Abstract
This study aimed to explore the role of mechanical tension in hypertrophic scars and the change in nerve density using hematoxylin-eosin staining and S100 immunohistochemistry, and to observe the expression of nerve growth factor by western blot analysis. The results demonstrated that mechanical tension contributed to the formation of a hyperplastic scar in the back skin of rats, in conjunction with increases in both nerve density and nerve growth factor expression in the scar tissue. These experimental findings indicate that the cutaneous nervous system plays a role in hypertrophic scar formation caused by mechanical tension.
Keywords: neural regeneration, innervation, nerve growth factor, hypertrophic scar, keloid, mechanical tension, wound healing, surgery, grants-supported paper, neuroregeneration
Research Highlights
(1) Mechanical strain contributes to the formation of hyperplastic scar in wounds.
(2) Nerve density and expression of nerve growth factor are increased in hyperplastic scar tissue.
(3) The cutaneous nervous system is involved in mechanical strain-caused hypertrophic scar development.
INTRODUCTION
Skin is the major protective barrier between an animal and its environment, and its integrity is important for survival. Hypertrophic scarring is a fibroproliferative disease that occurs following deep skin injury, such as burns, abrasion injuries, and deep skin graft donor sites. These scars are frequently painful, reddish, firm, and itchy. They often have a profound negative impact on the quality of life of individuals affected. During the past several decades, many studies have been performed in an effort to determine the etiology of hypertrophic scarring[1,2,3,4]. Potential etiologies thought to underlie human hypertrophic scar formation include mechanical loading, nerve factors, inflammation, and foreign-body reactions[5,6,7,8]. Unfortunately, insight into the pathophysiology of hypertrophic scar formation has been hindered by the absence of a reliable animal model[9,10,11,12]. Aarabi et al[13] demonstrated for the first time that mechanical tension applied to a healing wound is sufficient to produce hypertrophic scars in mice. The striking similarity to human hypertrophic scars suggested that this murine model would be useful to investigate the pathophysiology of human hypertrophic scarring.
Mechanical stimuli have been shown to influence fibroblast processes, such as cell differentiation, proliferation, survival, and matrix production. Mechanical tension is the greatest causative factor of hypertrophic scarring[14,15,16,17,18]. For several years it has been known that the nerve system of skin plays a role in adult mammalian wound healing and scarring, perhaps via the biological effects of neuropeptides, including the proliferation of epithelial, vascular and connective tissue[19].
Traditionally, nerve growth factor is considered as a chemoattractant that participates in the regulation of cell proliferation, differentiation and myelination of neurons[20]. However, currently available data suggest that the physiological role of nerve growth factor in the organism is much wider[21]. Nerve growth factor is secreted and synthesized by a variety of cells, such as inflammatory and repair cells, and its biological effects are diverse and closely related to the process of wound repair. Nerve growth factor participates in the regulatory process of cell proliferation and plays an important role in tissue repair[22]. Nerve growth factor has been linked to hypertrophic scar and keloid formation in a number of different ways, with strong and persistent expression of nerve growth factor and its receptors shown to occur in fibroblasts of post-burn hypertrophic scars[23].
We hypothesized that nerve growth factor is one of the mediators connecting mechanical tension and hypertrophic scarring. The aim of this study was to explore the role of mechanical tension in hypertrophic scarring in mice by quantifying nerve density in scar tissue and then examining nerve growth factor expression.
RESULTS
Quantitative analysis of experimental animals
A total of 40 mice were initially included in the study, and were equally and randomly assigned to the mechanical forces or control groups, receiving mechanical tension on the back skin or no treatment, respectively. All 40 mice were included in the final analysis.
Histological observation of scars
At 2 weeks after injury, the murine scars in the mechanical forces group were raised and showed epidermal thickening with an absence of adnexal structures and hair follicles in the dermis, indicating significantly higher scar thickness. The control group developed very little fibrosis after 2 weeks (Figures 1A, B). At 5 weeks after injury, the murine scars in the mechanical forces group remained visible. The control wounds healed with almost no scarring (Figures 1C, D).
