Abstract
Adrenocorticotropic hormone is recommended worldwide as an initial therapy for infantile spasms. However, infantile spasms in about 50% of children cannot be fully controlled by adrenocorticotropic hormone monotherapy, seizures recur in 33% of patients who initially respond to adrenocorticotropic hormone monotherapy, and side effects are relatively common during adrenocorticotropic hormone treatment. Topiramate, vitamin B6, and immunoglobulin are effective in some children with infantile spasms. In the present study, we hypothesized that combined therapy with adrenocorticotropic hormone, topiramate, vitamin B6, and immunoglobulin would effectively treat infantile spasms and have mild adverse effects. Thus, 51 children newly diagnosed with West syndrome including infantile spasms were enrolled and underwent polytherapy with the four drugs. Electroencephalographic hypsarrhythmia was significantly improved in a majority of patients, and these patients were seizure-free, had mild side effects, and low recurrence rates. The overall rates of effective treatment and loss of seizures were significantly higher in cryptogenic children compared with symptomatic children. The mean time to loss of seizures in cryptogenic children was significantly shorter than in symptomatic patients. These findings indicate that initial polytherapy with adrenocorticotropic hormone, topiramate, vitamin B6, and immunoglobulin effectively improves the prognosis of infantile spasms, and its effects were superior in cryptogenic children to symptomatic children.
Keywords: neural regeneration, brain injury, West syndrome, spasm, etiology, polytherapy, adrenocorticotropic hormone, topiramate, vitamin B6, intravenous immunoglobulin, seizure-free, electroencephalograph, neuroregeneration
Research Highlights
(1) Initial polytherapy with adrenocorticotropic hormone, topiramate, vitamin B6 and immunoglobulin in 51 children newly diagnosed with infantile spasms significantly improved their rate of seizures and electroencephalographic hypsarrhythmia, with low relapse and no severe adverse effects.
(2) The effects of this polytherapy in cryptogenic children were superior to symptomatic children.
INTRODUCTION
West syndrome is an age-specific epilepsy syndrome characterized by flexor, extensor, and mixed flexor-extensor spasms, which often occur in clusters and start during the first 2 years of life[1]. Patients exhibit a characteristic chaotic and high-voltage interictal electroencephalography pattern, which is called hypsarrhythmia when it has its typical characteristics[2,3,4,5]. Patients with West syndrome have poor developmental outcome, even following present treatment. Overall 5–12% of patients have normal mental and motor development. Approximately 50% are left with motor impairments, and 70–78% are mentally retarded[6,7,8,9,10].
The treatment of infantile spasms remains very challenging. The effects of monotherapies using adrenocorticotropic hormone[11,12,13,14], corticosteroid prednisone[15,16], topiramate[17,18], intravenous immunoglobulin[19,20], and vitamin B6[21] have been evaluated in the United States, Japan, the United Kingdom, Europe and China.
Previous studies have suggested that these drugs are effective for the treatment of infantile spasms. However, a majority of spasms cannot be fully controlled in patients by monotherapies, and these spasms deteriorate mental functions. Moreover, adrenocorticotropic hormone can have fatal infectious adverse effects[22,23]. All of these drugs have different mechanisms of actions, and intravenous immunoglobulin can improve immune system responses to infection in addition to its anticonvulsant effect[24]. It is important to note that vigabatrin is unfortunately not yet commercially available in China. We hypothesize that polytherapy using drugs with different mechanisms as an initial treatment for infantile spasms would be effective and have few adverse effects. In the present study, we aimed to assess the efficacy and tolerability of polytherapy using adrenocorticotropic hormone, topiramate, vitamin B6, and immunoglobulin as the initial treatment for patients with newly diagnosed West syndrome that included infantile spasms.
RESULTS
Quantitative analysis of participants
A total of 51 children newly diagnosed with West syndrome who underwent the initial polytherapy were included. They were divided into cryptogenic (n = 39) and symptomatic (n = 12) groups according to their etiology. Polytherapy included intravenous adrenocorticotropic hormone followed by oral prednisone, oral topiramate, intravenous immunoglobulin, and intravenous vitamin B6. All children were included in the final analysis.
Baseline information
Our subjects included boys and girls with a gender ratio of 2:1. The age of subjects was 2–27 months when the spasms began, with a median age of 6.6 months. The median time from the initial spasms to initiating the polytherapy was 14.5 months. Flexor spasms were the most common type of spasms, and accounted for 82.2% of the spasms. The general characteristics of the subjects, including the types of spasms they exhibited, are listed in Table 1.
