Abstract
Lentivirus carrying the Atoh1 gene can infect Corti's organ and express a hair-like cell surface marker in the supporting cell area. However, expression of the gene carried by adenovirus is instantaneous, which undoubtedly limits its clinical application. Lentivirus acts as a carrier that can stably and continuously express genes. In this study, the cochlear structure and hearing level were not affected, and Atoh1 gene carried by lentivirus promoted the production of hair-like cells in the cochlear supporting cell area. This led to expression of the hair-like cell surface marker myosin 7a 30 days after lentivirus carrying Atoh1 was microinjected into the cochlear round window of rats.
Keywords: neural regeneration, gene therapy, Atoh1, lentivirus, hair cells, myosin 7a, cochlea, auditory threshold, grants-supported paper, neuroregeneration
Research Highlights
(1) Numbers of hair cells and auditory function in normal rats were observed after microinjection of lentivirus carrying enhanced green fluorescent protein and Atoh1 into the rat cochlea, and the production of new hair cells in cochlea was explored.
(2) Recombinant lentivirus carrying a reporter gene, enhanced green fluorescent protein, and the target gene Atoh1 were constructed by gene engineering methods. Then, recombinant lentivirus was injected into the cochlea of normal rats using a postauricular approach via the round window membrane. The results showed that recombinant lentivirus has no impact on the hair cell numbers and auditory functions, can infect hair and supporting cells, and promotes supporting cells to transdifferentiate into hair cells.
(3) Recombinant lentivirus carrying enhanced green fluorescent protein and the Atoh1 gene caused no obvious damage to cochlear hair cells or auditory function. The target gene carried in lentivirus was continuously expressed in the transfected cells, providing evidence for lentivirus research.
INTRODUCTION
In the mammalian embryonic period, cochlear hair cells and supporting cells have common precursor cells[1]. Several genes control the differentiation of precursor cells and Atoh1 is an important one[2]. Expression of Atoh1 can generate extra hair cells[3,4,5], and many hair cell precursors die in Atoh1 null mice[6]; thus, Atoh1 is probably necessary and sufficient to drive hair cell differentiation in the ear.
Lentivirus vector, based on the human immunodeficiency virus, can mediate stable in vivo gene transfer into differentiated neurons and infect dividing and nondividing cells[7]. Lentivirus, as one of a number of retroviral vector systems, can integrate into the genomes of target cells[7]. Based on these features, lentivirus vectors are potential vectors for transfer of genes to the inner ear, being safe and causing only minimal inflammatory reactions[8]. Local cochlear injections of lentivirus do not enter the lungs, heart, femoral bone marrow, duodenum, contralateral ear[8] or brain tissue[9]. However, it is not clear whether local cochlear injection of lentivirus carrying Atoh1 would affect the auditory function and the number of hair cells, or induce generation of extra hair-like cells.
In this study, we aimed to observe the cochlear structure, hearing level and hair cell-like cells after microinjection of lentivirus carrying enhanced green fluorescent protein and Atoh1 into the cochlea of normal hearing rats via the cochlear round window by a postauricular approach.
RESULTS
Quantitative analysis of experimental animals
Forty adult Sprague-Dawley rats were randomly divided into four groups: a lentivirus-enhanced green fluorescent protein-Atoh1 microinjection group, a lentivirus-enhanced green fluorescent protein microinjection group, an artificial perilymph injection group and a non-injection group. Each group contained 10 rats. With the exception of the non-injection group, rats were injected respectively with lentivirus-enhanced green fluorescent protein-Atoh1, lentivirus-enhanced green fluorescent protein and artificial perilymph via the cochlear round window. No rats died, and all rats entered the final analysis.
General conditions of rats after injection
No rat had symptoms of wryneck, walking instability, paralysis, anorexia, or incision infection. There was no suppurative infection or tympanic membrane in the rat bony bulla.
Cochlear microinjection of recombinant lentivirus carrying extrinsic Atoh1 had no impact on rat hearing levels
The results of auditory brainstem response measurements revealed no significant differences in values among rats in the different groups before microinjection of lentivirus, and an insignificant difference after microinjection. The auditory brainstem response thresholds of rats in the same group did not differ significantly between before and after injection. The auditory brainstem response threshold valves are shown in Figure 1.
Figure 1.
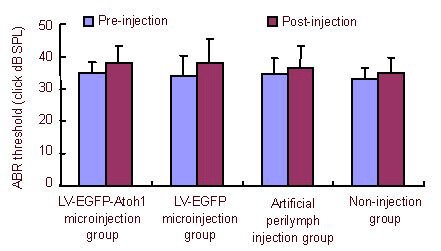
Effect of cochlear microinjection of recombinant lentivirus carrying extrinsic Atoh1 on rat hearing levels.
The auditory brainstem response (ABR) threshold in the same rat was not different before and after injection. The ABR threshold in the same group also did not differ before and after injection. The ABR thresholds in all rats were recorded before injection and 30 days after injection.
Data are expressed as mean ± SD. There are 10 rats in each group. Analysis of variance was used to compare ABR thresholds.
LV: Lentivirus; EGFP: enhanced green fluorescent protein; dB SPL: decibel sound pressure level.
