Abstract
Studies have shown that there are strong interactions between gustatory and visceral sensations in the central nervous system when rats ingest sweet foods or solutions. To investigate the role of the subdiaphragmatic vagi in transmitting general visceral information during the process of drinking sweet-tasting solutions, we examined the effects of subdiaphragmatic vagotomy on the intake of 0.5 mol/L sucrose, 0.005 mol/L saccharin or distilled water over the course of 1 hour in rats deprived of water. Results showed no significant difference in consumption of these three solutions in vagotomized rats. However, rats in the sham-surgery group drank more saccharin solution than sucrose solution or distilled water. Moreover, the intake of distilled water was similar between vagotomized rats and sham-surgery group rats, but significantly less sucrose and saccharin were consumed by vagotomized rats compared with rats in the sham-surgery group. These findings indicate that subdiaphragmatic vagotomy reduces intake of sweet-tasting solution in rats, and suggest that vagal and extravagal inputs play a balanced role in the control of the intake of sweet-tasting solutions. They also suggest that subdiaphragmatic vagotomy eliminates the difference in hedonic perception induced by sweet-tasting solutions compared with distilled water.
Keywords: neural regeneration, peripheral nerve injury, sweet taste, visceral sensation, vagotomy, nucleus of solitary tract, parabrachial nucleus, intake, hedonic, grants-supported paper, neuroregeneration
Research Highlights
(1) Previous studies in rats have focused on the effects of subdiaphragmatic vagotomy on the intake of solid foods, but not sweet-tasting solutions.
(2) This study highlights the influence of subdiaphragmatic vagotomy on the intake of sweet-tasting solutions such as sucrose and saccharin. Sucrose intake increased transmission of general visceral information, while saccharin intake reduced transmission.
(3) This study also investigated the influence of subdiaphragmatic vagotomy on body mass gain over a short period of time, in contrast with the long period of time observed in previous studies.
(4) The vagal and humoral pathways transmitting visceral information play a reciprocal-balancing role. Functional loss of the vagus nerve enhances negative feedback signals from the gastrointestinal tract and eliminates the differences in hedonic perception induced by sweet-tasting solutions compared with distilled water. In addition, subdiaphragmatic vagotomy has minimal effects on body mass gain over a short period of time.
INTRODUCTION
The sense of taste is an important oral chemical sense that plays a critical role in mammals. It not only strongly influences food and fluid intake, but also controls, to a large extent, the dietary choice of animals. When rats eat foods or drink liquids, tastants within the food or fluid act on the taste receptors of the tongue in the oral cavity. The taste information coming from the taste receptors is conducted to the rostral area of the nucleus of the solitary tract[1,2,3,4]. The gustatory information is further conducted into the higher nervous nuclei and gustatory cortex[5,6,7]. In this way, rats perceive the gustatory quality, strength, and hedonic properties of the foods or fluids, which are then swallowed, and digested and absorbed by the stomach and intestine. The actions of digestion and absorption result in the transmission of general visceral information[8]. Part of this visceral information is transmitted into the caudal area of the solitary tract nucleus via the vagal nerves[9,10,11]. The visceral information, processed initially in the nucleus of the solitary tract, then ascends to the higher level of the central nervous system along the visceral information-conducting pathway, which is parallel to the gustatory information-conducting pathway[12]. Another component of the visceral information ascends to the central nervous system through the humoral pathway[10]. Immunohistochemical studies have shown that visceral information and taste information produced by the intake of sweet solutions interact strongly in both the solitary tract nucleus and the parabrachial nucleus[13,14]. The gustatory information and the visceral information following food and liquid intake in the central nervous system can also negatively regulate food and liquid intake of rats[15]. Mordes et al[16] reported that the volume of food intake in rats was reduced after subdiaphragmatic vagotomy, and body weight gain was also less than that of control animals. In addition, rats with subdiaphragmatic vagotomy showed an increase in the intake of saccharin solution following the establishment of conditioned taste aversion to this solution[17]. These studies suggest that subdiaphragmatic vagotomy has an effect on food intake and psychological perception of gustatory solutions in rats.
Over the past few years, a number of studies on the effects of vagotomy on animals’ ingestion have focused on food intake and body weight gain[16,18,19,20,21]. Reports concerning the effects of vagotomy on the intake of gustatory solutions are few[22,23], particularly regarding the intake of sweet-tasting solutions. In the present study, we used sucrose and saccharin as sweet stimuli to investigate changes in the intake of sweet-tasting solutions in rats following total subdiaphragmatic vagotomy. We attempted to explore the role of the vagus nerve pathway, by which general visceral information is transmitted, in regulating the intake of these sweet solutions.