Table 1.
General characteristics of children with West syndrome

Etiologies of the patients with West syndrome
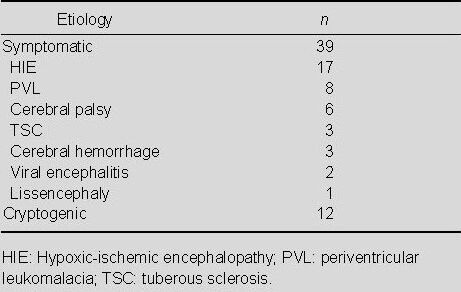
The etiologies of the 51 patients are shown in Table 2. Twelve (23.5%) patients had cryptogenic epilepsy. Among the 39 (76.5%) symptomatic subjects, hypoxic-ischemic encephalopathy was the most common underlying disease, followed by periventricular leukomalacia and cerebral palsy.
Table 2.
Etiologies of patients with West syndrome (n)
Efficacy of the polytherapy and electroencephalogram outcomes according to etiology
The efficacy of the polytherapy in children with West syndrome and electroencephalogram outcomes according to etiology are shown in Table 3 and Figure 1. Overall, 46 (90.2%) of the patients were spasm-free following the polytherapy, with two of the remaining children showing positive results and three not showing positive results. The total effective rate was 94.1%. Of the 39 symptomatic patients, 36 children were spasm-free following the polytherapy, with one of the remaining children showing positive results and two not showing positive results. Of the 12 cryptogenic patients, 10 children were spasm-free following the polytherapy, with one of the remaining children showing positive results and one not showing positive results.
Table 3.
Effectiveness of initial polytherapy in patients with West syndrome
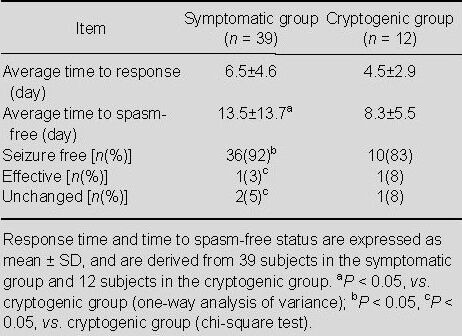
Figure 1.
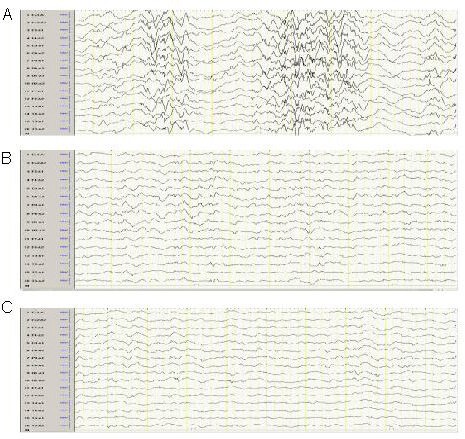
Electroencephalogram (EEG) outcomes of children with West syndrome after polytherapy.
(A) Representative EEG with demonstrated hypsarrhythmia in a subject with West syndrome.
(B) Representative EEG with spike and slow waves.
(C) Representative EEG with a normal background.
Accordingly, the effectiveness of this initial therapy in subjects newly diagnosed with West syndrome was better in cryptogenic than symptomatic subjects (P < 0.01). Moreover, the seizure-free rate was higher in cryptogenic than symptomatic subjects (P < 0.01; Table 3).
The time at which the subjects responded to the polytherapy was also recorded. Among the 48 responsive children, the fastest response time was 1 day, and the slowest response time was 23 days. Thirty-nine subjects appeared responsive within the first to the seventh day, and nine subjects responded after more than 7 days from the initiation of the polytherapy. Of the 37 responders with symptomatic West syndrome, the fastest response time was 2 days, and the slowest response time was 23 days. Within 1 week, 29 subjects showed positive results during the first week, and eight subjects responded after 1 week of polytherapy. Of the 12 cryptogenic subjects, 11 responded to the polytherapy. The fastest response time was 1 day, and the slowest responsive time was 11 days. Ten responders showed positive effects within 1 week, and only one subject required more than 1 week to respond. Although the response time in the cryptogenic group was shorter than in the symptomatic group, this difference was not statistically significant (P > 0.05; Table 3).