Cochlear microinjection of recombinant lentivirus carrying extrinsic Atoh1 had no impact on the number of rat cochlear hair cells
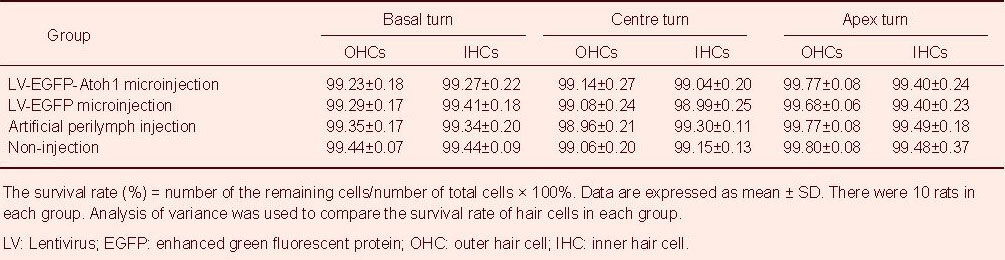
There were dispersive losses of inner or outer hair cells in all basal turns of cochlea (Figure 2). Among all groups, neither the number of hair cells nor the survival rate in the basal, middle and apex turns differed significantly (Tables 1, 2).
Figure 2.
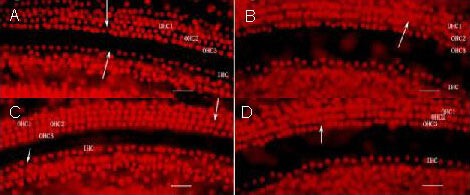
Effect of cochlear microinjection of recombinant lentivirus carrying extrinsic Atoh1 on rat cochlear hair cells (propidium iodide staining).
Three rows of OHCs (OHC1, OHC2, OHC3) showed no differences. Arrows indicate dispersive loss of OHCs or IHCs. There was no significant difference in the numbers of hair cells among different groups. Scale bars: 2.5 μm.
(A) Lentivirus-enhanced green fluorescent protein-Atoh1 microinjection group; (B) lentivirus-enhanced green fluorescent protein microinjection group; (C) artificial perilymph injection group; (D) non-injection group.
OHC: Outer hair cell; IHC: inner hair cell.
Table 1.
Effect of cochlear microinjection of recombinant lentivirus carrying extrinsic Atoh1 on the number of hair cells (/40-fold field; propidium iodide staining)
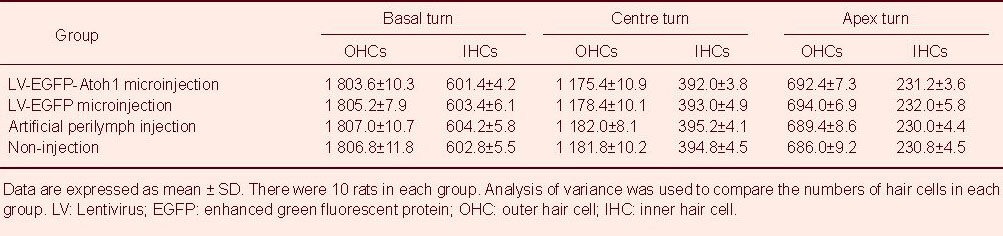
Table 2.
Effect of cochlear microinjection of recombinant lentivirus carrying extrinsic Atoh1 on the survival rate of hair cells (%; propidium iodide staining)
Cochlear microinjection of recombinant lentivirus carrying extrinsic Atoh1 promoted hair-like cell surface marker myosin 7a
When cochlear hair cells were transfected with lentivirus carrying enhanced green fluorescent protein-Atoh1, both enhanced green fluorescent protein and Atoh1 were expressed in rat cochlear hair cells as detected by immunochemical fluorescence staining.
In the lentivirus-enhanced green fluorescent protein-Atoh1 microinjection group, myosin 7a, which is a hair cell-related marker[10], could be expressed in both cochlear hair cells and supporting cells. This finding provides evidence that supporting cells can be transfected by lentivirus carrying enhanced green fluorescent protein and Atoh1, then expressed myosin 7a. In the lentivirus-enhanced green fluorescent protein microinjection group and the artificial perilymph injection group, myosin 7a was only expressed in cochlear hair cells (Figure 3).
Figure 3.
