Abstract
Interleukin-18 gene promoter polymorphisms are potential risk factors for ischemic cerebrovascular disease, and the –607C allele may increase ischemic stroke risk in the Han Chinese population. In the present study, we recruited 291 patients with ischemic cerebrovascular disease from the Affiliated Hospital of Qingdao University Medical College, China, and 226 healthy controls. Both patients and controls were from the Han population in northern China. Immunoresonance scattering assays detected increased serum amyloid A protein, C-reactive protein, and interleukin-18 levels in ischemic cerebrovascular disease patients compared with healthy controls. Analysis of the –607C/A (rs1946518) polymorphism in the interleukin-18 gene promoter showed ischemic cerebrovascular disease patients exhibited increased frequencies of the CC genotype and C alleles than healthy controls. Genotype and allele frequencies of the interleukin-18 –137G/C (rs187238) polymorphism and the –13T/C (rs11024595) polymorphism in the 5’-flanking region of serum amyloid A, showed no significant difference between the two groups. Multivariate logistic regression analysis on the interleukin-18 promoter A/C genetic locus, for correction of age, gender, history of smoking, hypertension, diabetes mellitus, hypercholesteremia, and an ischemic stroke family history, showed ischemic cerebrovascular disease risk in individuals without the A allele (C homozygotes) was 2.2-fold greater than in A allele carriers. Overall, our findings suggest that the –13T/C (rs11024595) polymorphism in the 5’-flanking region of serum amyloid A has no correlation with ischemic cerebrovascular disease, but the C allele of the –607C/A (rs1946518) polymorphism in the interleukin-18 promoter is a high-risk factor for ischemic cerebrovascular disease in the Han population of northern China. In addition, the A allele is likely a protective gene for ischemic cerebrovascular disease.
Keywords: neural regeneration, brain injury, interleukin-18, ischemic cerebrovascular disease, atherosclerosis, gene polymorphism, C-reactive protein, serum amyloid A protein, inflammation, neuroregeneration
Research Highlights
-
(1)
A serum amyloid A gene polymorphism is positively correlated with carotid intima-media thickness in the healthy Han Chinese population. However, the correlation between this serum amyloid A polymorphism and ischemic cerebrovascular disease is not yet known. Interleukin-18 is closely related to atherosclerotic plaque progression and instability. Interleukin-18 promoter gene polymorphisms may be associated with ischemic stroke pathogenesis, and the –607C allele increases ischemic stroke risk in the Han Chinese population. The frequency distribution of genetic polymorphisms varies among different populations, races, and living environments.
-
(2)
In the present study, we analyzed serum amyloid A and interleukin-18 levels in a Han Chinese population to investigate the relationship between serum amyloid A, interleukin-18, and C-reactive protein, as well as their role in ischemic cerebrovascular disease.
-
(3)
The –13T/C (rs11024595) polymorphism, in the 5’-flanking region of the serum amyloid A gene, shows no correlation with ischemic cerebrovascular disease. However, the C allele of the –607C/A (rs1946518) polymorphism in the interleukin-18 gene promoter is a strong risk factor for ischemic cerebrovascular disease in the Han population of northern China. In addition, the A allele is likely protective for ischemic cerebrovascular disease.
INTRODUCTION
Atherosclerosis and thrombotic incidents are a major cause of morbidity and mortality. The increasing incidence of ischemic cerebrovascular disease is due to certain risk factors, including atherosclerosis. Atherosclerosis can result in ruptured fibrous caps, which are strongly associated with a recent stroke history[1,2,3]. Moreover, atherosclerotic plaque rupture is involved in the etiology of cardiovascular events[4,5].
Many factors lead to plaque instability, with inflammation being an important one. Recent evidence supports inflammation as a major cause of the initiation, unstable progression, and eventual disruption of coronary plaques, and patients with a more pronounced vascular inflammatory response have a poorer outcome[6,7,8,9]. Inflammation plays an important role not only in the initiation and progression of atherosclerosis, but also in plaque rupture[10].