Of the 46 spasm-free patients, the fastest remission time was 1 day, and the longest time to achieve loss of spasms was 67 days. Cessation of spasms was reached within 1 week in 27 subjects. Eleven subjects achieved remission within the second week, and eight subjects achieved cessation of spasms over 15 days. The average time to loss of spasms was 12.3 days. Of the 36 symptomatic West syndrome patients who became spasm-free, the fastest time to remission was 5 days, and the longest time was 67 days. Of these subjects, 20 subjects achieved remission between 5 and 7 days, nine subjects within the second week, and seven subjects over 15 days. The average remission time in these subjects was 13.5 days. Of the 10 subjects with cryptogenic West syndrome who achieved remission, the fastest time to remission was 1 day, and the longest time 21 days. Seven subjects achieved remission within 1 week, two in the second week, and one over 15 days. The mean remission time was 8.3 days. The mean remission time was shorter in cryptogenic than symptomatic subjects (P < 0.05; Table 3).
Of the 46 patients who became spasm-free, hypsarrhythmia disappeared in 45 children, but not in one with lissencephaly. Normal 24-hour ambulatory electroencephalograms were achieved in 32 spasm-free children, whereas epileptic and slow spikes were seen in 14 spasm-free children. One spasm-free subject with lissencephaly retained hypsarrhythmia.
Adverse effects in children with West syndrome during polytherapy
A total of 33 (66%) patients exhibited adverse effects during the polytherapy (Table 4). These adverse effects included infections, electrolyte abnormalities, sleep disturbances, irritability, lethargy, and hypertension, and all were mild to moderate. Electrolyte abnormalities and infections were the most common adverse effects, and were especially prevalent during adrenocorticotropic hormone infusion. Infection was readily treated by antibiotics and/or antiviral drugs.
Table 4.
Adverse effects in children with West syndrome during polytherapy

Follow-up evaluations of spasm-free children with West syndrome following polytherapy
Of the 46 spasm-free subjects, three relapsed. All three had symptomatic West syndrome. The time to relapse ranged from 2 to 22 months. One subject was administered adrenocorticotropic hormone again, and became spasm-free. The other two refused further treatment. One of them was the subject with lissencephaly, who still had hypsarrhythmia after achieving a spasm-free status.
DISCUSSION
Although West syndrome was first reported more than 170 years ago, its management continues to pose many challenges to pediatric neurologists. Previous studies have surveyed the efficacy of drugs such as adrenocorticotropic hormone, vitamin B6, topiramate, and intravenous immunoglobulin. Adrenocorticotropic hormone has been widely used as an effective treatment for infantile spasms since 1958[25]. Outside China, the synthetic adrenocorticotropic hormone compound tetracosactide is frequently used. However, there has been no comparative study of tetracosactide and natural adrenocorticotropic hormone. Moreover, the form used in this study is the only animal-derived natural adrenocorticotropic hormone in China. Accordingly, in this study, we only discuss the efficacy of natural adrenocorticotropic hormone for West syndrome. Adrenocorticotropic hormone may reduce neuronal excitability in West syndrome by inducing steroid release or through a direct, steroid-independent action on melanocortin receptors. These combined effects may explain the robust, established clinical effects of adrenocorticotropic hormone in the therapy of West syndrome. Additionally, suppression of corticotropin-releasing hormone, an excitant neuropeptide, by adrenocorticotropic hormone/steroids has been proposed as another mechanism for adrenocorticotropic hormone treatment of infantile spasms[26]. However, data have shown that about 50% of patients are resistant to adrenocorticotropic hormone therapy. Moreover, adrenocorticotropic hormone should be used clinically with caution because of its potential side effects, such as severe infections, intracerebral hemorrhages, and potential lethality, especially at high doses[27].
Vitamin B6 is a coenzyme of glutamic acid decarboxylase and enhances gamma-aminobutyric acid synthesis. Low levels of gamma-aminobutyric acid in the cerebrospinal fluid of infants with infantile spasms have been reported. As such, vitamin B6 may improve gamma-aminobutyric acid levels in the cerebrospinal fluid of children with West syndrome. Vitamin B6 is the first clinical treatment of choice for infantile spasms in Japan[28]. Topiramate blocks voltage-sensitive sodium channels, enhances the activity of gamma-aminobutyric acid, blocks the action of glutamate, and is a weak carbonic anhydrates inhibitor[29]. Previous first-choice use or addition of topiramate therapy is effective in patients with infantile spasms. The use of intravenous immunoglobulin in intractable epilepsy is one of its oldest applications in medicine, starting from empirical observations of its beneficial effect on seizures. Immune system dysfunction may play a role in epilepsy by triggering, maintaining, or unexpectedly improving intractable seizures. Several laboratory and clinical investigations support an immunological basis for different forms of experimental and human epilepsies. A wide range of immune abnormalities have been reported, suggesting the existence of different subtypes of epileptic syndromes with different abnormalities of the immune system[30]. High-dose intravenous immunoglobulin treatment in West syndrome has proven effective in children with West syndrome. Considering possible interactions between their mechanisms, intravenous immunoglobulin may reduce the side effects of adrenocorticotropic hormone, especially for severe infections. Vitamin B6 may enhance the gamma-aminobutyric acid inhibitory effects of adrenocorticotropic hormone and topiramate may sustain a seizure-free status even after adrenocorticotropic hormone withdrawal. Accordingly, in the present study, we investigated the efficacy of initial polytherapy in children with West syndrome, and evaluated such spasm control, response time, time to loss of spasms, and electroencephalogram changes.