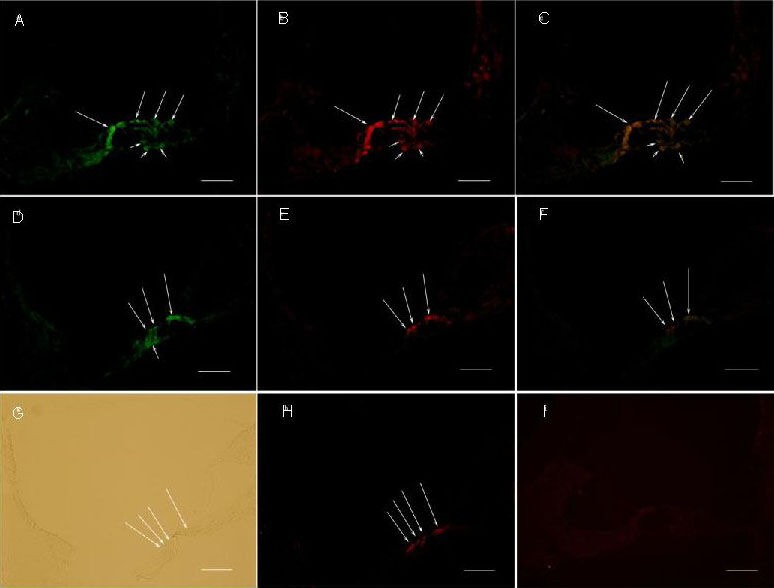
Effect of cochlear microinjection of recombinant lentivirus carrying extrinsic Atoh1 and enhanced green fluorescent protein (EGFP) on the cochlear central turn (immunochemical fluorescence staining).
Expression of EGFP displayed green fluorescence. Expression of myosin 7a displaying red fluorescence. LV-EGFP could infect both cochlear hair cells and supporting cells (A, D). Long arrows represent hair cells and short arrows represent supporting cells. Scale bars: 2.5 μm.
In the LV-EGFP-Atoh1 microinjection group: (A) The fluorescein isothiocyanate filter shows expression of EGFP. (B) The rhodamine filter shows expression of myosin 7a. (C) Merge of A and B.
In the LV-EGFP microinjection group: (D) The fluorescein isothiocyanate filter shows expression of EGFP. (E) The rhodamine filter shows expression of myosin 7a. (F) Merge of D and E.
In the artificial perilymph injection group: (G) Light microscopic view. (H) The rhodamine filter shows expression of myosin 7a. There were new hair-like cells formed in LV-EGFP-Atoh1 microinjection group, but no new hair-like cells in LV-EGFP microinjection group and artificial perilymph injection group.
(I) PBS instead of myosin 7a antibody as a negative control.
LV-EGFP: Lentivirus-enhanced green fluorescent protein.
DISCUSSION
Gene therapy is an important method for treating deafness caused by hair cell damage. Recently, lentivirus has been widely used as a gene carrier[11,12,13], because it has a number of advantages. Lentivirus can infect dividing cells and nondividing cells (such as neural cells, hematopoietic stem cells, and hepatic cells), and the viral genome integrates into the host genome to enable long-term expression of target genes, with little immunogenicity and good biological safety[14]. Studies have shown that lentivirus can be used as a carrier to express target genes[15,16]. We constructed a lentivirus based on human immunodeficiency virus and genetically exchanged the human immunodeficiency virus gp120 protein for G glycoproteins of the vesicular stomatitis virus, known as VSV-G, so that the lentivirus can target and infect cell types other than helper T-cells, which human immunodeficiency virus infects via binding of the gp120 protein to the CD4 receptor of helper T-cells.
At 30 days after injection, lentivirus successfully infected cochlear hair cells and supporting cells, and expressed green fluorescence. This finding indicates that lentivirus can infect the cochlea and express gene, as also reported by Pietola[8].
Atoh1, which codes for a basic helix-loop-helix transcription factor, is one of a number of members of the basic helix-loop-helix family[17]. Basic helix-loop-helix transcription factors control the development and differentiation of many systems in vertebrates[18], including cochlear hair cells[19]. Atoh1 (also known as math1) is essential for development of the peripheral and central nervous system[20,21,22], and for the formation of some non-neural cell types[23,24]. Atoh1 plays a critical role in hair cell differentiation during development[25,26]. An Atoh1 conditional knockout mouse line shows progressive loss of almost all the inner hair cells and the majority of outer hair cells within 3 weeks of birth[27]. In the neonatal and juvenile mouse, Atoh1 ectopic expression has converted partial cochlear Pillar and Deiters’ cells to immature hair cells[28]. In the postnatal mammalian cochlea, Atoh1 directs the formation of sensory mosaics and induces cell proliferation[29]. We microinjected lentivirus carrying enhanced green fluorescent protein-Atoh1 into rat cochlea and found that myosin 7a, a hair cell-related marker[10], was expressed in hair cells and supporting cells. However, myosin 7a was only expressed in hair cells when we microinjected lentivirus carrying enhanced green fluorescent protein or artificial perilymph into rat cochlea. This provides evidence that Atoh1 might transform some supporting cells into hair-like cells expressing myosin 7a in the normal adult rat cochlea. Kawamoto et al[30] observed the auditory sensory epithelium and surrounding tissue using immunochemical fluorescence methods, and reported expression of myosin 7a in Corti’s organ, interdental cells and Hensen cells, when Atoh1 carried by adenovirus was introduced into the cochlea of normal hearing guinea pigs. Izumikawa et al[2] revealed that myosin 7a reappeared in hair cells damaged by ear drug toxicity after inoculation of Atoh1 carried by adenovirus into cochlea of deaf guinea pigs. These findings indicated that overexpression of Atoh1 may promote generation of hair-like cells in the cochlea and that this approach could be used to treat sensorineural deafness. However, further studies assessing whether hair-like cells express myosin 7a and have normal morphological and physiological functions of hair cells are needed.