RESULTS
Quantitative analysis of experimental animals
Thirty-six rats were randomly assigned to one of two groups (n = 18): a vagotomy group (which underwent a total subdiaphragmatic vagotomy) and a sham-surgery group (which underwent sham operation) as a control. At the end of water intake training, the animals in each group were subdivided into three subgroups according to the type of gustatory solution: sucrose (0.5 mol/L), saccharin (0.005 mol/L) or distilled water, with six rats in each group. All 36 rats were included in the final analysis.
Subdiaphragmatic vagotomy reduced the intake of sweet-tasting solution in rats
The type of solution and the category of surgery were regarded as two factors, and the main effects of these two factors and their interaction on the solution intake in rats were analyzed using two-way analysis of variance. Results showed that the type of solution (F = 17.316, P < 0.001) and the category of surgery (F = 29.091, P < 0.001) affected fluid intake of the rats. The effect of their interaction on fluid intake was not significant (F = 3.057, P = 0.062).
We therefore further analyzed the main effects of the category of surgery on the solution intake by an independent sample t-test. Compared with sham-surgery rats, the consumption volume of 0.5 mol/L sucrose and 0.005 mol/L saccharin in vagotomized rats was significantly decreased (t = 2.73, P = 0.033 for sucrose; t = 4.82, P = 0.001 for saccharin). Subdiaphragmatic vagotomy reduced sucrose solution intake by 23.9% and saccharin solution intake by 30.5% during the 1-hour test. Comparison of distilled water intake in the 1-hour test showed that subdiaphragmatic vagotomy reduced distilled water intake by 13.4%, but this difference was not significant (t = 1.678, P = 0.124; Figure 1). These data show that subdiaphragmatic vagotomy affects the sweet-tasting solution intake of rats, but has minimal effect on distilled water intake.
Figure 1.
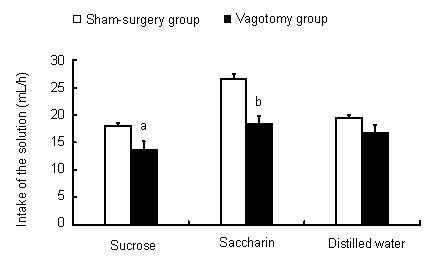
Comparison of solution intake (mL/hour) between vagotomy and sham-surgery groups for each solution.
The intake of each solution during a period of 1 hour was measured daily, and this measurement was continued for 7 days. The value is the average daily intake during this 1-hour period and is expressed as mean ± SEM of six animals for each group. aP < 0.05, bP < 0.01, vs. sham-surgery group using an independent sample t-test.
There was no significant difference in intake of sweet-tasting solutions or distilled water in rats undergoing subdiaphragmatic vagotomy
We also compared intake of the three different solutions within the vagotomy group or within the sham-surgery group. Results showed that intake of 0.005 mol/L saccharin was much greater than that of sucrose or distilled water in the sham-surgery group rats (saccharin vs. sucrose, t = 7.716, P < 0.01; saccharin vs. distilled water, t = 6.066, P < 0.01). For animals with intact vagal nerves, the consequence of ingestion of the two different sweet compounds was different. These animals preferred drinking saccharin solution to sucrose solution in response to thirst as a result of water restriction. Interestingly, there was no significant difference in the intake of sucrose, saccharin or distilled water in rats undergoing subdiaphragmatic vagotomy (F = 2.727, P = 0.098; Figure 2). This shows that the taste of the sweet compounds appears to have no regulatory function with respect to intake when there is a loss of transmission of general visceral information via the vagal nerves.
Figure 2.
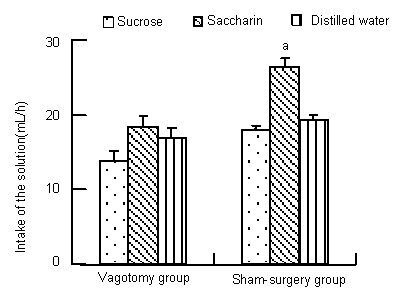
Effect of subdiaphragmatic vagotomy on the intake of three different solutions in rats.
The intake of each solution during a 1-hour period in rats undergoing subdiaphragmatic vagotomy and sham operation is shown. The intake of each solution was measured daily, and this measurement continued for 7 days. The values in the figure represent the average daily intake during this 1-hour period, and are expressed as mean ± SEM of six animals from each group. aP < 0.01, vs. sucrose and distilled water groups using one-way analysis of variance followed by Student’s t-test.