The results of our clinical trial show that the total rate of showing any positive effects was 94.1% (48/51) and the rate of loss of spasms was 90% (46/51) in the patients undergoing polytherapy. The American Academy of Neurology and the Child Neurology Society have supported the use of adrenocorticotropic hormone for the treatment of infantile spasms, though they did not specify the dose and duration of treatment[31]. Peltzer et al[32] found that six of 12 children treated with high-dose adrenocorticotropic hormone (88–180 U/M2 per day) achieved an elimination of their spasms within 1 month, but three later relapsed. Another study reported that nine children (47.4%) became seizure-free among 19 children with infantile spasms who received low-dose adrenocorticotropic hormone (25 U/day) for 3 weeks[33]. Hrachovy reported no statistical differences in efficacy with 50% responders in the high-dose group (150 IU/m2 per day for 3 weeks) and 58% response in the low-dose group (20 IU/day for 2 weeks)[34].
Currently, a relatively low-dose adrenocorticotropic hormone regime is extensively used clinically, which is why we chose to examine low-dose adrenocorticotropic hormone administration. Zou et al[35] conducted a prospective open study to assess the efficacy of topiramate as a first-choice drug in children with infantile spasms. Nine of the 54 patients (16.7%) were seizure-free for more than 24 months. A previous study investigated the efficacy of topiramate monotherapy for children newly diagnosed with West syndrome. In this study, four (22.1%) among 19 children eventually achieved seizure-free status over a treatment period of 0, 1, 8, or 69 months. Two uncontrolled prospective open-label studies of West syndrome showed that vitamin B6 is effective for infantile spasms. Monotherapy with vitamin B6 has eliminated spasms in 11% of patients[36,37]. Arrizumi et al[19] examined high-dose intravenous immunoglobulin therapy in early West syndrome. Six patients with cryptogenic West syndrome suffered from attacks over the previous 15 days to 6 months (mean 70 days) and five patients with symptomatic West syndrome suffered from attacks over the previous 14 days to 4 months (mean 32 days). All patients with cryptogenic West syndrome showed complete remission with intravenous immunoglobulin. Of the five patients with symptomatic West syndrome, one patient showed cessation of clinical seizures. In a prospective study, 23 children with infantile spasms received intravenous immunoglobulin at high doses. Complete normalization was obtained in five patients[38]. Compared with previous studies, the efficacy of our clinical investigation is superior to the previous monotherapies using four drugs for spasm control in children with West syndrome.
We also investigated the response time and time to elimination of spasms in detail. Such outcomes have not been extensively studied previously. The response time ranged from 1–23 days, and the time to elimination of spasms ranged from 1–67 days. Overwhelming responders achieved more than a 50% decrease in their spasm frequencies within a week, in both symptomatic and cryptogenic patients. If the patients did not respond to the drugs within 2 weeks, there was little chance they would subsequently respond to the polytherapy. A majority of patients achieved loss of spasms within a week, with some in the second week and later. No significant differences in the average time to respond were evident between the symptomatic and cryptogenic patients. However, the average time to spasm-free status in symptomatic patients was significantly longer than that in cryptogenic patients. These data suggest that spasms may be more difficult to control in symptomatic than cryptogenic patients, even though their spasm-free rates were not significantly different.
