Abstract
Parkinson's disease has a negative impact on health-related quality of life in Parkinson's disease patients. Depression, cognitive impairment, coping strategies, dyskinesia, gait disorders and complications of dopaminergic drugs are the variables that most affect health-related quality of life. The ecological model of human development focuses attention on both individual and social environmental factors as targets for health interventions. From this perspective, the aim of this cross-sectional survey was to evaluate the influence of gender, family size and perceived autonomy on health-related quality of life in Parkinson's disease patients in northeastern Sicily, Italy. Ninety Parkinson's disease patients, attending the Movement Disorders Clinic at IRCCS Centro Neurolesi “Bonino-Pulejo” (Messina), were consecutively enrolled. The Unified Parkinson Disease Rating Scale motor subscale (UPDRS-III) scores, the Parkinson Disease Questionnaire-39 Item scores (as a disease-specific measure of health-related quality of life), scores on the Short Form (36) Health Survey Questionnaire (as a generic measure), and answers to a brief checklist were recorded. A total of 85 Parkinson's disease patients (49% males and 51% females; mean age 70.8 ± 8.6 years; mean UPDRS-III 24.15 ± 6.55; mean disease duration 5.52 ± 4.65 years) completed the booklet of questionnaires. In the multivariate regression analysis, we included clinical and social variables as independent predictors of health-related quality of life. Our results suggest a potential compounding effect of ecological intrapersonal and interpersonal levels on health-related quality of life outcomes. Gender, self-evaluated autonomy and family size significantly impacted health-related quality of life. If quality of life is used as an indicator of treatment outcomes, an ecological perspective of the case history will be important to disclose relevant prognostic information and trigger personalized health care interventions.
Keywords: neural regeneration, neurodegenerative disease, health-related quality of life, Parkinson’s disease, ecological model, Parkinson’s Disease Questionnaire-39 Items, social variables, the Unified Parkinson Disease Rating Scale motor subscales, caregiver, grants-supported paper
Research Highlights
(1) Parkinson’s disease has a negative impact on patients’ health-related quality of life.
(2) Within an ecological framework, intrapersonal and interpersonal aspects may have a compounding effect on health-related quality of life outcomes.
(3) Gender, self-evaluated autonomy and family size significantly impact health-related quality of life in people with Parkinson’s disease.
(4) An ecological framework is important for determining personalized health care interventions and improving medical decision making.
INTRODUCTION
Parkinson’s disease is the second most common chronic neurodegenerative disorder after Alzheimer’s disease, affecting more than 1 in 1 000 people in Europe[1,2]. The core symptoms are bradykinesia, rigidity, rest tremor and postural instability. However, Parkinson’s disease may involve not only physical ability but also cognitive, emotional and social domains with important direct and indirect costs for patients and their families[3,4,5,6,7,8,9,10,11,12].
In fact, Parkinson’s disease patients may experience non-motor symptoms, such as impairments in mood (especially depression and anxiety), cognition (selective deficits or dementia), orthostatic hypotension and other autonomic symptoms, such as sleep disturbances, fatigue and impulse control disorders[13,14,15,16,17,18,19,20,21,22,23,24,25,26,27]. The assessment of health-related quality of life is an important index with which to better understand the patient’s point of view about her/his health and to relieve the burden of disease[28,29,30,31,32,33,34].
Clinical determinants of health-related quality of life, such as age, disease severity, motor and non-motor symptoms, have been thoroughly investigated in previous studies[28,29,30,31,32,33,34,35,36,37,38,39,40,41]. Depression, anxiety, comorbidity, disability and complications of dopaminergic drugs are the variables that most affect the quality of life of Parkinson’s disease patients[35,36,37,38,39,40,41].
Other factors also affect health-related quality of life. Bronfenbrenner’s ecological model of human development, the theoretical framework used in this study, focuses attention on individual and social environmental factors as targets for health interventions[42,43,44]. The multiple levels of analysis include intrapersonal, interpersonal, institutional and community aspects. All of these levels can affect the course of the disease or the patient’s perception of quality of life[18,45,46,47,48,49].