There are many inflammation factors involved in atherosclerosis. C-reactive protein is an acute-phase protein that participates in atherosclerosis formation and development. It has emerged as the most powerful predictor of cardiovascular disease because of its role in atherosclerosis, endothelial dysfunction, atherosclerotic-plaque formation, plaque maturation, and eventual rupture[11].
Serum amyloid A is another important inflammation factor. Animal experiments and clinical studies have found serum amyloid A levels are closely related to coronary heart disease, obesity, and carotid arteriosclerosis[12,13]. Serum amyloid A triggers inflammatory cell adhesion, chemotaxis, and aggregation, leading to cell infiltration in arteriosclerotic plaques. Although serum amyloid A is a sensitive marker for coronary artery disease, its specific effect on arteriosclerosis in vivo remains unclear.
The polymorphisms, rs12218 of the serum amyloid A1 gene, and rs2468844 of the serum amyloid A2 gene, are positively correlated with carotid intima-media thickness in the healthy Han Chinese population[14]. However, the correlation between serum amyloid A polymorphisms and ischemic cerebrovascular disease is still unknown. A recent study found serum amyloid A acts not only as an inflammatory factor, but also as an apolipoprotein, replacing apolipoprotein A1 as the major apolipoprotein of the high-density lipoprotein involved in atherosclerosis[14].
Interleukin-18 is directly involved in atherosclerotic plaque progression and instability[15,16]. Furthermore, interleukin-18 plays an important role in ischemic stroke pathogenesis, and polymorphisms within its promoter contribute to interleukin-18 expression levels[17]. Interleukin-18 gene promoter polymorphisms may also be associated with ischemic stroke pathogenesis, as the –607C allele has been shown to increase ischemic stroke risk in the Han Chinese population[17], although frequency distributions of genetic polymorphisms vary between different populations, races, and living environments. In the present study, we investigated a Han Chinese population.
We measured serum amyloid A and interleukin-18 levels in ischemic cerebrovascular disease patients to determine the relationship between serum amyloid A, interleukin-18, C-reactive protein, and ischemic cerebrovascular disease. This will improve our understanding of the mechanisms underlying serum amyloid A and interleukin-18 actions in cerebrovascular disease. We also explored the –13T/C polymorphism, found within the 5’-flanking region of the serum amyloid A gene, and two polymorphisms (–607C/A and –137G/C) found within the promoter region of the interleukin-18 gene. Our aim was to identify molecular genetic mechanisms involved in cerebrovascular disease pathogenesis.
RESULTS
Quantitative analysis and baseline data of study subjects
A total of 291 patients with ischemic cerebrovascular disease and 226 healthy controls were included in the final analysis. The ischemic cerebrovascular disease group matched the control group in age, number of male patients, and smoking status (P > 0.05). Both patients and controls were from the Han population living in northern China (north of Tsinling Mountains and Huai River).
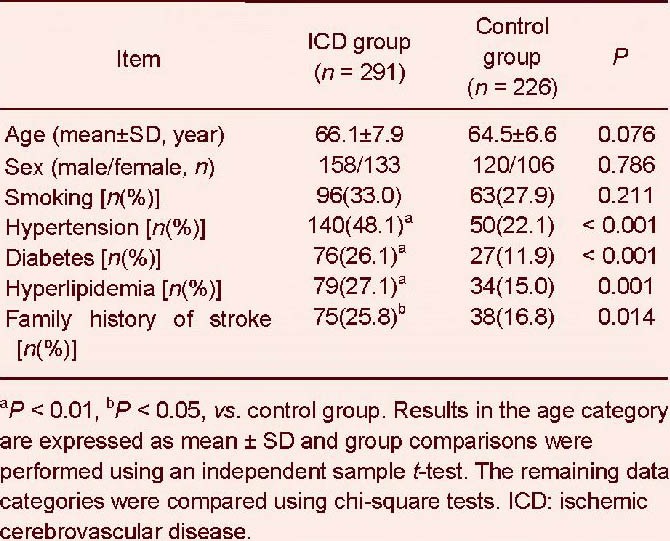
The number of cases with a disease history of hypertension, diabetes mellitus, hyperlipidemia, or a family history of stroke was significantly higher in the ischemic cerebrovascular disease group than the control group (P < 0.01 or P < 0.05; Table 1).