Hypsarrhythmia is a typical electroencephalogram pattern in children with West syndrome. Of the 46 patients who became spasm-free in the present study, hypsarrhythmia disappeared in 45 children, with the sole exception showing lissencephaly. Adrenocorticotropic hormone is effective for hypsarrhythmia in 21–23% of patients[39,40]. No data have been provided for the effectiveness of topiramate, vitamin B6, or intravenous immunoglobulin monotherapy on electroencephalogram changes in children with West syndrome. In the present study, approximately two thirds of the spasm-free patients achieved normal 24-hour ambulatory electroencephalograms. The rest showed epileptic spikes and slow spikes with normal backgrounds. Relapse was found in three symptomatic subjects of the 46 children who achieved elimination of their spasms after initial polytherapy, and the time to relapse was between 2–22 months after initiating the treatment. One subject who relapsed was treated with the polytherapy for a second time, and has thus far achieved remission. The other two subjects who relapse refused further treatment. Although a high rate of adverse effects have been found during the treatment of West syndrome, severe side effects such as septicemia were not evident in our subjects, and the infections that did occur were easily treated. This may be attributable to the use of intravenous immunoglobulin in our protocol. Moreover, this study also indicates that the efficacy of initial polytherapy is better in cryptogenic children than that in symptomatic subjects.
In all, the spasm-free rate obtained by initial polytherapy was higher than the rates reported for monotherapy using each of the four drugs that composed our polytherapy, namely adrenocorticotropic hormone, topiramate, vitamin B6, and intravenous immunoglobulin. The electroencephalogram changes were superior to those reported for monotherapy using adrenocorticotropic hormone. We propose that these high spasm-free rates and good electroencephalogram outcomes are related to synergistic effects between drugs with different mechanisms.
There are some shortcomings of this trial. The number of patients enrolled was not sufficiently large, and our design lacks appropriate controls. Furthermore, longer term outcomes should be determined carefully.
In summary, initial polytherapy with adrenocorticotropic hormone, topiramate, vitamin B6, and intravenous immunoglobulin in newly diagnosed subjects with West syndrome may be a worthwhile clinical choice. Further controlled studies are necessary to evaluate the benefit of this potentially effective treatment.
SUBJECTS AND METHODS
Design
Retrospective case analysis.
Time and setting
The study was performed at the Department of Pediatric Neurorehabilitation, First Hospital of Jilin University, China between December 2007 and April 2010.
Subjects
We retrospectively reviewed the medical records of 51 patients who were newly diagnosed with West syndrome and initially treated with corticosteroids (adrenocorticotropic hormone followed by prednisone), topiramate, intravenous immunoglobulin, and vitamin B6 between December 2007 and April 2010. The parents or caregivers of the subjects were informed of the study methods and clinical risks by the researchers. This study was conducted according to the Administrative Regulations for Medical Institutions issued by the State Council of China on September 1st, 1994[41].
The following criteria were used to diagnose West syndrome: (1) seizures characterized by axial muscle flexion, extension, or mixed spasms, (2) electroencephalogram recordings with demonstrated hypsarrhythmia and (3) an average of more than one cluster of spasms per day over 1 week before the start of polytherapy. This study included 34 male and 17 female subjects aged 3–18 months.
Methods
Drugs used for polytherapy and treatment protocol
Adrenocorticotropic hormone therapy was initiated by intravenous infusion over 6 hours per day at a dose of 25 IU. If a patient became spasm-free within 2 weeks, this regimen was continued for 2 more weeks and then tapered to 15 IU for 1 week. Next, adrenocorticotropic hormone (Shanghai No. 1 Biochemical Pharmaceutical Co., Ltd., Shanghai, China) was replaced by oral prednisone at a dose of 20 mg/d[34]. The prednisone was decreased by 5 mg every week until it was discontinued. If a patient did not achieve loss of spasms within 2 weeks, 40 IU of adrenocorticotropic hormone was used until the subject was spasm-free or for another 2 weeks. It was then tapered to 25 IU for 1 week and 15 IU for 1 week, and then the adrenocorticotropic hormone was replaced by prednisone as described above. In patients that did not exhibit loss of spasms with 40 IU of adrenocorticotropic hormone within 2 weeks, the adrenocorticotropic hormone was tapered to discontinuate as described above. Topiramate (Xi’an-Janssen Pharmaceutical Co., Ltd., Xi’an, China) was given at an initial dosage of 1 mg/kg per day with a rapid titration of 1 mg/kg per day until the subjects were receiving 5 mg/kg per day, if the patient became spasm-free within 5 days. A maintenance dosage of 10 mg/kg per day was administered if the spasms were not controlled within 5 days. Intravenous immunoglobulin (Chengdu Institute of Biological Products, Chengdu, China) was given for 5 days per month over 6 consecutive months at a dose of 0.4 g/kg per day. Vitamin B6 (Southwest Pharmaceutical Co., Ltd., Chongqing, China) was intravenously administered at a dose of 10 mg/kg per day for 10 consecutive days.