Figure 1.
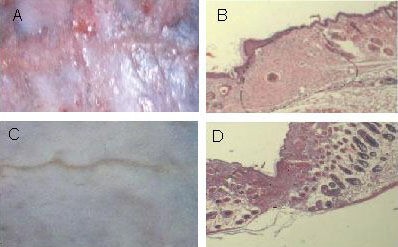
Histological observation of scars at 2 weeks post-injury.
Gross (A) and histological (B; hematoxylin-eosin staining, × 10) images showed that in the mechanical forces group, a loss of rete pegs, adnexae, and hair follicles, the classic histological features of human hypertrophic scars, were observed.
Gross (C) and histological (D; hematoxylin-eosin staining, × 10) images showed that very little fibrosis formed in the control group. The dashed lines in B and D show the scar tissue.
Nerve fibers in hypertrophic scars
At 2 weeks after injury, brown immunoreactive S100 positive nerve fibers were visible in the scar tissue layer in the mechanical forces group, with dot-, short bar- or beansprout-shaped morphologies (Figure 2). The nerve density was increased significantly compared with the control group.
Figure 2.
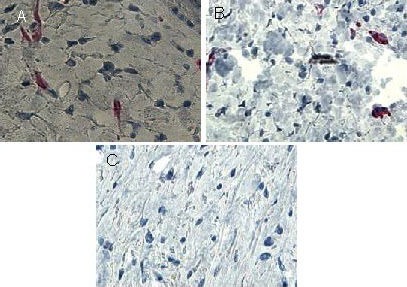
Immunoreactivity of S100 in skin scars after injury (immunohistochemical staining, × 400).
Brown immunoreactive S100 positive nerve fibers were visible in the scar tissue layer in the mechanical forces group at 2 weeks (A) and 5 weeks (B) post-injury, while they were nearly invisible in the scar tissue layer in the control group (C).
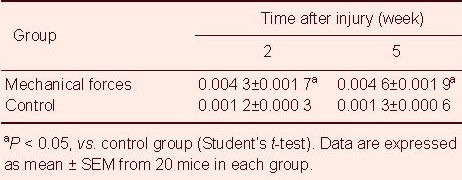
At 5 weeks after injury, the nerve density in the mechanical forces group remained significantly elevated compared with the control group. There were no significant differences in the nerve density between 2 weeks and 5 weeks post-injury in the mechanical forces group (Table 1).
Table 1.
Effect of mechanical tension on the nerve density (nerve number/total dermal area) in skin scar tissues
Nerve growth factor expression in skin scars
Western blot analysis was used to measure nerve growth factor production in the scars. At 2 weeks post-injury, nerve growth factor expression levels in the mechanical forces group were significantly upregulated compared with the control group. At 5 weeks post-injury, nerve growth factor levels in the mechanical forces group remained significantly elevated compared with the control. There were no significant differences in the nerve growth factor levels between 2 and 5 weeks post-injury in the mechanical forces group (Figure 3).
Figure 3.
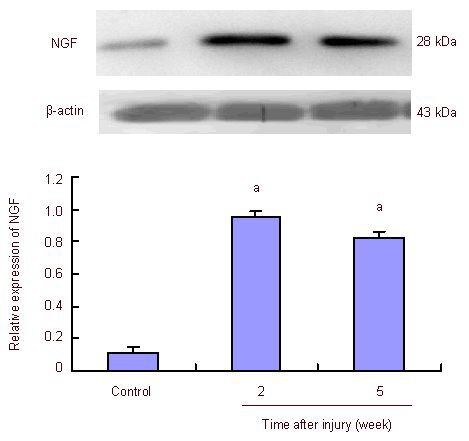
Effect of mechanical tension on the expression of nerve growth factor (NGF) in skin scars (western blot analysis).
aP < 0.01, vs. control group (Student's t-test). Data are expressed as mean ± SEM with absorbance ratio of NGF to β-actin.