This ecological framework can provide a better understanding of the implications of every neurodegenerative disease, such as Parkinson’s disease, for normal daily living of the patient and his/her family. The aim of the present study was to evaluate the effect of gender difference, family size and perceived autonomy on health-related quality of life in Parkinson’s disease patients from northeastern Sicily. Considered from an ecological perspective, these features may facilitate, sustain or modify perceived well-being.
RESULTS
Sociodemographic and clinical characteristics of Parkinson’s disease patients
Ninety Parkinson’s disease patients, attending the Movement Disorders Clinic at IRCCS Centro Neurolesi “Bonino-Pulejo” (Messina), were consecutively enrolled. Of the 90 recruited patients, five were excluded because their neuropsychological records were not complete. These five patients did not differ significantly from the others in terms of clinical and sociodemographic characteristics. A total of 85 Parkinson’s disease patients (49% males and 51% females; mean age 70.8 ± 8.6 years; mean Unified Parkinson Disease Rating Scale motor subscale (UPDRS-III) 24.2 ± 6.6; disease duration 5.5 ± 4.6 years) completed the assessments. Males were slightly younger than females (69.2 ± 7.7 vs. 72.3 ± 9.2 years old), while the mean disease duration was similar between males and females (5.88 ± 4.95 vs. 5.23 ± 4.43 years). Patients mostly lived at home with their own spouse (64%), while others lived alone (14%), several lived with their relatives (12%), and a small percentage cohabited with a professional carer (5%). Most patients considered themselves to be totally autonomous, and some (20%) needed help only outside their home. Among the 85 patients, 20% thought they sometimes needed help also at home and some patients (10%) felt they needed help all the time (Table 1).
Table 1.
Sociodemographic characterist (%) of 85 Parkinson's disease patients

Correlations between generic and specific disease measures of health-related quality of life in Parkinson’s disease patients
The subscale scores for the Parkinson’s Disease Questionnaire-39 Items (PDQ-39) and Short Form 36 Health Survey Questionnaire (SF-36) are shown in Table 2. The health-related quality of life reported by Parkinson’s disease patients was significantly lower than that of the healthy population[50].
Table 2.
Health-related quality of life scale scores of included Parkinson's disease patients

Physical disability assessed by UPDRS-III was correlated with perceived autonomy (rs = 0.26, P = 0.01). We analyzed the subscales of the generic measure of health-related quality of life, the SF-36, and the specific disease measure, the PDQ-39, and examined the correlation between them (Table 3).
Table 3.
Spearman correlations (rs) between PDQ-39 and SF-36 scores in Parkinson's disease patients

Comparisons of gender, autonomy and family size groups with respect to SF-36 and PDQ-39
We focused on three aspects: family size, autonomy and gender of patients. For each variable, we had growth of subgroups according to the answers given by Parkinson’s disease patients. Focusing on these variables, we used the Mann-Whitney U test to compare the patient’s subgroup (gender, autonomy, family size) with the SF-36 and PDQ-39 scores. The results of these comparisons are shown in Tables 4 and 5.
Table 4.
Gender, autonomy, and family size groups compared with respect to SF-36 scores
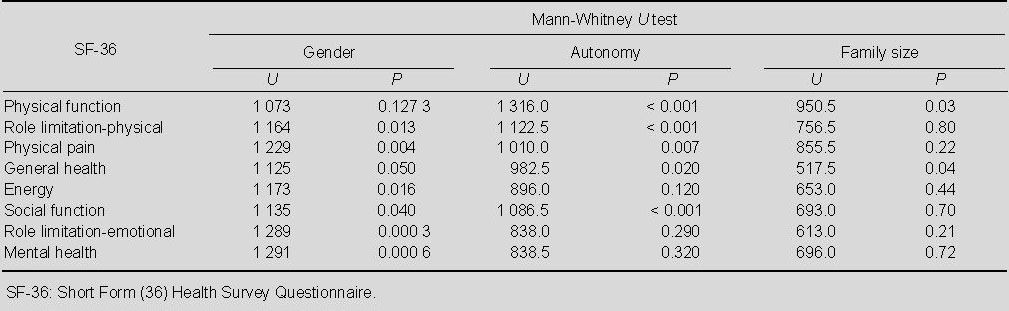
Table 5.