Informed consent and epilepsy diaries
To ensure that we obtained informed consent from the patients’ families, we provided them with detailed information regarding standard treatment options for West syndrome. We also provided the families with information on the efficacy and potential side effects of each drug used. Additionally, we educated them regarding their children’s spasms and asked them to maintain daily diaries to document the occurrence of the spasms.
Effectiveness of polytherapy for spasms and electroencephalogram changes and its adverse effects
Etiologies of 51 patients were analyzed and the underlying etiology was classified using the pediatric adaption of the International Statistical Classification of Disease and Related Health Problems 10th Revision. Patients were followed from the initial polytherapy. At 6 months after the initiation of polytherapy, we analyzed the effectiveness and tolerability of the drugs.
The evaluations of effectiveness were based on the frequency of spasms. Spasm-free status was defined as cessation of spasms over more than 1 week; the treatment was considered effective if the spasm frequency decreased to less than 50% of the baseline. The response time and time to spasm-free states were defined as the time from beginning the treatment to the time of effectiveness or the loss of spasms, respectively. These parameters were recorded and calculated as days for comparison between the etiologies. Twenty-four hour ambulatory electroencephalograms (Nihon Kohden, Tokyo, Japan) were recorded and analyzed after the subjects became spasm-free. Adverse effects were monitored and recorded.
We obtained blood and urine samples for routine laboratory examination during the study period. The routine laboratory examination included electroencephalogram, complete blood cell counts, electrolyte analysis, urinalysis, and assays for liver and renal function.
Statistical analysis
Response time and time to spasm cessation are expressed as mean ± SD. Normal distributions were verified using SPSS 13.0 software (SPSS, Chicago, IL, USA). Statistical differences in the response time and time to cessation of spasms were determined using one-way analysis of variance. The seizure-free rate was analyzed by chi-square test. A value of P < 0.05 was considered statistically significant.
Footnotes
Feiyong Jia, M.D., Associate professor, Master's supervisor.
Conflicts of interest: None declared.
Ethical approval: The study obtained full approval by the Medical Ethics Committee, the First Affiliated Hospital of Jilin University, China.
(Reviewed by Murnane K, Wysong S, Jiang B, Hu WL)
(Edited by Wang J, Su LL, Li CH, Song LP)
REFERENCES
- [1].Rho JM. Basic science behind the catastrophic epilepsies. Epilepsia. 2004;45(Suppl 5):5–11. doi: 10.1111/j.0013-9580.2004.05001.x. [DOI] [PubMed] [Google Scholar]
- [2].Malik MA, Tarrar MA, Qureshi AO, et al. Clinical spectrum of infantile spasm at presentation. J Coll Physicians Surg Pak. 2012;22(1):31–34. [PubMed] [Google Scholar]
- [3].Melani F, Mei D, Pisano T, et al. CDKL5 gene-related epileptic encephalopathy: electroclinical findings in the first year of life. Dev Med Child Neurol. 2011;53(4):354–360. doi: 10.1111/j.1469-8749.2010.03889.x. [DOI] [PubMed] [Google Scholar]
- [4].Kumaran A, Kar S, Kapoor RR, et al. The clinical problem of hyperinsulinemic hypoglycemia and resultant infantile spasms. Pediatrics. 2010;126(5):1231–1236. doi: 10.1542/peds.2009-2775. [DOI] [PubMed] [Google Scholar]
- [5].Halevy A, Kiviti S, Goldberg-Stern H, et al. Infantile spasms and modified hypsarrhythmia. Harefuah. 2011;150(4):373–377. [PubMed] [Google Scholar]
- [6].O’Callaghan FJ, Lux AL, Darke K, et al. The effect of lead time to treatment and of age of onset on developmental outcome at 4 years in infantile spasms: evidence from the United Kingdom Infantile Spasms Study. Epilepsia. 2011;52(7):1359–1364. doi: 10.1111/j.1528-1167.2011.03127.x. [DOI] [PubMed] [Google Scholar]