Effects of subdiaphragmatic vagotomy on the gain of body mass in rats
We recorded the body mass of the animals before and after the 1-week period of measuring fluid intake. The body mass of all animals was increased during the 1-week test period. One-way analysis of variance showed that there was no significant difference in the body mass between the groups before the test (F = 1.330, P = 0.278). There was also no difference between the groups after the 1-week test (F = 2.219, P = 0.078; Table 1). These data show that subdiaphragmatic vagotomy had no effect on body mass during a short-term behavioral test of sweet-tasting solution intake.
Table 1.
Effects of subdiaphragmatic vagotomy and sweet-tasting solution intake on body mass (g) in rats after a 1-week test

DISCUSSION
Ingestion of food or fluids by animals involves many sensory signals produced by various organs (tongue, laryngeal, epiglottis, esophagus, gastrointestinal tract, liver, and portal vein). Among these signals, taste information from the tongue and visceral information from the gastrointestinal tract (and parts of the portal vein of the liver) after swallowing of food and absorption of nutrients control the behaviors of the animals during food intake[24]. It is well known that subdiaphragmatic vagal nerves are an important pathway through which general visceral information following food intake is transmitted into the central nervous system[10]. Neuroanatomical studies[25,26,27,28] using anterograde tracing methods and electrophysiological studies[29,30,31,32] have shown that the main projection sites of the vagal nerves in the brain are the nuclei of the area postrema, the nucleus of the solitary tract, and the parabrachial nucleus which are also the projection sites of gustatory nerves. General visceral information is integrated and processed in the central nervous nuclei, which send commands to the peripheral system to rapidly regulate the subsequent ingestive behavior.
In addition, many experiments have shown that some of the general visceral information is also transmitted into the central nervous system through the sympathetic nervous system[32,33,34] and humoral pathways[10,35,36,37] in addition to the conducting pathway of parasympathetic nerves. In the gastrointestinal tract, food undergoes the action of digestion and absorption. The nutrient components of the foods are absorbed into the circulation to provide the body with nutrition and energy. The composition of the blood will therefore change, particularly the blood glucose concentration. For example, sweet-tasting stimulations elicit insulin release prior to increasing plasma glucose levels, a process called cephalic phase insulin release[38]. In addition, food intake not only changes the concentration of some circulating hormones, but also causes some organs to produce related gastrointestinal peptides that regulate ingestive behavior in a more long-term manner[18,19,24,39,40].
The ingestion of sweet-tasting solutions by an animal results in sweet taste information in the oral cavity. This information is transmitted into the gustatory central nervous nuclei through the gustatory afferent nerves. Furthermore, the action of digestion and absorption of these sweet compounds in the stomach and intestine causes the transmission of general visceral information into the central nervous system, largely through the vagal afferent neurons and humoral pathway. The ingestion of sweet solutions not only leads to perception of quantity of the sweet taste in the central region of the brain, but also causes hedonic psychological perception in rats[41,42,43]. Results from the present study showed that intake of sucrose and saccharin decreased following subdiaphragmatic vagotomy, whereas distilled water intake remained unchanged in the same conditions of vagotomy. The decreased intake of sweet solutions was not due to gastric distension or inhibition of gastric emptying caused by the vagotomy[16], because both vagotomized and sham-operated rats ingested the same amount of distilled water. The main reason for decreased intake of the sweet solutions is the loss of visceral signals transmitting to the central nervous system following their ingestion. Under normal conditions, these visceral signals transmitted by the vagal nerves will enhance the intake of sweet solutions. For instance, the signals of hedonic sensation which enhance food and liquid intake are lost after the subdiaphragmatic vagotomy. A microstructural analysis of licking behavior of rats with abdominal vagotomy showed that the decrease of the size of a milk meal was due to a more rapid decline in the rate of licking during the meal[22]. These results collectively indicate that there is an inhibitory effect of vagotomy on the intake of sweet solutions in rats. This result is consistent with data on the effects of vagotomy on food intake[16]. This previous study showed that vagotomy decreased the amount of food intake and reduced body mass gain in rats. The inhibition of food and fluid intake following vagotomy might be due to the enhanced strength of an extravagally-mediated negative feedback signal from the gastrointestinal tract[22]. It is known that negative feedback signals, which inhibit an animal’s food intake, are mediated by both the vagal and humoral pathways. The effect of vagotomy may contribute to the loss of a feeding signal carried by vagal afferent neurons, or to altered humoral signals, for example, increased production of a satiety hormone[20]. The vagal and humoral pathways transmitting visceral information thus play a reciprocal-balancing role in the control and guidance of ingestive behavior in animals. This is also a consequence of the interaction of visceral and taste information in the central nervous system (e.g. nucleus of the solitary tract, parabrachial nucleus and the central amygdala).