Table 1.
Demographic characteristics of subjects
Serum amyloid A and interleukin-18 levels were positively correlated with C-reactive protein levels in ischemic cerebrovascular disease patients
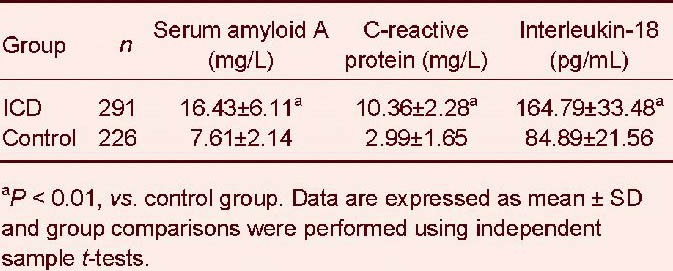
Immunoresonance scattering assays detected higher serum amyloid A and interleukin-18 levels in the ischemic cerebrovascular disease group than in the control group (P < 0.01; Table 2).
Table 2.
Comparison of serum amyloid A, interleukin-18, and C-reactive protein levels in ischemic cerebrovascular disease (ICD) and control groups
Pearson's correlation analysis identified a positive correlation between C-reactive protein and both serum amyloid A and interleukin-18 levels in ischemic cerebrovascular disease (r = 0.494, P < 0.01; Figure 1).
Figure 1.
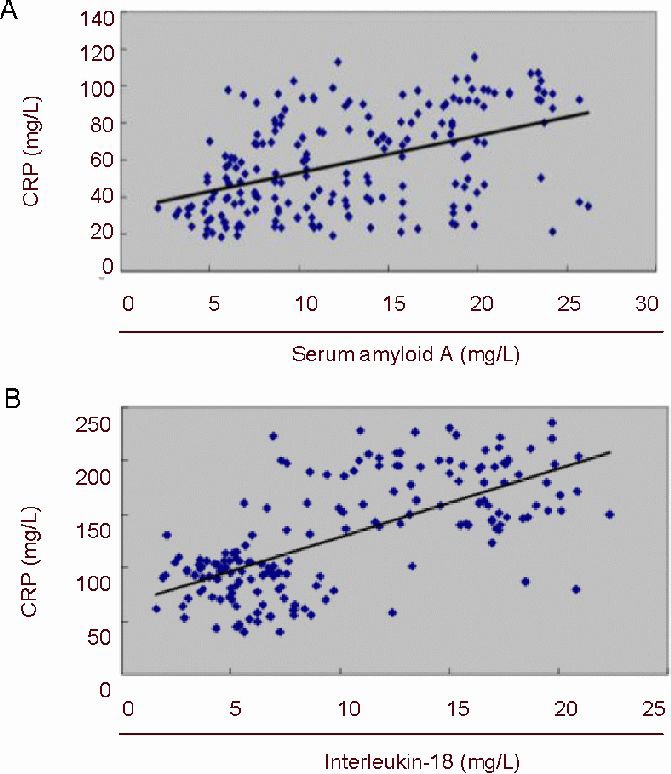
Pearson's correlation analysis of serum C-reactive protein (CRP), and serum amyloid A or interleukin-18 levels, in ischemic cerebrovascular disease patients.
Correlation coefficients (r) between serum CRP and serum amyloid A (A) or interleukin-18 (B) levels are 0.494 (P < 0.01) or 0.677 (P < 0.01), respectively, showing significant positive correlations in both cases.