DISCUSSION
In this study, we used murine models of hypertrophic scars that are grossly and histologically identical to human hypertrophic scars, produced by applying exogenous mechanical forces to healing murine wounds. Our experimental findings showed that the nerve density was increased in the scar tissue caused by mechanical tension. Our previous study suggested that compared with mature scars, hypertrophic scars exhibited a greater number of nerve fibers, with more serious pathologies[24]. These findings are consistent with the previous histological findings of increased nerve numbers in human hypertrophic scar samples from several investigators[25,26,27,28,29,30,31,32].
Furthermore, our study found that the expression levels of nerve growth factor in the scar tissue were increased by mechanical tension. Mechanical tension, including skin stretching, stimulates mechanosensitive nociceptors on sensory fibers in the skin. Stimulated fibers release neuropeptides and overexpressed nerve growth factor may induce the hyper-release of neuropeptides from sensory fibers, resulting in the accumulation of neuropeptides, even in the absence of mechanical tension, once the malignant cycle has begun[33,34,35,36,37,38,39,40].
In conclusion, nerve distribution in murine models of wound-healing mechanical tension-induced scars correlates well with human scars, thus providing additional evidence that this scar model may be valuable for understanding the pathophysiology of human hypertrophic scar formation.
MATERIALS AND METHODS
Design
A randomized, controlled, animal experiment.
Time and setting
The study was performed at the Laboratory Animal Center and Central Laboratory of Shandong Provincial Hospital Affiliated to Shandong University from November 2007 to June 2011.
Materials
A total of 40 clean, female C57BL/6 mice weighing 20 ± 5 g, 8 weeks old, were provided by the Laboratory Animal Center of Shandong University, China (license No. SCXK (Lu) 2003-0004). Animals received regular feeding and free water, with no adverse factors. Disposal of animals during the experiments was consistent with the Animal Ethics Committee of Shandong Provincial Hospital Affiliated to Shandong University, China.
Methods
Establishment of mouse scar models
Mice were anesthetized with intraperitoneal injection of 3% pentobarbital (1 mL/kg), and the back hair was removed. Murine models of hypertrophic scar were established according to Aarabi’s method[13] with some modifications. In brief, biomechanical loading devices were constructed from 22-mm expansion screws (Great Lakes Orthodontic Products, Tonawanda, NY, USA). A midline skin incision, approximately 2 cm long, was made on the dorsum of the mouse, which was subsequently closed with 6-0 nylon sutures. Four days later, the sutures were removed from the scars, and a loading device was carefully secured with 6-0 nylon sutures. The wound in the mechanical forces group was loaded every other day. Prior to applying tension, two points were identified on either side of the scars using a permanent marking pen. Tension on the wounds was created by carefully extracting the expansion screws by 2 mm on day 4 and 4 mm every other day thereafter. During the periods between extractions, stress relaxation was observed because of the natural elongation of skin, resulting in a continuous decrease in the force acting on the wounds. To compensate, tension was reapplied every other day up to 2 weeks. Scar tissue was harvested at 2 weeks after initiation of tension by carefully extracting the expansion screws.
The wound in the control group served as a control, in which the device was not activated (expansion screws were used without loading).
Collection of tissue samples
The backs of the animals were shaved prior to morphometric measurements. Scar tissue (0.5-cm in length) was harvested at 2 and 5 weeks after the initiation of tension, and then fixed in formalin, gradient ethanol dehydrated, paraffin-embedded, and sliced into 5-μm thick sections with a microtome (Leica CM3050S, Leica, Germany) for use in further experiments.
Hematoxylin-eosin staining
The sections were dewaxed using xylene and washed with alcohol and water, followed by hematoxylin staining for 5 minutes. Sections were washed with tap water, differentiated with hydrochloric acid for 30 seconds, soaked in tap water for 15 minutes, and stained with eosin for 2 minutes. Sections were then dehydrated, cleared and mounted. The microstructural changes in tissues were observed by microscopy (Type BX51, Olympus, Tokyo, Japan).