Interestingly, the sham-surgery group rats in this study drank more saccharin solution than either sucrose solution or distilled water, but there was no significant difference between intake of saccharin, sucrose or distilled water in rats in the vagotomy group. Sucrose and saccharin are commonly used as sweet compounds in research regarding sweet taste. Although normal rats experience the sweet sensation when they drink a solution of either of these two compounds, the perception of these two tastants is different. Saccharin is an artificial sweetener, and it results in little visceral information after consumption. In contrast, sucrose is a natural sweetener and it can generate much visceral information following intake[44,45,46]. The information processing of the central nervous system following ingestion of these two compounds is therefore different. Chen et al[47] reported that ingestion of sucrose induced a significant increase in c-Fos protein expression in the nucleus of the solitary tract and in the parabrachial nucleus compared with that in response to saccharin. This enhanced intensity of Fos expression is a response to the integrated effects of the sweet taste sensation and post-ingestive signals caused by ingestion of a sucrose solution. Some data also showed that the degree of preference to a sucrose concentration higher than 10% (about 0.3 mol/L) in rats was decreased[48]. This may be due to the “unhappy” feeling, and the level of satiety caused by drinking sucrose solution of a high concentration, which reduces further intake of this solution[49]. In contrast, the post-ingestive factors in response to saccharin intake are less than those of the sucrose intake. Post-ingestive factors induce transmission of general visceral information, most of which is conducted to the central nervous system by the vagal nerves. A subset of these factors is negative feedback signals which can lead to a reduction in further intake of the compound in question. All of these factors must be considered to explain why the rats drank more saccharin than either sucrose or distilled water. For the rats with intact vagal nerves, the strength of hedonic perception is greater for the 0.005 mol/L saccharin solution than for either 0.5 mol/L sucrose or distilled water. For the total subphragmatic vagotomized animals, the situation is different. The vagotomized animals drank similar amounts of 0.5 mol/L sucrose, 0.005 mol/L saccharin and distilled water. This indicates that although the animals still perceive the sweet taste after ingestion of sucrose and saccharin, as a result of vagotomy the sweet sensation is no longer a motivation to drive the thirsty rats to drink more fluid. Vagotomy thus eliminates the difference of hedonic perception caused by the sweet sensation of these fluids. This is consistent with an experiment conducted using food intake tests. When rats with vagotomy were administrated three types of food (food with the original taste sensation, food adulterating with saccharin, or food adulterated with quinine hydrochloride), the rats ingested the same amount of food[50], suggesting that food intake was no longer regulated by the gustatory information centers in the central nervous system, particularly the hedonic aspect of the food. It further suggests that the vagal nerves play an important role in the daily regulation of food and fluid intake.
We also measured body mass during this 1-week test in rats undergoing subdiaphragmatic vagotomy and those undergoing the sham operation. Our results showed that there was no difference in body mass between any of the groups before or after the 1-week behavioral test. This is consistent with previous experimental results[21] which showed that there was no significant difference in body mass change between a vagotomy group and control group from postoperative days 5 to 14. The influence of vagotomy on body mass gain may be a long-term effect. Mordes et al[16] found that vagotomized animals maintained a body weight 14–30% less than controls due to decreased food intake over the period from 30–300 days. In the present study, the recovery period after surgery is approximately two weeks; this may be the reason that we did not see a difference in body mass gain between the vagotomy and sham-surgery groups. A difference in body weight gain might be expected if we performed the behavioral intake test for a longer period of time.
In conclusion, intake of a sweet solution was not only related to the quality of the taste compounds (different compounds have differential properties of gustatory hedonic sensation), but also associated with the function of the vagal nerves. The loss of vagal nerve function inhibited the intake of sweet-tasting solutions through two essential factors. Dysfunction of the vagal nerves abolished the difference in hedonic perception of the different compounds, while vagotomy enhanced the strength of extravagally-mediated negative feedback signals from the intestine. The vagal nerves therefore play an important role in the gustatory psychological perception of the tastants and in the regulation of intake of sweet solutions.
MATERIALS AND METHODS
Design
A neurobehavioral study.
Time and setting
The experiments were performed at the laboratory in the Institute of Neurobiology, Henan University, China from December 2011 to May 2012.
Materials
Thirty-six 3-month-old male Sprague-Dawley rats, weighing 200–220 g, were provided by the Laboratory Animal Center of Henan University College of Medicine, China (license No. 08-016). The rats were housed separately in polycarbonate cages in a colony room except during recovery from surgery, when they were housed in stainless steel hanging wire cages. The rats were maintained in a room in 12-hour light: dark cycle at 20–25°C, which was automatically controlled. They were allowed free access to food and water. All the manipulations were performed during the light phase. The animals were housed in the above environments for 5 days prior to surgery. The protocols were performed in accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals, formulated by the Ministry of Science and Technology of China[51].