Generally speaking, surgical methods for injecting vectors or cells into the animal cochlea include ventral[31,32] and postaural[33] approaches. Damage to the trachea or large artery and vein branches during ventral approach surgery could induce animal death during or after surgery. However, relatively few important structures are exposed in postaural approach surgery. A postaural approach was adopted in our study. After cutting the posterior auricular sulcus skin and platysma muscle and pulling back the sternocleidomastoid muscle, we can expose the triangle region surrounded by the facial nerve and posterior belly of the digastrics muscle. When soft tissue and fascia overlapping the osseous tissue below the facial nerve and in front of the posterior belly of the digastrics muscle are separated, the bony bulla is exposed. The internal carotid artery is in the front of the bony bulla. Care should be taken to avoid damage to the internal carotid artery. The post-inferior region of the bony bulla is opened as a window, which is large enough to expose the stapedia artery and round window niche.
To detect an effect on auditory function caused by lentivirus microinjection, auditory brainstem response thresholds were measured before and after injection among the four groups. To avoid a probable influence of assessing different ears, the left ear was chosen for experiments. In the non-injection group, as in the normal control group, there was no significant difference in auditory brainstem response thresholds between before and after injection. In the artificial perilymph injection group, as in the surgery control group, there was also no significant difference. This provides evidence that surgery for injections did not affect the auditory functions of experimental animals. In the lentivirus-enhanced green fluorescent protein-Atoh1 microinjection group and the lentivirus-enhanced green fluorescent protein microinjection group, as experimental groups, no significant difference in auditory brainstem response thresholds was observed, indicating that the lentivirus had no impact on the auditory functions of experimental animals. Thus, lentivirus microinjection via the round window represents a potential method for treating inner ear diseases. It should be pointed out that different microinjection locations may have different influences on auditory functions. In our previous study, microinjection via the cochlear scala media lateral wall caused a higher auditory brainstem response threshold than was seen in non-injected rats[34]. We think that injection via the cochlear lateral wall may damage the bony labyrinth or cause endolymph leakage. Microinjection via the round window can maximally reduce bony labyrinth structural damage and avoid perilymph leakage, which prevents any loss of hearing. However, there is some debate about whether auditory function is affected by microinjecting via the round window. Chen et al[35] reported that auditory thresholds did not differ after microinjecting through an intact round window membrane in guinea pigs. However, Xu et al[36] found that auditory function was severely damaged after microinjecting through an intact round window membrane in mouse, mainly due to rupture of the round window membrane.
To detect damage to hair cells caused by injection and lentivirus, we counted the numbers of hair cells among the four groups after injection. Our results demonstrated dispersive hair cell loss and no significant difference was seen among the four groups. Dispersive hair cell loss among the four groups may be caused during the preparation of the cochlear membrane. Under an optical microscope, cochlear structures showed no obvious damage after injection. This indicates that microinjection via the round window did not cause significant mechanical damage to hair cells or the cochlea. We speculate that the reasons for this are as follows: (1) injection slowly at a speed of 1 μL/min maximally avoids damage to cochlear structures and the degeneration of hair cells; (2) lentivirus might not change the microenvironment of the perilymph; (3) microinjection via the round window membrane and the subsequent fascia cover ensures inner ear integrity and avoids structural damage after injection. Consistent with this, Fu et al[37] found no significant hair cell or stereocilia loss after neural stem cells were transplanted into the rat cochlea via the round window membrane.
Although we microinjected lentivirus carrying enhanced green fluorescent protein-Atoh1 and found that myosin 7a was expressed in supporting cells and hair cells, an urgent requirement is verification of whether the supporting cells expressing myosin 7a have the physiological properties of hair cells. Thus, the present study is only a preliminary study on the injection of lentivirus carrying Atoh1 into the cochlea via the round window. Extended observation periods and more detailed physiologic measures will be necessary to examine the long-term effects of this approach.
MATERIALS AND METHODS
Design
A randomized, controlled animal experiment.
Time and setting
The experiment was performed in the State Key Laboratory of Diagnosis and Treatment of Infectious Diseases, the First Affiliated Hospital of Zhejiang University in China from October 2010 to November 2011.
Materials
Forty Sprague-Dawley male rats, specific pathogen-free grade, weighing 150–200 g, were purchased from the Laboratory Animal Center of Zhejiang University in China (No. 2011-015) and fed at room temperature, under natural lighting and air with relative humidity about 50%. All experimental disposals were in accordance with the Guidelines and Suggestions for the Care and Use of Laboratory Animals, formulated by the Animal Care Committees of Zhejiang University, China.