- [7].Perret EV, von Elm E, Lienert C, et al. Infantile spasms: does season influence onset and long-term outcome? Pediatr Neurol. 2010;43(2):92–96. doi: 10.1016/j.pediatrneurol.2010.03.006. [DOI] [PubMed] [Google Scholar]
- [8].Riikonen R. A long-term follow-up study of 214 children with the syndrome of infantile spasms. Neuropediatrics. 1982;13(1):14–23. doi: 10.1055/s-2008-1059590. [DOI] [PubMed] [Google Scholar]
- [9].Riikonen R. Long-term otucome of West syndrome: a study of adults with a history of infantile spasms. Epilepsia. 1996;37(4):367–372. doi: 10.1111/j.1528-1157.1996.tb00573.x. [DOI] [PubMed] [Google Scholar]
- [10].Sharma NL, Vishwanthan V. Outcome in West syndrome. Indian Pediatr. 2008;45(7):559–563. [PubMed] [Google Scholar]
- [11].Yang G, Zou LP, He B, et al. NR3C1 gene polymorphism for genetic susceptibility to infantile spasms in a Chinese population. Life Sci. 2012;91(1-2):37–43. doi: 10.1016/j.lfs.2012.06.010. [DOI] [PubMed] [Google Scholar]
- [12].Calcaterra V, Bottazzi A, Tzialla C, et al. Iatrogenic diabetes mellitus during ACTH therapy in an infant with West syndrome. Acta Diabetol. 2011;48(4):345–347. doi: 10.1007/s00592-011-0253-5. [DOI] [PubMed] [Google Scholar]
- [13].Fukuyama Y. The Japanese scheme of ACTH therapy in West syndrome. Epilepsia. 2010;51(10):2216–2218. doi: 10.1111/j.1528-1167.2010.02718.x. [DOI] [PubMed] [Google Scholar]
- [14].Wray CD, Benke TA. Effect of price increase of adrenocorticotropic hormone on treatment practices of infantile spasms. Pediatr Neurol. 2010;43(3):163–166. doi: 10.1016/j.pediatrneurol.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Verrotti A, Manco R, Coppola GG, et al. Update of the medical treatment of West syndrome. Minerva Pediatr. 2007;59(3):249–253. [PubMed] [Google Scholar]
- [16].Eidlitz-Markus T, Snir M, Kivity S, et al. Long-term follow-up for ophthalmologic sequelae in children treated with corticosteroids for infantile spasms. J Child Neurol. 2012;27(3):332–336. doi: 10.1177/0883073811420494. [DOI] [PubMed] [Google Scholar]
- [17].Lee GM, Lee KS, Lee EH, et al. Short term outcomes of topiramate monotherapy as a first-line treatment in newly diagnosed West syndrome. Korean J Pediatr. 2011;54(9):380–384. doi: 10.3345/kjp.2011.54.9.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Korinthenberg R, Schreiner A. Topiramate in children with west syndrome: a retrospective multicenter evaluation of 100 patients. J Child Neurol. 2007;22(3):302–306. doi: 10.1177/0883073807300535. [DOI] [PubMed] [Google Scholar]
- [19].Ariizumi M, Baba K, Hibio S, et al. Immunoglobulin therapy in the West syndrome. Brain Dev. 1987;9(4):422–425. doi: 10.1016/s0387-7604(87)80117-1. [DOI] [PubMed] [Google Scholar]
- [20].Mikati MA, Kurdi R, El-Khoury Z, et al. Intravenous immunoglobulin therapy in intractable childhood epilepsy: open-label study and review of the literature. Epilepsy Behav. 2010;17(1):90–94. doi: 10.1016/j.yebeh.2009.10.020. [DOI] [PubMed] [Google Scholar]
- [21].Imai Y, Yoshinaga H, Ishizaki Y, et al. Reappraisal of vitamin B6 therapy for West syndrome. No To Hattatsu. 2009;41(6):457–461. [PubMed] [Google Scholar]
- [22].Ohya T, Nagai T, Araki Y, et al. A pilot study on the changes in immunity after ACTH therapy in patients with West syndrome. Brain Dev. 2009;31(10):739–743. doi: 10.1016/j.braindev.2008.11.007. [DOI] [PubMed] [Google Scholar]
- [23].Partikian A, Mitchell WG. Major adverse events associated with treatment of infantile spasms. J Child Neurol. 2007;22(12):1360–1366. doi: 10.1177/0883073807310988. [DOI] [PubMed] [Google Scholar]
- [24].Geva-Dayan K, Shorer Z, Menascu S, et al. Immunoglobulin treatment for severe childhood epilepsy. Pediatr Neurol. 2012;46(6):375–381. doi: 10.1016/j.pediatrneurol.2012.03.015. [DOI] [PubMed] [Google Scholar]