Methods
Subdiaphragmatic vagotomy
All animals were fasted for 12 hours before surgery. The rats were deeply anesthetized with an intraperitoneal injection of 2% sodium pentobarbital (50 mg/kg). For the rats receiving bilateral subdiaphragmatic vagotomy, the surgery was conducted according to a previously published method[16]. Briefly, a longitudinal midline incision was made, and the liver was exposed after the skin and muscle were opened. The left lobes of the liver were pulled out from the peritoneal cavity, held aside, and covered with saline gauze. The stomach was gently pushed aside toward the posterior of the animal to expose the esophagus. Under a surgical microscope (Shanghai Batuo Instrument Co., Ltd., Shanghai, China) with 20 × magnification, the left and right vagus nerves (located anterior and posterior to the esophagus) were separated. The left and right trunks were identified and transected immediately below the diaphragm, and a length of nerve of approximately 1 cm was excised.
For the rats receiving the sham operations, the surgical procedures were identical except that the vagus nerves were visualized and, rather than being transected, were subjected to gentle traction applied to their fascial investiture. The stomach and lobes of the liver were then pulled back to their original place. The abdomen was closed with absorbable sutures and the skin was closed with surgical sutures. The wound was sterilized and penicillin was injected. Rats were returned to their home cage and maintained on their daily diet.
Animal training
The rats were allowed to recover from the surgery for 14 days, followed by water intake training for 4 consecutive days[52]. Briefly, the rats were allowed free access to water from 10:00 until 15:00, and then the water bottle was removed from the cage for water deprivation. The animals were habituated to this water intake training for 4 days and there was no food restriction during this training.
Test procedures
Body mass was recorded at the end of water intake training. Each group of animals was semi-randomly divided into three subgroups according to the type of gustatory solution: 0.5 mol/L sucrose (Xi’an Chemical Reagent Factory, Xi’an, Shaanxi Province, China), 0.005 mol/L saccharin (Zhengzhou Reagent Factory, Zhengzhou, Henan Province, China) or distilled water. Each animal was given the appropriate solution for drinking from 10:00 until 11:00. The weight of the bottle was measured with an electrical balance (Shanghai Youzhongheng Electronic Co., Ltd., Shanghai, China) before and after being given to the rat. The amount of solution consumed during this 1-hour period was calculated. The bottle containing the test solution was replaced with a bottle containing water at 11:00. The animals were allowed free access to water until 15:00. The water bottles were removed from the cage for water deprivation until 10:00 the next morning. This procedure was conducted for 7 consecutive days. The average intake for each animal was calculated as the amount of solution consumed by the animal during this 1-hour period. The body mass of each animal was measured at the end of the experiment.
Statistical analysis
All data were expressed as mean ± SEM and analyzed using SPSS 13.0 software (SPSS, Chicago, IL, USA). The volume of solution intake was analyzed using a two-way group (between subjects) × solution (within subjects) analysis of variance to assess the main group and solution effects and their interaction. t-tests of independent samples were used to analyze the group differences for each of the three solutions. One-way analysis of variance was applied to detect differences in intake between vagotomy and sham-surgery groups, as well as to compare group differences in body mass before and after the 1-week behavioral test. The rejection criterion (e.g., alpha) for all statistical tests was set at the conventional value of 0.05.
Acknowledgments
We thank Dr. Jiexin Deng, School of Nursing, Henan University, China for providing language help and Peixi Wang, Institute of Public Hygiene, School of Nursing, Henan University, China for help with statistical analysis.
Footnotes
Enshe Jiang, Ph.D., Associate professor.
Funding: This work was financially supported by the National Natural Science Foundation of China, No. 81071029; and the Joint Funds of the Natural Science Foundation Committee with Henan Province Government for Fostering Talents, No. U1204809.
Conflicts of interest: None declared.
Ethical approval: This experiment was approved by the Animal Ethics Committee of Henan University, China.