Methods
Construction of recombinant lentivirus vectors
The recombinant lentivirus vectors were produced as previously described[38]. Four plasmids were used to produce third-generation self-inactivating human immunodeficiency virus-1-based lentivirus vectors as follows: (1) the packaging constructs pMDIg-pRRE (Shanghai Televector Co., Ltd., Shanghai, China), in which the cytomegalovirus early promoter drove the synthesis of viral proteins including gag and pol; (2) the packaging constructs pRSV-REV (Shanghai Televector Co., Ltd.), which were rev cDNA expression plasmids, in which the joined second exons of human immunodeficiency virus-1 rev were under the transcriptional control of the rous sarcoma virus U3; (3) the plasmid pMD2G (Shanghai Televector Co., Ltd.), which produced the pseudotyping envelope vesicular stomatitis virus glycoprotein; and (4) the expression vector pLenti-enhanced green fluorescent protein-Neo (Shanghai Televector Co., Ltd.), in which the transgene was flanked by the human immunodeficiency virus-1 derived cis-acting sequences necessary for packaging, reverse transcription, and integration. Atoh1 cDNA, coding for the 1.056 kDa form of murine Atoh1 and bearing a site of SmaI and KpnI on its 5′ and 3′ ends, was cloned by PCR from the template Atoh1 open reading frame sequence (FulenGen Co., Ltd., Guangzhou, Guangdong Province, China). Then, the Atoh1 cDNA was inserted into the unique Smal and KpnI sites of the pLenti-enhanced green fluorescent protein-Neo plasmid to acquire pLenti-enhanced green fluorescent protein-Atoh1 plasmid, which was controlled by the cytomegalovirus early promoter (Figure 4).
Figure 4.
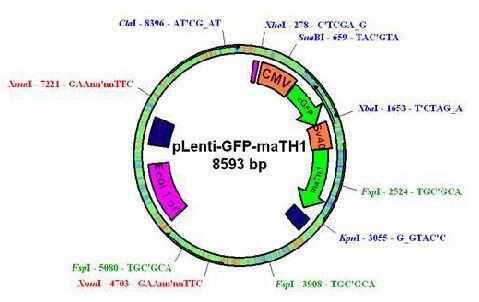
Structure of recombinant lentivirus.
The PCR primers (Shanghai Sangon Biological Engineering Technology & Services Co., Ltd., Shanghai, China) were as follows: forward 5′-ATG TCC CGC CTG CTG CAT GC-3′ and reverse 5′-GGG GTA CCC TAA CTG GCC TCA TCA GAG TC-3′. Viral particles of lentivirus carrying enhanced green fluorescent protein-Atoh1 were produced by transiently cotransfecting the transfer plasmid pLenti- enhanced green fluorescent protein-Atoh1 (20 μg), the packaging plasmids pRev-REV (10 μg) and pMDIg- pRRE (15 μg), and the vesicular stomatitis virus glycoprotein envelope plasmid pMD2G (7.5 μg) into subconfluent 293T cells using the calcium chloride method. The medium was replaced 12 hours later and the culture supernatant containing viral particles were collected at 48 hours post-transfection and stored at −80°C. Viral titer was determined in Hela cells transduced with 10 and 100 times serial dilutions of concentrated viral supernatant and was found to be about 3 × 108 TU/mL.
Microinjection of recombinant lentivirus into cochlea
Rats were placed on a homothermal pad (37°C) after being anesthetized with ketamine (40 mg/kg) and chlorpromazine (20 mg/kg) intramuscularly. An incision was made along the post-helical sulcus after skin preparation and disinfection. The facial nerve and sternocleidomastoid muscle were well exposed after the skin was cut and the platysma was separated. We then separated the triangle surrounded by the facial nerve and sternocleidomastoid muscle deeply, so that the bony bulla could be exposed within the triangle surrounded by the facial nerve and digastric muscle (Figure 5A). The stapedial artery and round window niche were exposed after a part of bulla bone was removed (Figure 5B).
Figure 5.
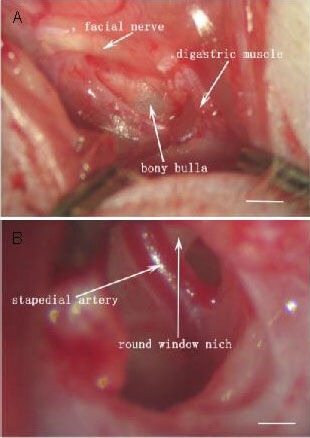
Exposure of the cochlear round window for lentivirus injection. Scale bars: 25 μm.
(A) When the skin is cut and the tissue is separated, the bony bulla is exposed.
(B) When a part of the bulla bone is removed, the round window is exposed.
A 20-μL microsyringe (Exmire Microsyringe; Ito Corporation, Fuji, Shizuoka, Japan), which was the same sort used in our previous study[37], was inserted into the round window membrane. Then, 5 μL of lentivirus carrying enhanced green fluorescent protein-Atoh1, lentivirus carrying enhanced green fluorescent protein, or artificial perilymph (NaCl 125 mmol/L, KCl 3.5 mmol/L, MgCl2 1.2 mmol/L, NaH2PO4 0.75 mmol/L, NaHCO3 25 mmol/L, glucose 5 mmol/L) was slowly microinjected into the scala tympani at the speed of 1 μL/min in the corresponding injection groups. The needle of the microsyringe was kept in the inner ear for 10 minutes to prevent backflow. A small piece of fascia was placed over the round window, and the incision was closed with sutures.