Gender, autonomy and family size groups compared with respect to PDQ-39 scores

Regression analysis
Table 6 shows the gender, age, UPDRS-III score, family size, and autonomy scores, which were found to be independent predictors of PDQ-39 subscale scores. In particular, gender was found to be an independent determinant of mobility. Age was found to be an independent determinant of stigma. Disease duration was found to be an independent determinant of mobility, activities of daily living, cognition, communication and bodily discomfort. Family size was found to be an independent determinant of activities of daily living.
Table 6.
Multivariate regression analysis of PDQ-39

Also, autonomy was found to be an independent determinant of mobility, activities of daily living and emotional well-being. Gender, disease duration and autonomy were identified as independent determinants of mobility and were able to explain 51.4% (adjusted R2) of the variance in this score. Disease duration, family size and autonomy explained 42.3% (adjusted R2) of the variance in activities of daily living scores.
Table 7 showed the same variables considered as potential determinant also of SF-36 subscale scores.
Table 7.
Multivariate regression analysis of SF-36

Gender was found to be an independent determinant of physical pain and role limitation-emotional and mental health. Disease duration was found to be an independent determinant of mobility, social function and role limitation-emotional. Family size was found to be an independent determinant of physical pain and general health. Autonomy was found to be an independent determinant of physical function, role limitation-physical, general health and social function. Comorbidity was found to be an independent determinant of role limitation-physical, physical pain, general health, energy and social function. Regarding SF-36 subscales, disease duration and autonomy explained 58% (adjusted R2) of the variance in physical function score.
DISCUSSION
The well-being of the Parkinson’s disease patients and their ability to perform occupational and social roles are important to better understand the personal and social implications of the disease and to improve treatments[51,52]. Motor and non-motor symptoms influence health-related quality of life in Parkinson’s disease patients[3,11,15,16,17,18,19,20,21,22,23,53]. In our sample, gender, family size and perceived autonomy were found to significantly affect health-related quality of life. In particular, gender differences significantly affected self-evaluation of mobility, emotional and psychological well-being. Family size influenced the perception of personal skills in everyday life and perceived general health. A previous study showed that marital status was not correlated with health-related quality of life[10]. In the current study, we focused on family size: if the patient lived with family member(s), and not only the spouse. Living with someone has positive implications for health-related quality of life in our sample.
The perception of pain varies significantly depending on gender differences and family size. In agreement with the literature, females experienced a worse quality of life than males[26,54]. Parkinson’s disease patients have a pragmatic idea about the impact of motor symptoms on their disability and the assessment of motor symptoms by medical practitioners agreed with the patient’s evaluation about their own autonomy in daily life. In contemporary society, autonomy is flaunted as a value and dependence is perceived as a weakness. Perceived autonomy was an independent predictor of social function, general health, physical and emotional well-being. This outcome, together with Kleiner-Fisman’s results[55], is an important reminder that loss of independence may be an important source of morbidity in individuals with Parkinson’s disease. In fact, self-evaluated autonomy is an important predictor of many aspects of health-related quality of life and a crucial aspect of disease course.
It is interesting to note that clinically evaluated mobility (UPDRS-III score) did not influence PDQ-39 and SF-36 subscales in our sample. Health-related quality of life, evaluated by selected measures, reflects the Parkinson’s disease patients’ point of view about their well-being and is influenced by personal and social aspects in everyday life.
These data show how the perception of quality of life is influenced not only by clinical symptoms of the disease but also “ecological” aspects such as gender, family size and perceived autonomy. If health-related quality of life is used as an indicator of treatment outcomes, the influence of these factors will have to be considered in the evaluation of clinical outcomes. The promotion of autonomy as a goal of a non-pharmacological treatment may increase the perceived well-being of the patient. Our results suggest a potential compounding effect of ecological intrapersonal and interpersonal levels on health-related quality of life and, in general, on the medical history.
Our data should be interpreted in the context of the limitations of this study. It is organized as a pragmatic research study and we cannot exclude the possibility of selection bias. The sample size is small and we studied only a few variables. In future, it would be useful to also study the social networks, annual income and other sociodemographic information about patients and caregivers to deepen our understanding of the influences of these factors at the community and institution level.
