;186(2):168–175. doi: 10.1016/j.bbr.2007.08.004. [DOI] [PubMed] [Google Scholar]
- [29].Gandal MJ, Ehrlichman RS, Rudnick ND, et al. A novel electrophysiological model of chemotherapy-induced cognitive impairments in mice. Neuroscience. 2008;157(1):95–104. doi: 10.1016/j.neuroscience.2008.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Seigers R, Schagen SB, Coppens CM, et al. Methotrexate decreases hippocampal cell proliferation and induces memory deficits in rats. Behav Brain Res. 2009;201(2):279–284. doi: 10.1016/j.bbr.2009.02.025. [DOI] [PubMed] [Google Scholar]
- [31].Li Y, Vijayanathan V, Gulinello ME, et al. Systemic methotrexate induces spatial memory deficits and depletes cerebrospinal fluid folate in rats. Pharmacol Biochem Behav. 2010;94(3):454–463. doi: 10.1016/j.pbb.2009.10.008. [DOI] [PubMed] [Google Scholar]
- [32].Seigers R, Timmermans J, van der Horn HJ, et al. Methotrexate reduces hippocampal blood vessel density and activates microglia in rats but does not elevate central cytokine release. Behav Brain Res. 2010;207(2):265–272. doi: 10.1016/j.bbr.2009.10.009. [DOI] [PubMed] [Google Scholar]
- [33].Seigers R, Pourtau L, Schagen SB, et al. Inhibition of hippocampal cell proliferation by methotrexate in rats is not potentiated by the presence of a tumor. Brain Res Bull. 2010;81(4-5):472–476. doi: 10.1016/j.brainresbull.2009.10.006. [DOI] [PubMed] [Google Scholar]
- [34].Yang M, Kim JS, Kim J, et al. Neurotoxicity of methotrexate to hippocampal cells in vivo and in vitro. Biochem Pharmacol. 2011;82(1):72–80. doi: 10.1016/j.bcp.2011.03.020. [DOI] [PubMed] [Google Scholar]
- [35].Yang M, Kim J, Kim SH, et al. Temporal profiles of synaptic plasticity-related signals in adult mouse hippocampus with methotrexate treatment. Neural Regen Res. 2012;7(21):1651–1658. doi: 10.3969/j.issn.1673-5374.2012.21.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Yang M, Kim JS, Kim J, et al. Acute treatment with methotrexate induces hippocampal dysfunction in a mouse model of breast cancer. Brain Res Bull. 2012;89(1-2):50–56. doi: 10.1016/j.brainresbull.2012.07.003. [DOI] [PubMed] [Google Scholar]
- [37].Temple S, Alvarez-Buylla A. Stem cells in the adult mammalian central nervous system. Curr Opin Neurobiol. 1999;9(1):135–141. doi: 10.1016/s0959-4388(99)80017-8. [DOI] [PubMed] [Google Scholar]
- [38].Cameron HA, Woolley CS, Gould E. Adrenal steroid receptor immunoreactivity in cells born in the adult rat dentate gyrus. Brain Res. 1993;611(2):342–346. doi: 10.1016/0006-8993(93)90524-q. [DOI] [PubMed] [Google Scholar]
- [39].Kim JS, Jung J, Lee HJ, et al. Differences in immunoreactivities of Ki-67 and doublecortin in the adult hippocampus in three strains of mice. Acta Histochem. 2009;111(2):150–156. doi: 10.1016/j.acthis.2008.05.002. [DOI] [PubMed] [Google Scholar]
- [40].Kim JS, Lee HJ, Kim JC, et al. Transient impairment of hippocampus-dependent learning and memory in relatively low-dose of acute radiation syndrome is associated with inhibition of hippocampal neurogenesis. J Radiat Res. 2008;49(5):517–526. doi: 10.1269/jrr.08020. [DOI] [PubMed] [Google Scholar]
- [41].Kronenberg G, Reuter K, Steiner B, et al. Subpopulations of proliferating cells of the adult hippocampus respond differently to physiologic neurogenic stimuli. J Comp Neurol. 2003;467(4):455–463. doi: 10.1002/cne.10945. [DOI] [PubMed] [Google Scholar]
- [42].Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16(6):2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Parent JM, Yu TW, Leibowitz RT, et al. Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. J Neurosci. 1997;17(10):3727–3738. doi: 10.1523/JNEUROSCI.17-10-03727.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Scott BW, Wang S, Burnham WM, et al. Kindling-induced neurogenesis in the dentate gyrus of the rat. Neurosci Lett. 1998;248(2):73–76. doi: 10.1016/s0304-3940(98)00355-3. [DOI] [PubMed] [Google Scholar]