Examination of the serum amyloid A –13T/C polymorphism, and the –607C/A and –137G/C polymorphisms within the interleukin-18 promoter
Chi-square goodness-of-fit tests showed the genotype distributions and frequencies for the –13T/C polymorphism (within the serum amyloid A gene), and –607C/A and –137G/C polymorphisms (within the interleukin-18 gene promoter region), all reached Hardy-Weinberg equilibrium in both ischemic cerebrovascular disease patients and healthy controls (serum amyloid A, ischemic cerebrovascular disease P = 0.052, controls P = 0.054; interleukin-18 –137G/C, ischemic cerebrovascular disease P = 0.12, controls P = 0.813; interleukin-18 –607C/A, ischemic cerebrovascular disease P = 0.635, controls P = 0.566). Thus, indicating all subjects are from the same population. Target gene fragments were readily detected by PCR amplification (Figures 2, 3).
Figure 2.
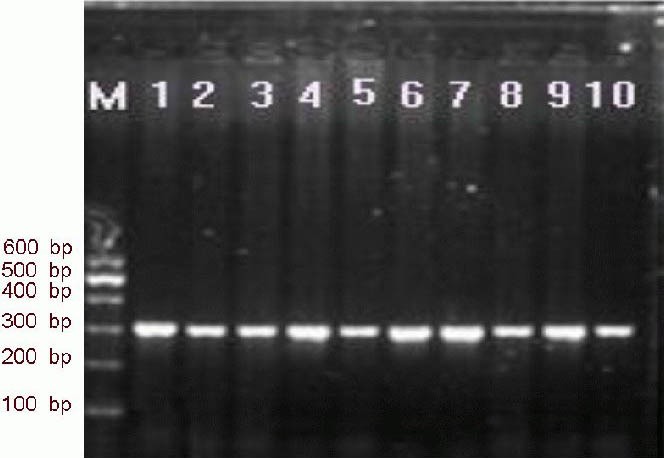
Electrophoresis of PCR fragments of the serum amyloid A gene polymorphism (–13T/C) in ischemic cerebrovascular disease patients.
1–10: Serum samples from 10 patients with ischemic cerebrovascular disease; PCR fragment: 303 bp; M: marker.
Figure 3.
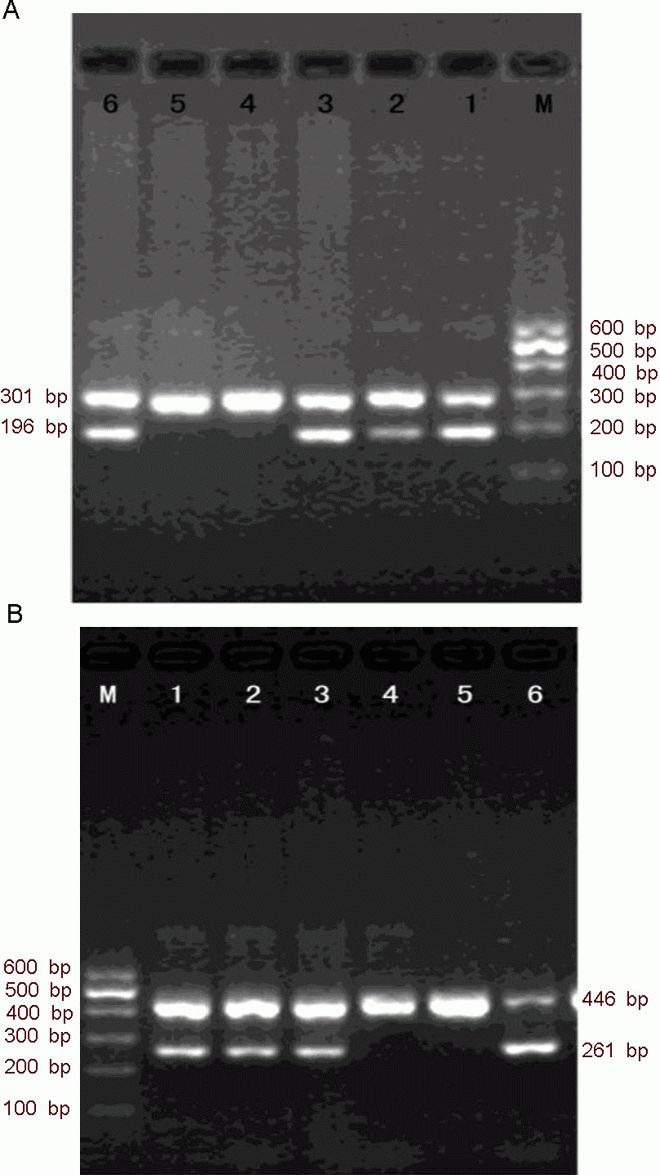
Electrophoresis of PCR fragments of the interleukin-18 polymorphisms (–607C/A and –137G/C) in ischemic cerebrovascular disease patients.