In conclusion, these data validate the importance of applying an ecological framework in clinical practice to better understand the implications of intrapersonal and interpersonal aspects on the clinical course of Parkinson’s disease. An ecological perspective in the care of Parkinson’s disease patients can disclose important prognostic information and help with planning individual and personalized care.
SUBJECTS AND METHODS
Design
A cross-sectional survey.
Time and setting
Subjects were recruited between November 2009 and March 2010 at the Movement Disorders Clinic at IRCCS Centro Neurolesi “Bonino-Pulejo”, Italy.
Subjects
Ninety Parkinson’s disease patients were enrolled in this cross-sectional survey. Inclusion criteria included clinical diagnosis of Parkinson’s disease (United Kingdom Parkinson’s Disease Society Brain Bank Criteria); Mini-Mental State Examination (MMSE[56]) > 24; and provision of informed consent. Suspected Parkinson’s disease was diagnosed by a neurology expert. Exclusion criteria were: (1) diagnosis of dementia according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM IV-TR[57]) criteria and MMSE < 24; (2) history of neurological disorders other than Parkinson’s disease; (3) evidence of significant psychiatric disorders, such as psychosis, depression and anxiety, according to DSM IV-TR; and (4) substance abuse. All patients were treated with levodopa and/or dopaminergic agonist. The clinical study was conducted in accordance with the Declaration of Helsinki (1964 and subsequent amendments)[58]. A total of 85 Parkinson’s disease patients completed the booklet of questionnaires.
Methods
Neurological and psychological assessments
Neurological and psychological assessments were performed in patients who were hospitalized. Disease severity was graded using the UPDRS-III[59] score. The subjects completed a booklet of questionnaires, which included the PDQ-39[60], as a disease-specific measure of subjective health status, the SF-36[50,61], as a generic measure[62], and an ecological variables checklist. This checklist included gender, age at time of symptom onset, comorbidity (intrapersonal level), and family size (interpersonal level). Every subject had to provide a judgment about self-autonomy. Autonomy is an important aspect of neurodegenerative diseases for the central physical and emotional burden on the patient and their family[63]. It provides a link between intrapersonal and interpersonal levels of analysis in the ecological perspective. All responses were coded on a Likert scale.
Statistical analysis
The software R 2.13 (http://cran.stat.unipd.it/) was used for statistical analysis. Correlations between data were analyzed by Spearman’s rank correlation. The t-test was used to compare if the data followed normal distribution (Kolmogorov-Smirnov test). If a normal distribution was not present, group comparisons were performed by means of the Mann-Whitney U test (two independent groups). In the multivariate regression analysis, the R2 method was used to explore the variability accounted for in independent predictors.
Footnotes
Funding: The research was supported by a grant from the Ministry of Health (Research for the Strategic Program 2007).
Conflicts of interest: None declared.
Ethical approval: The study was approved by the Ethical Committee of Istituto di Ricovero e Cura a Carattere Scientifico Centro Neurolesi “Bonino-Pulejo” via Provinciale Palermo, Messina, Italy.