Auditory brainstem response measurement for detecting hearing threshold variations
All animals were recorded for auditory brainstem response threshold before injection and 30 days after injection. Animals were intramuscularly anesthetized with ketamine (40 mg/kg) and chlorpromazine (20 mg/kg) before auditory brainstem response measurement. A Nicolet Compass system (Nicolet Diagnostics Ltd., San Carlos, CA, USA) was used at 10 ms duration, 11.1/s repetition rate, 150–2 000 Hz filtering band, and 1 000 times superposition, and the stimulation sound was a 0.1 ms broad band click. A recording electrode was inserted into the scalp in the middle position between the two ears. A reference electrode was inserted below the left ear and a ground electrode was inserted contralaterally. The threshold was defined as the lowest decibel sound pressure level (dB SPL) of stimulus at which a positive waveform in the evoked response tracing was evident and should be repeated[39].
Expression of myosin 7a in cochlear cells detected by immunochemical fluorescence staining
Ten anesthetized rats in three injection groups were sacrificed 30 days after injection. They were perfused intracardially with normal saline, followed by 4% paraformaldehyde in PBS. The left temporal bones were removed, then the cochlea were dissected and immersed in the same fixative at 4°C for 4 hours. After decalcification with 0.1 mol/L ethylene diamine tetraacetic acid for 7 days at 4°C, cochlea were incubated with 20% sucrose for 2 days and optimal cutting temperature compound for 1 hour before freezing. Cryostat sections (10 mm) of the cochlea were prepared. The sections were immersed in PBS for 10 minutes, then permeabilized with 0.3% Triton X-100 in PBS for 10 minutes. Non-specific binding of secondary antibody was blocked by incubation with 5% goat serum in PBS for 30 minutes. The sections were incubated overnight with mouse anti-rat enhanced green fluorescent protein monoclonal antibody (1:200; Beyotime Institute of Biotechnology, Haimen, Jiangsu Province, China), or rabbit anti-myosin7a polyclonal antibody (1:400; Santa Cruz Biotechnology, Santa Cruz, CA, USA) at 4°C using PBS as a negative control. After three PBS washes for 10 minutes each, the sections were incubated with secondary antibodies, rhodamine-conjugated goat anti-rabbit IgG (Santa Cruz Biotechnology; 1:400) or fluorescein- conjugated affinipure goat anti-mouse IgG (Jackson ImmunoResearch Newmarket, UK; 1:400), at room temperature for 1 hour. The sections were examined and photographed using a Leica DMRB epifluorescence microscope with a digital camera (Spot RT, Diagnostic Instrument or Polaroid DMC le, Sterling Heights, MI, USA).
Assessing the numbers of cochlear hair cells using propidium iodide staining
At 30 days after injection, the numbers of hair cells were counted by the method of Ding et al[40]. Five rats from each group were anesthetized and perfused intracardially with normal saline, followed by 4% paraformaldehyde in PBS. The left temporal bones were removed; then, the cochlea were dissected and immersed in the same fixative at 4°C for 4 hours. After cochlea was exposed, the spiral ligament, stria vascularis and tectorial membrane were removed. The basilar membranes were permeabilized with 0.3% Triton X-100 in PBS for 10 minutes and 0.5% propidium iodide in PBS for 20 minutes, then rinsed with PBS three times for 5 minutes each. The sections were mounted on slides containing 20% glycerol, then examined and photographed using a Leica DMRB epifluorescence microscope with a digital camera (Spot RT, Diagnostic Instrument or Polaroid DMC le). Whole basilar membranes were divided into three parts, consisting of the basal, center and apex turns.
Statistical analysis
Data are expressed as mean ± SD and were statistically analyzed using SPSS 16.0 software (IBM Corporation, New York, NY, USA). Analysis of variance was used to compare values among groups. A value of P less than 0.05 was considered statistically significant.
Footnotes
Song Pan, M.D., Attending physician.
Funding: This study was supported by grants from the National Basic Research Program of China (973 Program), No. 2012CB967900, 2012CB967904; the National Natural Science Foundation of China, No. 81070782; the Natural Science Foundation of Zhejiang Province, China, No. 30672308; the Qianjiang Talent Project of Science and Technology Ministry in Zhejiang Province, No. 2011R10014; and the Natural Science Foundation of Ningbo, No. 2011A610042.
Conflicts of interest: None declared.
Ethical approval: The care and use of the animals described in this study were approved by the Animal Care and Use Committee of Zhejiang University in China.