Detection of S100 immunoreactivity in the scar by immunohistochemistry
The specimens were embedded in paraffin and cut into 4-μm-thick sections for immunohistochemical staining. Tissue sections were deparaffinized and microwaved for antigen retrieval, followed by incubation in 3% H2O2 for 10 minutes. Nonspecific binding of antibodies was inhibited by incubation in 5% normal goat serum for 20 minutes in a humidified chamber. Sections were washed with PBS three times, and incubated with rabbit anti-S100 polyclonal antibody (1:100; Abcam, Cambridge, MA, USA) for 40 hours at 4°C. After three washes with PBS, tissue sections were incubated with biotinylated goat anti-rabbit IgG (1:1 000; Boster, Wuhan, Hubei Province, China) for 30 minutes at room temperature. After washing with PBS, slides were incubated in streptavidin-peroxidase complex for 30 minutes at 37°C, washed three times, visualized using diazoaminobenzene, and counterstained with hematoxylin. As a negative control, sections were stained without the addition of a primary antibody. Contents of S100 were analyzed using morphological analysis software (Leica Qwind Pro V3.3.1) to perform semi-quantitative analysis. All measurements were undertaken in a blinded manner.
Detection of nerve growth factor expression in the scar by western blot analysis
Total protein was extracted from scar tissue samples using tissue protein extraction reagent (Pierce, Rockford, IL, USA). Protein concentrations were determined using the Bradford method (Bio-Rad, Richmond, CA, USA). Protein extracts from the scar tissue (20 μg) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Blots were transferred to a nitrocellulose membrane (Bio-Rad), and blocked with blocking solution (3% bovine serum albumin in 1 Tween 20-Tris buffered saline, consisting of 20 mmol/L Tris HCl, pH 7.4, 150 mmol/L NaCl, and 0.1% Tween 20) for 2 hours at 4°C. Then, tissues were probed with rabbit anti-nerve growth factor polyclonal antibody (1:100; Abcam). Rabbit anti-mouse beta-actin antibody (mouse monoclonal; 1:5 000; Sigma-Aldrich, St. Louis, MO, USA) was used as an internal loading control. The blots were incubated overnight at 4°C with each antibody. Peroxidase goat anti-rabbit IgG (1:2 000; Santa Cruz Biotechnology, Santa Cruz, CA, USA) was used as a secondary antibody. Immunoreactivity was detected by enhanced chemiluminescence kit (Amersham Biosciences, Little Chalfont, Buckinghamshire, UK). Film autoradiograms were exposed for 10–30 minutes. The absorbance values of the target protein were measured using the image analysis program ‘Image J’ (Toronto Western Research Institute University Health Network, Toronto, ON, Canada). The absorbance ratio of target protein to internal reference was represented as the relative amount of target protein.
Statistical analysis
Data were analyzed by SPSS 10.0 statistical analysis software (SPSS, Chicago, IL, China), and experimental data were expressed as mean ± SEM. The differences between the two groups were analyzed by Student’s t-test. A value of P < 0.05 was considered significantly significant.
Footnotes
Hu Xiao, M.D., Associate chief physician.
Funding: This study was supported by the Shandong Excellent Young Scientist Research Award Fund of the Natural Science Foundation of Shandong Province, No. BS2009YY043; Shandong Medical and Health Science and Technology Development Program for Youth Fund, No. 2009QZ023; and the National Natural Science Foundation of China, No. 81272099.
Conflicts of interest: None declared.
Ethical approval: This research was approved by the Animal Ethics Committee of Shandong Provincial Hospital Affiliated to Shandong University, China.