- [45].Segi-Nishida E, Warner-Schmidt JL, Duman RS. Electroconvulsive seizure and VEGF increase the proliferation of neural stem-like cells in rat hippocampus. Proc Natl Acad Sci U S A. 2008;105(32):11352–11357. doi: 10.1073/pnas.0710858105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Tada E, Parent JM, Lowenstein DH, et al. X-irradiation causes a prolonged reduction in cell proliferation in the dentate gyrus of adult rats. Neuroscience. 2000;99(1):33–41. doi: 10.1016/s0306-4522(00)00151-2. [DOI] [PubMed] [Google Scholar]
- [47].Seo HS, Yang M, Song MS, et al. Toluene inhibits hippocampal neurogenesis in adult mice. Pharmacol Biochem Behav. 2010;94(4):588–594. doi: 10.1016/j.pbb.2009.11.015. [DOI] [PubMed] [Google Scholar]
- [48].Young D, Lawlor PA, Leone P, et al. Environmental enrichment inhibits spontaneous apoptosis, prevents seizures and is neuroprotective. Nat Med. 1999;5(4):448–453. doi: 10.1038/7449. [DOI] [PubMed] [Google Scholar]
- [49].Duman RS, Malberg J, Nakagawa S. Regulation of adult neurogenesis by psychotropic drugs and stress. J Pharmacol Exp Ther. 2001;299(2):401–407. [PubMed] [Google Scholar]
- [50].Aonurm-Helm A, Jurgenson M, Zharkovsky T, et al. Depression-like behaviour in neural cell adhesion molecule (NCAM)-deficient mice and its reversal by an NCAM-derived peptide, FGL. Eur J Neurosci. 2008;28(8):1618–1628. doi: 10.1111/j.1460-9568.2008.06471.x. [DOI] [PubMed] [Google Scholar]
- [51].Cameron HA, Tanapat P, Gould E. Adrenal steroids and N-methyl-D-aspartate receptor activation regulate neurogenesis in the dentate gyrus of adult rats through a common pathway. Neuroscience. 1998;82(2):349–354. doi: 10.1016/s0306-4522(97)00303-5. [DOI] [PubMed] [Google Scholar]
- [52].Gould E, Tanapat P. Stress and hippocampal neurogenesis. Biol Psychiatry. 1999;46(11):1472–1479. doi: 10.1016/s0006-3223(99)00247-4. [DOI] [PubMed] [Google Scholar]
- [53].Jacobs BL, van Praag H, Gage FH. Adult brain neurogenesis and psychiatry: a novel theory of depression. Mol Psychiatry. 2000;5(3):262–269. doi: 10.1038/sj.mp.4000712. [DOI] [PubMed] [Google Scholar]
- [54].van Praag H, Christie BR, Sejnowski TJ, et al. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci U S A. 1999;96(23):13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Vaidya VA, Fernandes K, Jha S. Regulation of adult hippocampal neurogenesis: relevance to depression. Expert Rev Neurother. 2007;7(7):853–864. doi: 10.1586/14737175.7.7.853. [DOI] [PubMed] [Google Scholar]
- [56].Mesulam MM. Neuroplasticity failure in Alzheimer's disease: bridging the gap between plaques and tangles. Neuron. 1999;24(3):521–529. doi: 10.1016/s0896-6273(00)81109-5. [DOI] [PubMed] [Google Scholar]
- [57].Neves G, Cooke SF, Bliss TV. Synaptic plasticity, memory and the hippocampus: a neural network approach to causality. Nat Rev Neurosci. 2008;9(1):65–75. doi: 10.1038/nrn2303. [DOI] [PubMed] [Google Scholar]
- [58].Wang Y. Probing the role of AMPAR endocytosis and long-term depression in behavioural sensitization: relevance to treatment of brain disorders, including drug addiction. Br J Pharmacol. 2008;153(Suppl 1):S389–395. doi: 10.1038/sj.bjp.0707616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Crews L, Rockenstein E, Masliah E. APP transgenic modeling of Alzheimer's disease: mechanisms of neurodegeneration and aberrant neurogenesis. Brain Struct Funct. 2010;214(2):111–126. doi: 10.1007/s00429-009-0232-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Hu Q, Fu H, Song H, et al. Low-level lead exposure attenuates the expression of three major isoforms of neural cell adhesion molecule. Neurotoxicology. 2011;32(2):255–260. doi: 10.1016/j.neuro.2010.12.007. [DOI] [PubMed] [Google Scholar]
- [61].Pessoa L. Emergent processes in cognitive-emotional interactions. Dialogues Clin Neurosci. 2010;12(4):433–448. doi: 10.31887/DCNS.2010.12.4/lpessoa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Pasupathy A, Miller EK. Different time courses of learning-related activity in the prefrontal cortex and striatum. Nature. 2005;433(7028):873–876. doi: 10.1038/nature03287. [DOI] [PubMed] [Google Scholar]