(Reviewed by McGowan D, Frenchman B, Miao YZ, Liu LF)
(Edited by Yu J, Yang Y, Li CH, Song LP)
REFERENCES
- [1].Fekete DM. Cell fate specification in the inner ear. Curr Opin Neurobiol. 1996;6(4):533–541. doi: 10.1016/s0959-4388(96)80061-4. [DOI] [PubMed] [Google Scholar]
- [2].Izumikawa M, Minoda R, Kawamoto K, et al. Auditory hair cell replacement and hearing improvement by Atoh1 gene therapy in deaf mammals. Nat Med. 2005;11(3):271–276. doi: 10.1038/nm1193. [DOI] [PubMed] [Google Scholar]
- [3].Praetorius M, Hsu C, Baker K, et al. Adenovector-mediated hair cell regeneration is affected by promoter type. Acta Otolaryngol. 2010;130(2):215–222. doi: 10.3109/00016480903019251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Gubbels SP, Woessner DW, Mitchell JC, et al. Functional auditory hair cells produced in the mammalian cochlea by in utero gene transfer. Nature. 2008;455(7212):537–541. doi: 10.1038/nature07265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Zheng JL, Gao WQ. Overexpression of Math1 induces robust production of extra hair cells in postnatal rat inner ears. Nat Neurosci. 2000;3(6):580–586. doi: 10.1038/75753. [DOI] [PubMed] [Google Scholar]
- [6].Chen P, Johnson JE, Zoghbi HY, et al. The role of Math1 in inner ear development: Uncoupling the establishment of the sensory primordium from hair cell fate determination. Development. 2002;129(10):2495–2505. doi: 10.1242/dev.129.10.2495. [DOI] [PubMed] [Google Scholar]
- [7].Naldini L, Blömer U, Gallay P, et al. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272(5259):263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- [8].Pietola L, Aarnisalo AA, Joensuu J, et al. HOX-GFP and WOX-GFP lentivirus vectors for inner ear gene transfer. Acta Otolaryngol. 2008;128(6):613–620. doi: 10.1080/00016480701663409. [DOI] [PubMed] [Google Scholar]
- [9].Duan M, Mi Q. Local delivery of reporter gene to the cochlea does not spread to brain tissue in an animal model. Acta Otolaryngol. 2010;130(1):25–30. doi: 10.3109/00016480902963053. [DOI] [PubMed] [Google Scholar]
- [10].Ouji Y, Ishizaka S, Nakamura-Uchiyama F, et al. In vitro differentiation of mouse embryonic stem cells into inner ear hair cell-like cells using stromal cell conditioned medium. Cell Death Dis. 2012;3:e314. doi: 10.1038/cddis.2012.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Liu Y, He P, Zhang M, et al. Silencing of the human SET gene in vitro with lentivirus-mediated RNA interference. Mol Med Rep. 2013;7(3):843–847. doi: 10.3892/mmr.2013.1275. [DOI] [PubMed] [Google Scholar]
- [12].Zhang N, Zhao L, Ma S, et al. Lentivirus-mediated expression of Drosophila melanogaster deoxyribonucleoside kinase driven by the hTERT promoter combined with gemcitabine: a potential strategy for cancer therapy. Int J Mol Med. 2012;30(3):659–665. doi: 10.3892/ijmm.2012.1033. [DOI] [PubMed] [Google Scholar]
- [13].Liu W, Huang ZF, Ye QF, et al. Transfusion of endothelial cells with lentivirus-mediated expression of fas ligand prolonged survival of rat liver allograft. Transplant Proc. 2012;44(5):1399–1403. doi: 10.1016/j.transproceed.2011.11.071. [DOI] [PubMed] [Google Scholar]
- [14].Cockrell AS, Kafri T. Gene delivery by lentivirus vectors. Mol Biotechnol. 2007;36(3):184–204. doi: 10.1007/s12033-007-0010-8. [DOI] [PubMed] [Google Scholar]
- [15].Li TT, Wang XD, Tian SQ, et al. Umbilical cord blood mesenchymal stem cells traced and transfected by recombinant lentivirus vector with enhanced green fluorescent protein for treatment of ischemic necrosis of the femoral head in rabbits. Zhongguo Zuzhi Gongcheng Yanjiu yu Lnchuang Kangfu. 2011;15(10):1897–1900. [Google Scholar]
- [16].Doiron B, Hu W, Norton L, et al. Lentivirus shRNA Grb10 targeting the pancreas induces apoptosis and improved glucose tolerance due to decreased plasma glucagon levels. Diabetologia. 2012;55(3):719–728. doi: 10.1007/s00125-011-2414-z. [DOI] [PubMed] [Google Scholar]
- [17].Akazawa C, Ishibashi M, Shimizu C, et al. A mammalian helix-loop-helix factor structurally related to the product of Drosophila proneural gene atonal is a positive transcriptional regulator expressed in the developing nervous system. J Biol Chem. 1995;270(15):8730–8738. doi: 10.1074/jbc.270.15.8730. [DOI] [PubMed] [Google Scholar]
- [18].Vetter ML, Brown NL. The role of basic helix-loop-helix genes in vertebrate retinogenesis. Semin Cell Dev Biol. 2001;12(6):491–498. doi: 10.1006/scdb.2001.0273. [DOI] [PubMed] [Google Scholar]
- [19].Bermingham NA, Hassan BA, Price SD, et al. Math1: an essential gene for the generation of inner ear hair cells. Science. 1999;284(5421):1837–1841. doi: 10.1126/science.284.5421.1837. [DOI] [PubMed] [Google Scholar]