(Reviewed by McGowan D, Raye Z, Dushanova J, Zhang B)
(Edited by Li CH, Song LP)
REFERENCES
- [1].Musicco M. Epidemiologia descrittiva e analitica della malattia di parkinson. Malattia di Parkinson e parkinsonismi. 2009:23–27. [Google Scholar]
- [2].von Campenhausen S, Bornschein B, Wick R, et al. Prevalence and incidence of Parkinson's disease in Europe. Eur Neuropsychopharmacol. 2005;15(4):473–490. doi: 10.1016/j.euroneuro.2005.04.007. [DOI] [PubMed] [Google Scholar]
- [3].Pohar SL, Allyson Jones C. The burden of Parkinson disease (PD) and concomitant comorbidities. Arch Gerontol Geriatr. 2009;49(2):317–321. doi: 10.1016/j.archger.2008.11.006. [DOI] [PubMed] [Google Scholar]
- [4].Rahman S, Griffin HJ, Quinn NP, et al. Quality of life in Parkinson's disease: The relative importance of the symptoms. Mov Disord. 2008;23(10):1428–1434. doi: 10.1002/mds.21667. [DOI] [PubMed] [Google Scholar]
- [5].Lindgren P, von Campenhausen S, Spottke E, et al. Cost of Parkinson's disease in Europe. Eur J Neurol. 2005;12(Suppl 1):68–73. doi: 10.1111/j.1468-1331.2005.01197.x. [DOI] [PubMed] [Google Scholar]
- [6].Morris ME, Watts JJ, Iansek R, et al. Quantifying the profile and progression of impairments, activity, participation, and quality of life in people with Parkinson disease: Protocol for a prospective cohort study. BMC Geriatr. 2009;9:2. doi: 10.1186/1471-2318-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].von Campenhausen S, Winter Y, Rodrigues e Silva A, et al. Costs of illness and care in Parkinson's disease: an evaluation in six countries. Eur Neuropsychopharmacol. 2011;21(2):180–191. doi: 10.1016/j.euroneuro.2010.08.002. [DOI] [PubMed] [Google Scholar]
- [8].Leroi I, McDonald K, Pantula H, et al. Cognitive impairment in Parkinson disease: impact on quality of life, disability, and caregiver burden. J Geriatr Psychiatry Neurol. 2012;25(4):208–214. doi: 10.1177/0891988712464823. [DOI] [PubMed] [Google Scholar]
- [9].Shin H, Youn J, Kim JS, et al. Caregiver burden in Parkinson disease with dementia compared to Alzheimer disease in Korea. J Geriatr Psychiatry Neurol. 2012;25(4):222–226. doi: 10.1177/0891988712464819. [DOI] [PubMed] [Google Scholar]
- [10].Kelly DH, McGinley JL, Huxham FE, et al. Health-related quality of life and strain in caregivers of Australians with Parkinson's disease: an observational study. BMC Neurol. 2012;12:57. doi: 10.1186/1471-2377-12-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Martinez-Martin P, Rodriguez-Blazquez C, Forjaz MJ. Quality of life and burden in caregivers for patients with Parkinson's disease: concepts, assessment and related factors. Expert Rev Pharmacoecon Outcomes Res. 2012;12(2):221–230. doi: 10.1586/erp.11.106. [DOI] [PubMed] [Google Scholar]
- [12].Bach JP, Riedel O, Klotsche J, et al. Impact of complications and comorbidities on treatment costs and health-related quality of life of patients with Parkinson's disease. J Neurol Sci. 2012;314(1-2):41–47. doi: 10.1016/j.jns.2011.11.002. [DOI] [PubMed] [Google Scholar]
- [13].Barone P, Antonini A, Colosimo C, et al. The PRIAMO study: A multicenter assessment of nonmotor symptoms and their impact on quality of life in Parkinson's disease. Mov Disord. 2009;24(11):1641–1649. doi: 10.1002/mds.22643. [DOI] [PubMed] [Google Scholar]
- [14].Chaudhuri KR, Odin P, Antonini A, et al. Parkinson's disease: the non-motor issues. Parkinsonism Relat Disord. 2011;17(10):717–723. doi: 10.1016/j.parkreldis.2011.02.018. [DOI] [PubMed] [Google Scholar]
- [15].Leroi I, Ahearn DJ, Andrews M, et al. Behavioural disorders, disability and quality of life in Parkinson's disease. Age Ageing. 2011;40(5):614–621. doi: 10.1093/ageing/afr078. [DOI] [PubMed] [Google Scholar]