(Reviewed by Barnett M, Stow A, Yuan TF, Tian ZZ)
(Edited by Wang LM, Su LL, Li CH, Song LP)
REFERENCES
- [1].Hamilton RB, Norgren R. Central projections of gustatory nerves in the rat. J Comp Neurol. 1984;222(4):560–577. doi: 10.1002/cne.902220408. [DOI] [PubMed] [Google Scholar]
- [2].Herrera DG, Robertson HA. Activation of c-fos in the brain. Prog Neurobiol. 1996;50(2-3):83–107. doi: 10.1016/s0301-0082(96)00021-4. [DOI] [PubMed] [Google Scholar]
- [3].Chen JY, Victor JD, Di Lorenzo PM. Temporal coding of intensity of NaCl and HCl in the nucleus of the solitary tract of the rat. J Neurophysiol. 2011;105(2):697–711. doi: 10.1152/jn.00539.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kwak Y, Rhyu MR, Bai SJ, et al. c-Fos expression in the nucleus of the solitary tract in response to salt stimulation in rats. Korean J Physiol Pharmacol. 2011;15(6):437–443. doi: 10.4196/kjpp.2011.15.6.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Lundy RF, Jr, Norgren R. Activity in the hypothalamus, amygdala, and cortex generates bilateral and convergent modulation of pontine gustatory neurons. J Neurophysiol. 2004;91(3):1143–1157. doi: 10.1152/jn.00840.2003. [DOI] [PubMed] [Google Scholar]
- [6].Li J, Chen K, Yan J, et al. Increased sucrose intake and corresponding c-Fos in amygdala and parabrachial nucleus of dietary obese rats. Neurosci Lett. 2012;525(2):111–116. doi: 10.1016/j.neulet.2012.07.053. [DOI] [PubMed] [Google Scholar]
- [7].Rosen AM, Victor JD, Di Lorenzo PM. Temporal coding of taste in the parabrachial nucleus of the pons of the rat. J Neurophysiol. 2011;105(4):1889–1896. doi: 10.1152/jn.00836.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Goehler LE, Finger TE. Functional organization of vagal reflex systems in the brain stem of the goldfish, Carassius auratus. J Comp Neurol. 1992;319(4):463–478. doi: 10.1002/cne.903190402. [DOI] [PubMed] [Google Scholar]
- [9].McDougal DH, Hermann GE, Rogers RC. Vagal afferent stimulation activates astrocytes in the nucleus of the solitary tract via AMPA receptors: evidence of an atypical neural-glial interaction in the brainstem. J Neurosci. 2011;31(39):14037–14045. doi: 10.1523/JNEUROSCI.2855-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Tome D, Schwarz J, Darcel N, et al. Protein, amino acids, vagus nerve signaling, and the brain. Am J Clin Nutr. 2009;90(3):838S–843S. doi: 10.3945/ajcn.2009.27462W. [DOI] [PubMed] [Google Scholar]
- [11].Schwartz GJ. Brainstem integrative function in the central nervous system control of food intake. Forum Nutr. 2010;63:141–51. doi: 10.1159/000264402. [DOI] [PubMed] [Google Scholar]
- [12].Rinaman L. Ascending projections from the caudal visceral nucleus of the solitary tract to brain regions involved in food intake and energy expenditure. Brain Res. 2010;1350:18–34. doi: 10.1016/j.brainres.2010.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Jiang ES, Yan JQ, Song XA. The effects of gustatory and visceral stimulation on c-Fos expression in parabrachial nucleus in rats. Xi’an Jiaotong Daxue Xuebao: Yixue Ban. 2003;24(1):20–23. [Google Scholar]
- [14].Jiang ES, Yan JQ, Song XA. The influence of gustatory and visceral stimulation on FOS expression in rat NST. Zhongguo Linchuang Kangfu. 2003;7(13):1898–1899. [Google Scholar]
- [15].Yamamoto T. Neural mechanisms of taste aversion learning. Neurosci Res. 1993;16(3):181–185. doi: 10.1016/0168-0102(93)90122-7. [DOI] [PubMed] [Google Scholar]
- [16].Mordes JP, el Lozy M, Herrera MG, et al. Effects of vagotomy with and without pyloroplasty on weight and food intake in rats. Am J Physiol. 1979;236(1):R61–66. doi: 10.1152/ajpregu.1979.236.1.R61. [DOI] [PubMed] [Google Scholar]
- [17].Kiefer SW, Rusiniak KW, Garcia J, et al. Vagotomy facilitates extinction of conditioned taste aversions in rats. J Comp Physiol Psychol. 1981;95(1):114–122. [PubMed] [Google Scholar]