- [20].Ben-Arie N, Bellen HJ, Armstrong DL, et al. Math1 is essential for genesis of cerebellar granule neurons. Nature. 1997;390(6656):169–172. doi: 10.1038/36579. [DOI] [PubMed] [Google Scholar]
- [21].Helms AW, Johnson JE. Progenitors of dorsal commissural interneurons are defined by MATH1 expression. Development. 1998;125(5):919–928. doi: 10.1242/dev.125.5.919. [DOI] [PubMed] [Google Scholar]
- [22].Bermingham NA, Hassan BA, Wang VY, et al. Proprioceptor pathway development is dependent on Math1. Neuron. 2001;30(2):411–422. doi: 10.1016/s0896-6273(01)00305-1. [DOI] [PubMed] [Google Scholar]
- [23].Van Keymeulen A, Mascre G, Youseff KK, et al. Epidermal progenitors give rise to Merkel cells during embryonic development and adult homeostasis. J Cell Biol. 2009;187(1):91–100. doi: 10.1083/jcb.200907080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Yang Q, Bermingham NA, Finegold MJ, et al. Requirement of Math1 for secretory cell lineage commitment in the mouse intestine. Science. 2001;294(5549):2155–2158. doi: 10.1126/science.1065718. [DOI] [PubMed] [Google Scholar]
- [25].Woods C, Montcouquiol M, Kelley MW. Math1 regulates development of the sensory epithelium in the mammalian cochlea. Nat Neurosci. 2004;7(12):1310–1318. doi: 10.1038/nn1349. [DOI] [PubMed] [Google Scholar]
- [26].Lewis RM, Hume CR, Stone JS. Atoh1 expression and function during auditory hair cell regeneration in post-hatch chickens. Hear Res. 2012;289(1-2):74–85. doi: 10.1016/j.heares.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Pan N, Jahan I, Kersigo J, et al. A novel Atoh1 “self-terminating” mouse model reveals the necessity of proper Atoh1 level and duration for hair cell differentiation and viability. PLoS One. 2012;7(1):e30358. doi: 10.1371/journal.pone.0030358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Liu Z, Dearman JA, Cox BC, et al. Age-dependent in vivo conversion of mouse cochlear pillar and Deiters’ cells to immature hair cells by Atoh1 ectopic expression. J Neurosci. 2012;32(19):6600–6610. doi: 10.1523/JNEUROSCI.0818-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kelly MC, Chang Q, Pan A, et al. Atoh1 directs the formation of sensory mosaics and induces cell proliferation in the postnatal mammalian cochlea in vivo. J Neurosci. 2012;32(19):6699–6710. doi: 10.1523/JNEUROSCI.5420-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kawamoto K, Ishimoto S, Minoda R, et al. Math1 gene transfer generates new cochlear hair cells in mature guinea pigs in vivo. J Neurosci. 2003;23(11):4395–4400. doi: 10.1523/JNEUROSCI.23-11-04395.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Pinilla M, Ramírez-Camacho R, Jorge E, et al. Ventral approach to the rat middle ear for otologic research. Otolaryngol Head Neck Surg. 2001;124(5):515–517. doi: 10.1067/mhn.2001.115370. [DOI] [PubMed] [Google Scholar]
- [32].Qiu J, Olivius P, Tong B, et al. Ventral approach to rat inner ear preserves cochlear function. Acta Otolaryngol. 2009;187(1):91–100. doi: 10.1080/00016480600818104. [DOI] [PubMed] [Google Scholar]
- [33].Bogaerts S, Douglas S, Corlette T, et al. J Neurosci Methods. 2008;168(1):156–163. doi: 10.1016/j.jneumeth.2007.09.016. [DOI] [PubMed] [Google Scholar]
- [34].Pan S, Fu Y, Liu J, et al. A study of a postauricular approach to rat cochlear scala media lateral wall for microinject lentivirus with EGFP. Chongqing Yixue. 2010;39(19):2593–2595. [Google Scholar]
- [35].Chen W, Yang SM, Guo W, et al. Cochlear Ad-EGFP expression by transferred gene through an intact round window membrane in guinea pigs. Zhonghua Erke Xue Zazhi. 2007;5(2):231–234. [Google Scholar]
- [36].Xu YJ, Hu YY, Zhai SQ, et al. The study of a new approach to postauricular microinjection via the round window membrane for cochlear gene transfection in mouse. Tingli Xue yu Yanyu Jibing Zazhi. 2009;17(3):279–282. [Google Scholar]
- [37].Fu Y, Wang S, Liu Y, et al. Study on neural stem cell transplantation into natural rat cochlea via round window. Am J Otolaryngol. 2009;30(1):8–16. doi: 10.1016/j.amjoto.2007.12.006. [DOI] [PubMed] [Google Scholar]
- [38].Dull T, Zufferey R, Kelly M, et al. A third-generation lentivirus vector with a conditional packaging system. J Virol. 1998;72(11):8463–8471. doi: 10.1128/jvi.72.11.8463-8471.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kalkanis JG, Whitworth C, Rybak LP. Vitamin E reduces cisplatin ototoxicity. Laryngoscope. 2004;114(3):538–542. doi: 10.1097/00005537-200403000-00028. [DOI] [PubMed] [Google Scholar]
- [40].Ding DL, Li M, Jiang SC. Harbin: Heilongjiang Science & Technology Press; 2001. Morphology of the Inner Ear. [Google Scholar]