- [16].Raggi A, Leonardi M, Carella F, et al. Impact of nonmotor symptoms on disability in patients with Parkinson's disease. Int J Rehabil Res. 2011;34(4):316–320. doi: 10.1097/MRR.0b013e32834d4b66. [DOI] [PubMed] [Google Scholar]
- [17].Adler CH. Premotor symptoms and early diagnosis of Parkinson's disease. Int J Neurosci. 2011;121(Suppl 2):3–8. doi: 10.3109/00207454.2011.620192. [DOI] [PubMed] [Google Scholar]
- [18].Miwa H, Miwa T. Fatigue in patients with Parkinson's disease: impact on quality of life. Intern Med. 2011;50(15):1553–1558. doi: 10.2169/internalmedicine.50.4954. [DOI] [PubMed] [Google Scholar]
- [19].Simuni T, Sethi K. Nonmotor manifestations of Parkinson's disease. Ann Neurol. 2008;64(Suppl 2):S65–80. doi: 10.1002/ana.21472. [DOI] [PubMed] [Google Scholar]
- [20].Tan LC. Mood disorders in Parkinson's disease. Parkinsonism Relat Disord. 2012;18(Suppl 1):S74–76. doi: 10.1016/S1353-8020(11)70024-4. [DOI] [PubMed] [Google Scholar]
- [21].Gallagher DA, Schrag A. Psychosis, apathy, depression and anxiety in Parkinson's disease. Neurobiol Dis. 2012;46(3):581–589. doi: 10.1016/j.nbd.2011.12.041. [DOI] [PubMed] [Google Scholar]
- [22].Kasten M, Kertelge L, Tadic V, et al. Depression and quality of life in monogenic compared to idiopathic, early-onset Parkinson's disease. Mov Disord. 2012;27(6):754–759. doi: 10.1002/mds.24999. [DOI] [PubMed] [Google Scholar]
- [23].Cupidi C, Realmuto S, Lo Coco G, et al. Sleep quality in caregivers of patients with Alzheimer's disease and Parkinson's disease and its relationship to quality of life. Int Psychogeriatr. 2012;24(11):1827–1835. doi: 10.1017/S1041610212001032. [DOI] [PubMed] [Google Scholar]
- [24].Storch A, Schneider CB, Wolz M, et al. Nonmotor fluctuations in Parkinson disease: Severity and correlation with motor complications. Neurology. 2013;80(9):800–809. doi: 10.1212/WNL.0b013e318285c0ed. [DOI] [PubMed] [Google Scholar]
- [25].Velseboer DC, Broeders M, Post B, et al. Prognostic factors of motor impairment, disability, and quality of life in newly diagnosed PD. Neurology. 2013;80(7):627–633. doi: 10.1212/WNL.0b013e318281cc99. [DOI] [PubMed] [Google Scholar]
- [26].Behari M, Srivastava AK, Pandey RM. Quality of life in patients with Parkinson's disease. Parkinsonism Relat Disord. 2005;11(4):221–226. doi: 10.1016/j.parkreldis.2004.12.005. [DOI] [PubMed] [Google Scholar]
- [27].Slawek J, Derejko M, Lass P. Factors affecting the quality of life of patients with idiopathic Parkinson's disease-a cross-sectional study in an outpatient clinic attendees. Parkinsonism Relat Disord. 2005;11(7):465–468. doi: 10.1016/j.parkreldis.2005.04.006. [DOI] [PubMed] [Google Scholar]
- [28].Reuther M, Spottke E, Klotsche J, et al. Assessing health-related quality of life in patients with Parkinson's disease in aprospective longitudinal study. Parkinsonism Relat Disord. 2007;13(2):108–114. doi: 10.1016/j.parkreldis.2006.07.009. [DOI] [PubMed] [Google Scholar]
- [29].Gomez-Esteban JC, Zarranz JJ, Lezcano E, et al. Influence of motor symptoms upon the quality of life of patients with Parkinson's disease. Eur Neurol. 2007;57(3):161–165. doi: 10.1159/000098468. [DOI] [PubMed] [Google Scholar]
- [30].Hinnell C, Hurt CS, Landau S, et al. Nonmotor versus motor symptoms: how much do they matter to health status in Parkinson's disease? Mov Disord. 2012;27(2):236–2341. doi: 10.1002/mds.23961. [DOI] [PubMed] [Google Scholar]
- [31].Opara JA, Brola W, Leonardi M, et al. Quality of life in Parkinson's disease. J Med Life. 2012;5(4):375–381. [PMC free article] [PubMed] [Google Scholar]
- [32].Uitti RJ. Treatment of Parkinson's disease: focus on quality of life issues. Parkinsonism Relat Disord. 2012;18(Suppl 1):S34–6. doi: 10.1016/S1353-8020(11)70013-X. [DOI] [PubMed] [Google Scholar]