- [25].Gupta R, Appleton R. Corticosteroids in the management of the paediatric epilepsies. Arch Dis Child. 2005;90(4):379–384. doi: 10.1136/adc.2004.051375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Stafstrom CE, Arnason BG, Baram TZ, et al. Treatment of infantile spasms: emerging insights from clinical and basic science perspectives. J Child Neurol. 2011;26(11):1411–421. doi: 10.1177/0883073811413129. [DOI] [PubMed] [Google Scholar]
- [27].Riikonen R, Donner M. ACTH therapy in infantile spasms: side effects. Arch Dis Child. 1980;55(9):664–672. doi: 10.1136/adc.55.9.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ito M, Mikawa H, Taniguchi T, et al. Cerebrospinal fluid GABA levels in children with infantile spasms. Neurology. 1984;34(2):235–238. doi: 10.1212/wnl.34.2.235. [DOI] [PubMed] [Google Scholar]
- [29].Verrotti A, Scaparrotta A, Agostinelli S, et al. Topiramate-induced weight loss: a review. Epilepsy Res. 2011;95(3):189–199. doi: 10.1016/j.eplepsyres.2011.05.014. [DOI] [PubMed] [Google Scholar]
- [30].Villani F, Avanzini G. The use of immunoglobulins in the treatment of human epilepsy. Neurol Sci. 2002;23(Suppl 1):33–37. doi: 10.1007/s100720200013. [DOI] [PubMed] [Google Scholar]
- [31].Mackay MT, Weiss SK, Adams-Webber T, et al. Practice parameter: medical treatment of infantile spasms: report of the American Academy of Neurology and the Child Neurology Society. Neurology. 2004;62(10):1668–1681. doi: 10.1212/01.wnl.0000127773.72699.c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Peltzer B, Alonso WD, Porter BE. Topiramate and adrenocorticotropic hormone (ACTH) as initial treatment for infantile spasms. J Child Neurol. 2009;24(4):400–405. doi: 10.1177/0883073808324538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Zou LP, Wang X, Dong CH, et al. Three-week combination treatment with ACTH + magnesium sulfate versus ACTH monotherapy for infantile spasms: a 24-week, randomized, open-label, follow-up study in China. Clin Ther. 2010;32(4):692–700. doi: 10.1016/j.clinthera.2010.04.008. [DOI] [PubMed] [Google Scholar]
- [34].Hrachovy RA, Frost JD, Jr, Glaze DG. (1994b) High-dose, long-duration versus low-dose, short-duration corticotropin therapy for infantile spasms. J Pediatr. 1994;124(5):803–806. doi: 10.1016/s0022-3476(05)81379-4. [DOI] [PubMed] [Google Scholar]
- [35].Zou LP, Ding CH, Fang F, et al. Prospective study of first-choice topiramate therapy in newly diagnosed infantile spasms. Clin Neuropharmacol. 2006;29(6):343–349. doi: 10.1097/01.WNF.0000236768.54150.8C. [DOI] [PubMed] [Google Scholar]
- [36].Ohtsuka Y, Matsuda M, Ogino T, et al. Treatment of the West syndrome with high-dose pyridoxal phosphate. Brain Dev. 1987;9(4):418–421. doi: 10.1016/s0387-7604(87)80116-x. [DOI] [PubMed] [Google Scholar]
- [37].Pietz J, Benninger C, Schafer H, et al. Treatment of infantile spasms with high-dosage vitamin B6. Epilepsia. 1993;34(4):757–763. doi: 10.1111/j.1528-1157.1993.tb00458.x. [DOI] [PubMed] [Google Scholar]
- [38].Echenne B, Dulac O, Parayre-Chanez MJ, et al. Treatment of infantile spasms with intravenous gamma-globulins. Brain Dev. 1991;13(5):313–319. doi: 10.1016/s0387-7604(12)80125-2. [DOI] [PubMed] [Google Scholar]
- [39].Yanagaki S, Oguni H, Hayashi K. A comparative study of high-dose and low-dose ACTH therapy for West syndrome. Brain Dev. 1999;21(7):461–467. doi: 10.1016/s0387-7604(99)00053-4. [DOI] [PubMed] [Google Scholar]
- [40].Hrachovy RA, Frost JD, Jr, Kellaway P, et al. Double-blind study of ACTH vs prednisone therapy in infantile spasms. J Pediatr. 1983;103(4):641–645. doi: 10.1016/s0022-3476(83)80606-4. [DOI] [PubMed] [Google Scholar]
- [41].Administrative Regulations on Medical Institution; 1994. State Council of the People's Republic of China. [Google Scholar]