- [18].Ladyman SR, Sapsford TJ, Grattan DR. Loss of acute satiety response to cholecystokinin in pregnant rats. J Neuroendocrinol. 2011;23(11):1091–1098. doi: 10.1111/j.1365-2826.2011.02191.x. [DOI] [PubMed] [Google Scholar]
- [19].deFonseka A, Kaunitz J. Gut sensing mechanisms. Curr Gastroenterol Rep. 2009;11(6):442–447. doi: 10.1007/s11894-009-0068-5. [DOI] [PubMed] [Google Scholar]
- [20].Furness JB, Koopmans HS, Robbins HL, et al. Effects of vagal and splanchnic section on food intake, weight, serum leptin and hypothalamic neuropeptide Y in rat. Auton Neurosci. 2001;92(1-2):28–36. doi: 10.1016/S1566-0702(01)00311-3. [DOI] [PubMed] [Google Scholar]
- [21].Kanno H, Kiyama T, Fujita I, et al. Vagotomy suppresses body weight gain in a rat model of gastric banding. Dig Surg. 2010;27(4):302–306. doi: 10.1159/000288701. [DOI] [PubMed] [Google Scholar]
- [22].Davis JD, Smith GP, Kung TM. Abdominal vagotomy alters the structure of the ingestive behavior of rats ingesting liquid diets. Behav Neurosci. 1994;108(4):767–779. [PubMed] [Google Scholar]
- [23].Radhakrishnan V, Sharma KN. Effect of selective gastric vagotomy on gustatory behavior in rats. J Auton Nerv Syst. 1986;16(2):127–136. doi: 10.1016/0165-1838(86)90004-4. [DOI] [PubMed] [Google Scholar]
- [24].Kitamura A, Torii K, Uneyama H, et al. Role played by afferent signals from olfactory, gustatory and gastrointestinal sensors in regulation of autonomic nerve activity. Biol Pharm Bull. 2010;33(11):1778–1782. doi: 10.1248/bpb.33.1778. [DOI] [PubMed] [Google Scholar]
- [25].Altschuler SM, Bao XM, Bieger D, et al. Viscerotopic representation of the upper alimentary tract in the rat: sensory ganglia and nuclei of the solitary and spinal trigeminal tracts. J Comp Neurol. 1989;283(2):248–268. doi: 10.1002/cne.902830207. [DOI] [PubMed] [Google Scholar]
- [26].Norgren R, Smith GP. Central distribution of subdiaphragmatic vagal branches in the rat. J Comp Neurol. 1988;273(2):207–223. doi: 10.1002/cne.902730206. [DOI] [PubMed] [Google Scholar]
- [27].Shapiro RE, Miselis RR. The central organization of the vagus nerve innervating the stomach of the rat. J Comp Neurol. 1985;238(4):473–488. doi: 10.1002/cne.902380411. [DOI] [PubMed] [Google Scholar]
- [28].Adachi A, Shimizu N, Oomura Y, et al. Convergence of hepatoportal glucose-sensitive afferent signals to glucose-sensitive units within the nucleus of the solitary tract. Neurosci Lett. 1984;46(2):215–218. doi: 10.1016/0304-3940(84)90444-0. [DOI] [PubMed] [Google Scholar]
- [29].Barber WD, Yuan CS. Brain stem responses to electrical stimulation of ventral vagal gastric fibers. Am J Physiol. 1989;257(1 Pt 1):G24–29. doi: 10.1152/ajpgi.1989.257.1.G24. [DOI] [PubMed] [Google Scholar]
- [30].Chambert G, Kobashi M, Adachi A. Convergence of gastric and hepatic information in brain stem neurons of the rat. Brain Res Bull. 1993;32(5):525–529. doi: 10.1016/0361-9230(93)90302-r. [DOI] [PubMed] [Google Scholar]
- [31].Yuan CS, Barber WD. Parabrachial nucleus: neuronal evoked responses to gastric vagal and greater splanchnic nerve stimulation. Brain Res Bull. 1991;27(6):797–803. doi: 10.1016/0361-9230(91)90211-2. [DOI] [PubMed] [Google Scholar]
- [32].Kobashi M, Adachi A. Effect of topical administration of glucose on neurons innervating abdominal viscera in dorsal motor nucleus of vagus in rats. Jpn J Physiol. 1994;44(6):729–734. doi: 10.2170/jjphysiol.44.729. [DOI] [PubMed] [Google Scholar]
- [33].Niijima A, Yamamoto T. The effects of lithium chloride on the activity of the afferent nerve fibers from the abdominal visceral organs in the rat. Brain Res Bull. 1994;35(2):141–145. doi: 10.1016/0361-9230(94)90094-9. [DOI] [PubMed] [Google Scholar]
- [34].Traub RJ, Lim F, Sengupta JN, et al. Noxious distention of viscera results in differential c-Fos expression in second order sensory neurons receiving ‘sympathetic’ or ‘parasympathetic’ input. Neurosci Lett. 1994;180(1):71–75. doi: 10.1016/0304-3940(94)90916-4. [DOI] [PubMed] [Google Scholar]