- [33].Leonardi M, Raggi A, Pagani M, et al. Relationships between disability, quality of life and prevalence of nonmotor symptoms in Parkinson's disease. Parkinsonism Relat Disord. 2012;18(1):35–39. doi: 10.1016/j.parkreldis.2011.08.011. [DOI] [PubMed] [Google Scholar]
- [34].Shearer J, Green C, Counsell CE, et al. The impact of motor and non motor symptoms on health state values in newly diagnosed idiopathic Parkinson's disease. J Neurol. 2012;259(3):462–468. doi: 10.1007/s00415-011-6202-y. [DOI] [PubMed] [Google Scholar]
- [35].Carod-Artal FJ, Ziomkowski S, Mourão Mesquita H, et al. Anxiety and depression: Main determinants of health-related quality of life in brazilian patients with Parkinson's disease. Parkinsonism Relat Disord. 2008;14(2):102–108. doi: 10.1016/j.parkreldis.2007.06.011. [DOI] [PubMed] [Google Scholar]
- [36].Klepac N, Hajnsek S, Trkulja V. Impact of pre-morbid depression on health-related quality of life in non-demented Parkinson's disease patients. Parkinsonism Relat Disord. 2010;16(1):21–27. doi: 10.1016/j.parkreldis.2009.07.003. [DOI] [PubMed] [Google Scholar]
- [37].Visser M, van Rooden S, Verbaan D, et al. A comprehensive model of health-related quality of life in Parkinson's disease. J Neurol. 2008;255(10):1580–1587. doi: 10.1007/s00415-008-0994-4. [DOI] [PubMed] [Google Scholar]
- [38].Visser M, Verbran D, van Rooden S, et al. A longitudinal evaluation of health-related quality of life of patients with parkinson's disease. Value Health. 2009;12(2):392–396. doi: 10.1111/j.1524-4733.2008.00430.x. [DOI] [PubMed] [Google Scholar]
- [39].Qin Z, Zhang L, Sun F, et al. Health related quality of life in early parkinson's disease: Impact of motor and non-motor symptoms, results from chinese levodopa exposed cohort. Parkinsonism Relat Disord. 2009;15(10):767–771. doi: 10.1016/j.parkreldis.2009.05.011. [DOI] [PubMed] [Google Scholar]
- [40].Cubo E, Rojo A, Ramos S, et al. The importance of educational and psychological factors in parkinson's disease quality of life. Eur J Neurol. 2002;9(6):589–593. doi: 10.1046/j.1468-1331.2002.00484.x. [DOI] [PubMed] [Google Scholar]
- [41].Klepac N, Trkulja V, Relja M, et al. Is quality of life in non-demented Parkinson's disease patients related to cognitive performance? A clinic-based cross-sectional study. Eur J Neurol. 2008;15(2):128–133. doi: 10.1111/j.1468-1331.2007.02011.x. [DOI] [PubMed] [Google Scholar]
- [42].Bronfenbrenner U. Cambridge, MA: Harvard University Press; 1981. The Ecology of Human Development: Experiments by Nature and Design. [Google Scholar]
- [43].Ceci SJ. Urie Bronfenbrenner (1917-2005) Am Psychol. 2006;61(2):173–174. doi: 10.1037/0003-066X.61.2.173. [DOI] [PubMed] [Google Scholar]
- [44].Sallis JF, Owen N, Fisher EB. Ecological models of health behavior. In: Glanz K, Rimer BK, Viswanath K, editors. Health Behavior and Health Education: Theory, research, and practice. Hoboken, NJ: John Wiley & Sons; 2008. [Google Scholar]
- [45].Klepac N, Pikija S, Kraljic T, et al. Association of rural life setting and poorer quality of life in Parkinson's disease patients: A cross-sectional study in croatia. Eur J Neurol. 2007;14(2):194–198. doi: 10.1111/j.1468-1331.2006.01604.x. [DOI] [PubMed] [Google Scholar]
- [46].Winter Y, von Campenhausen S, Gasser J, et al. Social and clinical determinants of quality of life in Parkinson's disease in austria: A cohort study. J Neurol. 2010;257(4):638–645. doi: 10.1007/s00415-009-5389-7. [DOI] [PubMed] [Google Scholar]
- [47].Den Oudsten BL, Lucas-Carrasco R, Green AM, et al. Perceptions of persons with Parkinson's disease, family and professionals on quality of life: an international focus group study. Disabil Rehabil. 2011;33(25-26):2490–2508. doi: 10.3109/09638288.2011.575527. [DOI] [PubMed] [Google Scholar]