(Reviewed by Wallace M, Norman C, Zhang GM, Ma R)
(Edited by Yang Y, Li CH, Song LP)
REFERENCES
- [1].Perry DM, McGrouther DA, Bayat A. Current tools for noninvasive objective assessment of skin scars. Plast Reconstr Surg. 2010;126(3):912–923. doi: 10.1097/PRS.0b013e3181e6046b. [DOI] [PubMed] [Google Scholar]
- [2].van der Veer WM, Bloemen MC, Ulrich MM, et al. Potential cellular and molecular causes of hypertrophic scar formation. Burns. 2009;35(1):15–29. doi: 10.1016/j.burns.2008.06.020. [DOI] [PubMed] [Google Scholar]
- [3].Bloemen MC, van der Veer WM, Ulrich MM, et al. Prevention and curative management of hypertrophic scar formation. Burns. 2009;35(4):463–475. doi: 10.1016/j.burns.2008.07.016. [DOI] [PubMed] [Google Scholar]
- [4].Ghahary A, Ghaffari A. Role of keratinocyte-fibroblast cross-talk in development of hypertrophic scar. Wound Repair Regen. 2007;15(Suppl 1):S46–53. doi: 10.1111/j.1524-475X.2007.00225.x. [DOI] [PubMed] [Google Scholar]
- [5].Moore ML, Dewey WS, Richard RL. Rehabilitation of the burned hand. Hand Clin. 2009;25(4):529–541. doi: 10.1016/j.hcl.2009.06.005. [DOI] [PubMed] [Google Scholar]
- [6].Schouten HJ, Nieuwenhuis MK, van Zuijlen PP. A review on static splinting therapy to prevent burn scar contracture: do clinical and experimental data warrant its clinical application? Burns. 2012;38(1):19–25. doi: 10.1016/j.burns.2011.06.003. [DOI] [PubMed] [Google Scholar]
- [7].Gabriel V. Hypertrophic scar. Phys Med Rehabil Clin N Am. 2011;22(2):301–310. doi: 10.1016/j.pmr.2011.02.002. [DOI] [PubMed] [Google Scholar]
- [8].Ramos ML, Gragnani A, Ferreira LM. Is there an ideal animal model to study hypertrophic scarring? J Burn Care Res. 2008;29(2):363–368. doi: 10.1097/BCR.0b013e3181667557. [DOI] [PubMed] [Google Scholar]
- [9].Henderson J, Ferguson MW, Terenghi G. The reinnervation and revascularization of wounds is temporarily altered after treatment with interleukin 10. Wound Repair Regen. 2011;19(2):268–273. doi: 10.1111/j.1524-475X.2011.00667.x. [DOI] [PubMed] [Google Scholar]
- [10].Anzarut A, Olson J, Singh P, et al. The effectiveness of pressure garment therapy for the prevention of abnormal scarring after burn injury: a meta-analysis. J Plast Reconstr Aesthet Surg. 2009;62(1):77–84. doi: 10.1016/j.bjps.2007.10.052. [DOI] [PubMed] [Google Scholar]
- [11].Feng YQ, Li X, Zhang R, et al. Remodeling of skin nerve fibers during burn wound healing. Neural Regen Res. 2010;5(19):1515–1520. [Google Scholar]
- [12].Yagmur C, Akaishi S, Ogawa R, et al. Mechanical receptor-related mechanisms in scar management: a review and hypothesis. Plast Reconstr Surg. 2010;126(2):426–434. doi: 10.1097/PRS.0b013e3181df715d. [DOI] [PubMed] [Google Scholar]
- [13].Aarabi S, Bhatt KA, Shi Y, et al. Mechanical load initiates hypertrophic scar formation through decreased cellular apoptosis. FASEB J. 2007;21(12):3250–3261. doi: 10.1096/fj.07-8218com. [DOI] [PubMed] [Google Scholar]
- [14].Ward RS, Tuckett RP, English KB, et al. Substance P axons and sensory threshold increase in burn-graft human skin. J Surg Res. 2004;118(2):154–160. doi: 10.1016/S0022-4804(03)00350-0. [DOI] [PubMed] [Google Scholar]