- [35].Yamamoto T, Shimura T, Sako N, et al. C-fos expression in the rat brain after intraperitoneal injection of lithium chloride. Neuroreport. 1992;3(12):1049–1052. doi: 10.1097/00001756-199212000-00004. [DOI] [PubMed] [Google Scholar]
- [36].Kalia M, Sullivan JM. Brainstem projections of sensory and motor components of the vagus nerve in the rat. J Comp Neurol. 1982;211(3):248–265. doi: 10.1002/cne.902110304. [DOI] [PubMed] [Google Scholar]
- [37].McCann SM, Gutkowska J, Antunes-Rodrigues J. Neuroendocrine control of body fluid homeostasis. Braz J Med Biol Res. 2003;36(2):165–181. doi: 10.1590/s0100-879x2003000200003. [DOI] [PubMed] [Google Scholar]
- [38].Louis-Sylvestre J. Preabsorptive insulin release and hypoglycemia in rats. Am J Physiol. 1976;230(1):56–60. doi: 10.1152/ajplegacy.1976.230.1.56. [DOI] [PubMed] [Google Scholar]
- [39].Rozengurt E, Sternini C. Taste receptor signaling in the mammalian gut. Curr Opin Pharmacol. 2007;7(6):557–562. doi: 10.1016/j.coph.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Raybould HE, Glatzle J, Freeman SL, et al. Detection of macronutrients in the intestinal wall. Auton Neurosci. 2006;125(1-2):28–33. doi: 10.1016/j.autneu.2006.01.016. [DOI] [PubMed] [Google Scholar]
- [41].Pepino MY, Mennella JA. Habituation to the pleasure elicited by sweetness in lean and obese women. Appetite. 2012;58(3):800–805. doi: 10.1016/j.appet.2012.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Ginane C, Baumont R, Favreau-Peigne A. Perception and hedonic value of basic tastes in domestic ruminants. Physiol Behav. 2011;104(5):666–674. doi: 10.1016/j.physbeh.2011.07.011. [DOI] [PubMed] [Google Scholar]
- [43].Accolla R, Carleton A. Internal body state influences topographical plasticity of sensory representations in the rat gustatory cortex. Proc Natl Acad Sci. 2008;105(10):4010–4015. doi: 10.1073/pnas.0708927105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Kitamura A, Sato W, Uneyama H, et al. Effects of intragastric infusion of inosine monophosphate and L: -glutamate on vagal gastric afferent activity and subsequent autonomic reflexes. J Physiol Sci. 2011;61(1):65–71. doi: 10.1007/s12576-010-0121-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Yamamoto T, Sawa K. Comparison of c-fos-like immunoreactivity in the brainstem following intraoral and intragastric infusions of chemical solutions in rats. Brain Res. 2000;866(1-2):144–151. doi: 10.1016/s0006-8993(00)02242-3. [DOI] [PubMed] [Google Scholar]
- [46].Yamamoto T, Sawa K. c-Fos-like immunoreactivity in the brainstem following gastric loads of various chemical solutions in rats. Brain Res. 2000;866(1-2):135–143. doi: 10.1016/s0006-8993(00)02241-1. [DOI] [PubMed] [Google Scholar]
- [47].Chen K, Yan J, Li J, et al. c-Fos expression in rat brainstem following intake of sucrose or saccharin. Front Med. 2011;5(3):294–301. doi: 10.1007/s11684-011-0144-8. [DOI] [PubMed] [Google Scholar]
- [48].Spector AC, Smith JC. A detailed analysis of sucrose drinking in the rat. Physiol Behav. 1984;33(1):127–136. doi: 10.1016/0031-9384(84)90023-4. [DOI] [PubMed] [Google Scholar]
- [49].Kelly L, Morales S, Smith BK, et al. Capsaicin-treated rats permanently overingest low- but not high-concentration sucrose solutions. Am J Physiol Regul Integr Comp Physiol. 2000;279(5):R1805–1812. doi: 10.1152/ajpregu.2000.279.5.R1805. [DOI] [PubMed] [Google Scholar]
- [50].Louis-Sylvestre J, Giachetti I, Le Magnen J. Vagotomy abolishes the differential palatability of food. Appetite. 1983;4(4):295–299. doi: 10.1016/s0195-6663(83)80022-1. [DOI] [PubMed] [Google Scholar]
- [51].The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006 Sep 30; [Google Scholar]
- [52].Jiang ES, Yan JQ, Song XA. Effects of sucrose/NaCl mixtures stimulation on c-fos-like immunoreactivity in the taste-related nuclei in rats. Xi’an Jiaotong Daxue Xuebao: Yingwen Ban. 2003;15(2):189–192. [Google Scholar]