- [48].Mazanderani F, Locock L, Powell J. Being differently the same: the mediation of identity tensions in the sharing of illness experiences. Soc Sci Med. 2012;74(4):546–553. doi: 10.1016/j.socscimed.2011.10.036. [DOI] [PubMed] [Google Scholar]
- [49].Khlebtovsky A, Rigbi A, Melamed E, et al. Patient and caregiver perceptions of the social impact of advanced Parkinson's disease and dyskinesias. J Neural Transm. 2012;119(11):1367–1371. doi: 10.1007/s00702-012-0796-9. [DOI] [PubMed] [Google Scholar]
- [50].Apolone G, Mosconi P. The Italian SF-36 Health Survey: translation, validation and norming. J Clin Epidemiol. 1998;51(11):1025–1036. doi: 10.1016/s0895-4356(98)00094-8. [DOI] [PubMed] [Google Scholar]
- [51].Grosset D, Taurah L, Burn D, et al. A multicentre longitudinal observational study of changes in self reported health status in people with Parkinson's disease left untreated at diagnosis. J Neurol Neurosurg Psychiatry. 2007;78(5):465–469. doi: 10.1136/jnnp.2006.098327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Hagell P, Reimer J, Nyberg P. Whose quality of life? ethical implications in patient-reported health outcome measurement. Value Health. 2009;12(4):613–617. doi: 10.1111/j.1524-4733.2008.00488.x. [DOI] [PubMed] [Google Scholar]
- [53].Schrag A, Jahanshahi M, Quinn N. What contributes to quality of life in patients with Parkinson's disease? J Neurol Neurosurg Psychiatry. 2000;69(3):308–312. doi: 10.1136/jnnp.69.3.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Winter Y, von Campenhausen S, Gasser J, et al. Social and clinical determinants of quality of life in Parkinson's disease in austria: A cohort study. J Neurol. 2010;257(4):638–645. doi: 10.1007/s00415-009-5389-7. [DOI] [PubMed] [Google Scholar]
- [55].Kleiner-Fisman G, Stern MB, Fisman DN. Health-related quality of life in Parkinson disease: correlation between Health Utilities Index III and Unified Parkinson's Disease Rating Scale (UPDRS) in U.S. male veterans. Health Qual Life Outcomes. 2010;30(8):91. doi: 10.1186/1477-7525-8-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- [57].Andreoli V, Cassano G, Rossi R. DSM-IV. Manuale diagnostico e statistico dei disturbi mentali. Elsevier Srl. 2002 [Google Scholar]
- [58].World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. J Postgrad Med. 2002;48(3):206–208. [PubMed] [Google Scholar]
- [59].Martinez-Martin P, Gil-Nagel A, Gracia LM, et al. Unified Parkinson's disease rating scale characteristics and structure. Mov Disord. 1994;9(1):76–83. doi: 10.1002/mds.870090112. [DOI] [PubMed] [Google Scholar]
- [60].Peto V, Jenkinson C, Fitzpatrick R, et al. The development and validation of a short measure of functioning and well being for individuals with Parkinson's disease. Qual Life Res. 1995;4(3):241–248. doi: 10.1007/BF02260863. [DOI] [PubMed] [Google Scholar]
- [61].Ware J, Snow KK, Kosinski M, et al. Boston: The Health Institute, New England Medical Center; 1993. SF-36 health survey. Manual and interpretation guide. [Google Scholar]
- [62].Brown CA, Cheng EM, Hays RD, et al. SF-36 includes less Parkinson disease (PD)-targeted content but is more responsive to change than two PD-targeted health-related quality of life measures. Qual Life Res. 2009;18(9):1219–1237. doi: 10.1007/s11136-009-9530-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Hariz GM, Forsgren L. Activities of daily living and quality of life in persons with newly diagnosed Parkinson's disease according to subtype of disease, and in comparison to healthy controls. Acta Neurol Scand. 2011;123(1):20–27. doi: 10.1111/j.1600-0404.2010.01344.x. [DOI] [PubMed] [Google Scholar]