- [63].Drevets WC, Videen TO, Price JL, et al. A functional anatomical study of unipolar depression. J Neurosci. 1992;12(9):3628–3641. doi: 10.1523/JNEUROSCI.12-09-03628.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Beinert T, Masuhr F, Mwela E, et al. Neuropathy under chemotherapy. Eur J Med Res. 2000;5(10):415–423. [PubMed] [Google Scholar]
- [65].Wilson CJ, Finch CE, Cohen HJ. Cytokines and cognition--the case for a head-to-toe inflammatory paradigm. J Am Geriatr Soc. 2002;50(12):2041–2056. doi: 10.1046/j.1532-5415.2002.50619.x. [DOI] [PubMed] [Google Scholar]
- [66].Bell IR, Edman JS, Morrow FD, et al. Brief communication. Vitamin B1, B2, and B6 augmentation of tricyclic antidepressant treatment in geriatric depression with cognitive dysfunction. J Am Coll Nutr. 1992;11(2):159–163. [PubMed] [Google Scholar]
- [67].Kruman II, Mouton PR, Emokpae R, et al. Folate deficiency inhibits proliferation of adult hippocampal progenitors. Neuroreport. 2005;16(10):1055–1059. doi: 10.1097/00001756-200507130-00005. [DOI] [PubMed] [Google Scholar]
- [68].Riggs KM, Spiro A, 3rd, Tucker K, et al. Relations of vitamin B-12, vitamin B-6, folate, and homocysteine to cognitive performance in the Normative Aging Study. Am J Clin Nutr. 1996;63(3):306–314. doi: 10.1093/ajcn/63.3.306. [DOI] [PubMed] [Google Scholar]
- [69].Pyter LM, Cochrane SF, Ouwenga RL, et al. Mammary tumors induce select cognitive impairments. Brain Behav Immun. 2010;24(6):903–907. doi: 10.1016/j.bbi.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Pyter LM, Pineros V, Galang JA, et al. Peripheral tumors induce depressive-like behaviros and cytokine production and alter hypothalamic-pituitary-adrenal axis regulation. Proc Natl Acad Sci U S A. 2009;106(22):9069–9074. doi: 10.1073/pnas.0811949106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Reddy AT, Witek K. Neurologic complications of chemotherapy for children with cancer. Curr Neurol Neurosci Rep. 2003;3(2):137–142. doi: 10.1007/s11910-003-0065-2. [DOI] [PubMed] [Google Scholar]
- [72].Ricard D, Taillia H, Renard JL. Brain damage from anticancer treatments in adults. Curr Opin Oncol. 2009;21(6):559. doi: 10.1097/CCO.0b013e328330c669. [DOI] [PubMed] [Google Scholar]
- [73].Myers JS, Pierce J, Pazdernik T. Neurotoxicology of chemotherapy in relation to cytokine release, the blood-brain barrier, and cognitive impairment. Oncol Nurs Forum. 2008;35(6):916–920. doi: 10.1188/08.ONF.916-920. [DOI] [PubMed] [Google Scholar]
- [74].Bigotte L, Arvidson B, Olsson Y. Cytofluorescence localization of adriamycin in the nervous system. I. Distribution of the drug in the central nervous system of normal adult mice after intravenous injection. Acta Neuropathol. 1982;57(2-3):121–129. doi: 10.1007/BF00685379. [DOI] [PubMed] [Google Scholar]
- [75].Petri B, Bootz A, Khalansky A, et al. Chemotherapy of brain tumour using doxorubicin bound to surfactant-coated poly(butyl cyanoacrylate) nanoparticles: revisiting the role of surfactants. J Control Release. 2007;117(1):51–58. doi: 10.1016/j.jconrel.2006.10.015. [DOI] [PubMed] [Google Scholar]
- [76].Tilloy S, Monnaert V, Fenart L, et al. Methylated beta-cyclodextrin as P-gp modulators for deliverance of doxorubicin across an in vitro model of blood-brain barrier. Bioorg Med Chem Lett. 2006;16(8):2154–2157. doi: 10.1016/j.bmcl.2006.01.049. [DOI] [PubMed] [Google Scholar]
- [77].Saykin A, Ahles T, McDonald B. Mechanisms of chemotherapy-induced cognitive disorders: neuropsychological, pathophysiological, and neuroimaging perspectives. Semin Clin Neuropsychiatry. 2003;8(4):201–206. [PubMed] [Google Scholar]
- [78].Myers R, Rogers MA, Hornsey S. A reappraisal of the roles of glial and vascular elements in the development of white matter necrosis in irradiated rat spinal cord. Br J Cancer Suppl. 1986;7:221–223. [PMC free article] [PubMed] [Google Scholar]
- [79].van der Kogel AJ. Radiation-induced damage in the central nervous system: an interpretation of target cell responses. Br J Cancer Suppl. 1986;7:207–217. [PMC free article] [PubMed] [Google Scholar]
- [80].Okamoto M, Suzuki Y, Shirai K, et al. Effect of radiation on the development of immature hippocampal neurons in vitro. Radiat Res. 2009;172(6):718–724. doi: 10.1667/RR1741.1. [DOI] [PubMed] [Google Scholar]