- [15].Henderson J, Ferguson MW, Terenghi G. The reinnervation pattern of wounds and scars after treatment with transforming growth factor β isoforms. J Plast Reconstr Aesthet Surg. 2012;65(4):e80–86. doi: 10.1016/j.bjps.2011.12.013. [DOI] [PubMed] [Google Scholar]
- [16].Yagmur C, Guneren E, Kefeli M, et al. The effect of surgical denervation on prevention of excessive dermal scarring: a study on rabbit ear hypertrophic scar model. J Plast Reconstr Aesthet Surg. 2011;64(10):1359–1365. doi: 10.1016/j.bjps.2011.04.028. [DOI] [PubMed] [Google Scholar]
- [17].Citak M, Backhaus M, Meindl R, et al. Rare complication after VAC-therapy in the treatment of deep sore ulcers in a paraplegic patient. Arch Orthop Trauma Surg. 2010;130(12):1511–1514. doi: 10.1007/s00402-010-1091-6. [DOI] [PubMed] [Google Scholar]
- [18].Elsharawy MA, Naim M, Greish S. Human CD34+ stem cells promote healing of diabetic foot ulcers in rats. Interact Cardiovasc Thorac Surg. 2012;14(3):288–293. doi: 10.1093/icvts/ivr068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Erba P, Wettstein R, Tolnay M, et al. Neurocutaneous sural flap in paraplegic patients. J Plast Reconstr Aesthet Surg. 2009;62(8):1094–1098. doi: 10.1016/j.bjps.2008.02.010. [DOI] [PubMed] [Google Scholar]
- [20].Zhang LQ, Laato M. Innervation of normal and hypertrophic human scars and experimental wounds in the rat. Ann Chir Gynaecol. 2001;90(Suppl 215):29–32. [PubMed] [Google Scholar]
- [21].Altun V, Hakvoort TE, van Zuijlen PP, et al. Nerve outgrowth and neuropeptide expression during the remodeling of human burn wound scars. A 7-month follow-up study of 22 patients. Burns. 2001;27:717–722. doi: 10.1016/s0305-4179(01)00026-2. [DOI] [PubMed] [Google Scholar]
- [22].Paterno J, Vial IN, Wong VW, et al. Akt-mediated mechanotransduction in murine fibroblasts during hypertrophic scar formation. Wound Repair Regen. 2011;19(1):49–58. doi: 10.1111/j.1524-475X.2010.00643.x. [DOI] [PubMed] [Google Scholar]
- [23].Micera A, Lambiase A, Stampachiacchiere B, et al. Nerve growth factor and tissue repair remodeling: trkA(NGFR) and p75(NTR), two receptors one fate. Cytokine Growth Factor Rev. 2007;18(3-4):245–256. doi: 10.1016/j.cytogfr.2007.04.004. [DOI] [PubMed] [Google Scholar]
- [24].Derderian CA, Bastidas N, Lerman OZ, et al. Mechanical strain alters gene expression in an in vitro model of hypertrophic scarring. Ann Plast Surg. 2005;55(1):69–75. doi: 10.1097/01.sap.0000168160.86221.e9. [DOI] [PubMed] [Google Scholar]
- [25].Ogawa R. Mechanobiology of scarring. Wound Repair Regen. 2011;19(Suppl 1):s2–9. doi: 10.1111/j.1524-475X.2011.00707.x. [DOI] [PubMed] [Google Scholar]
- [26].Kawamoto K, Matsuda H. Nerve growth factor and wound healing. Prog Brain Res. 2004;146:369–384. doi: 10.1016/S0079-6123(03)46023-8. [DOI] [PubMed] [Google Scholar]
- [27].Wang YB, Li X, Zhang R, et al. Quantitative and morphological differences of nerve fibers between proliferative and mature scars in two- and three-dimensional spaces. Neural Regen Res. 2010;5(2):132–137. [Google Scholar]
- [28].Henderson G, Terenghi DA, McGrouther D, et al. The reinnervation pattern of wounds and scars may explain their sensory symptoms. J Plast Reconstr Aesthet Surg. 2006;9:942–950. doi: 10.1016/j.bjps.2005.11.038. [DOI] [PubMed] [Google Scholar]
- [29].Akaishi S, Ogawa R, Hyakusoku H. Keloid and hypertrophic scar: neurogenic inflammation hypotheses. Med Hypotheses. 2008;71(1):32–38. doi: 10.1016/j.mehy.2008.01.032. [DOI] [PubMed] [Google Scholar]
- [30].Scott JR, Muangman P, Gibran NS. Making sense of hypertrophic scar: a role for nerves. Wound Repair Regen. 2007;15(Suppl 1):S27–31. doi: 10.1111/j.1524-475X.2007.00222.x. [DOI] [PubMed] [Google Scholar]
- [31].Scott JR, Muangman PR, Tamura RN, et al. Substance P levels and neutral endopeptidase activity in acute burn wounds and hypertrophic scar. Plast Reconstr Surg. 2005;115(4):1095–1102. doi: 10.1097/01.prs.0000156151.54042.da. [DOI] [PubMed] [Google Scholar]
- [32].Liang Z, Engrav LH, Muangman P, et al. Nerve quantification in female red Duroc pig (FRDP) scar compared to human hypertrophic scar. Burns. 2004;30(1):57–64. doi: 10.1016/j.burns.2003.09.004. [DOI] [PubMed] [Google Scholar]
- [33].Liu AT, Lin Q, Jiang H, et al. Facial reanimation by one-stage microneurovascular free abductor hallucis muscle transplantation: personal experience and long-term outcomes. Plast Reconstr Surg. 2012;130(2):325–335. doi: 10.1097/PRS.0b013e3182589d27. [DOI] [PubMed] [Google Scholar]
- [34].Isoardo G, Stella M, Cocito D, et al. Neuropathic pain in post-burn hypertrophic scars: a psychophysical and neurophysiological study. Muscle Nerve. 2012;45(6):883–890. doi: 10.1002/mus.23259. [DOI] [PubMed] [Google Scholar]
- [35].Tan J, Peng X, Luo G, et al. Investigating the role of P311 in the hypertrophic scar. PLoS One. 2010;5(4):e9995. doi: 10.1371/journal.pone.0009995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Wong VW, Paterno J, Sorkin M, et al. Mechanical force prolongs acute inflammation via T-cell-dependent pathways during scar formation. FASEB J. 2011;25(12):4498–4510. doi: 10.1096/fj.10-178087. [DOI] [PubMed] [Google Scholar]
- [37].Sarrazy V, Billet F, Micallef L, et al. Mechanisms of pathological scarring: role of myofibroblasts and current developments. Wound Repair Regen. 2011;19(Suppl 1):s10–15. doi: 10.1111/j.1524-475X.2011.00708.x. [DOI] [PubMed] [Google Scholar]
- [38].Gurtner GC, Dauskardt RH, Wong VW, et al. Improving cutaneous scar formation by controlling the mechanical environment: large animal and phase I studies. Ann Surg. 2011;254(2):217–225. doi: 10.1097/SLA.0b013e318220b159. [DOI] [PubMed] [Google Scholar]
- [39].Miyamoto J, Nagasao T, Miyamoto S, et al. Biomechanical analysis of stresses occurring in vertical and transverse scars on the lower leg. Plast Reconstr Surg. 2009;124(6):1974–1979. doi: 10.1097/PRS.0b013e3181bcf137. [DOI] [PubMed] [Google Scholar]
- [40].Junker JP, Kratz C, Tollbäck A, et al. Mechanical tension stimulates the transdifferentiation of fibroblasts into myofibroblasts in human burn scars. Burns. 2008;34(7):942–946. doi: 10.1016/j.burns.2008.01.010. [DOI] [PubMed] [Google Scholar]