(A) The –607C/A polymorphism. M: Marker. Lanes 1, 3, and 5: three samples analyzed with specific primer 1 (matching allele A), intra-control forward primer, and general reverse primer. Lanes 2, 4, and 6: three samples analyzed with specific primer 2 (matching allele C), intra-control forward primer, and general reverse primer. The genotype of the three samples is CA, AA, and CC. The length of specific and reference products are 196 bp and 301 bp, respectively.
(B) The –137G/C polymorphism. M: Marker. Lanes 1, 3, and 5: three samples analyzed with specific primer 1 (matching allele G), intra-control forward primer, and general reverse primer. Lanes 2, 4, and 6: three samples analyzed with specific primer 2 (matching allele C), intra-control forward primer, and general reverse primer. The genotype of the three samples is GC, GG, and CC. The length of specific and reference products are 261 bp and 446 bp, respectively.
The serum amyloid A –13T/C (rs11024595) polymorphism is characterized by three genotypes, specifically, homozygous TT, heterozygous TC, and homozygous CC (Figure 4). The genotype and allele distributions of serum amyloid A showed no significant difference between ischemic cerebrovascular disease and control groups (P > 0.05; Tables 3, 4).
Figure 4.
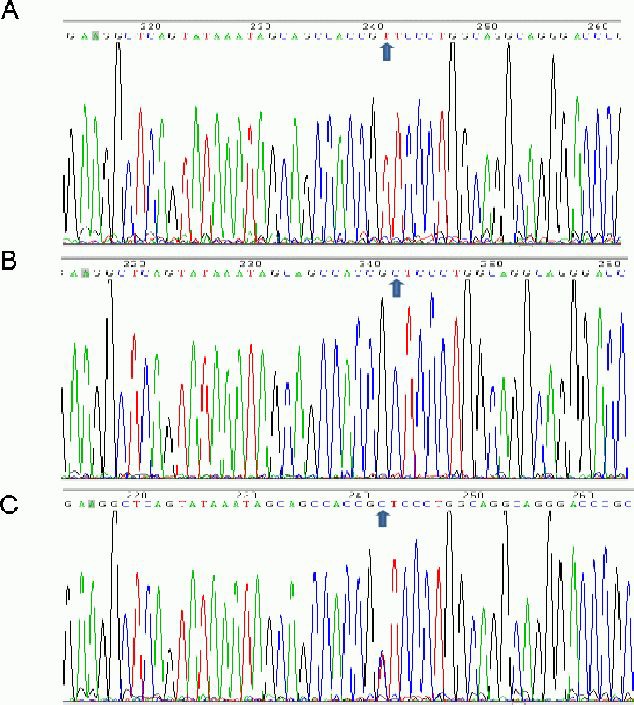
The –13T/C (rs11024595) polymorphism of the serum amyloid A gene in ischemic cerebrovascular disease.
(A) Homozygous TT; (B) heterozygous TC;
(C) homozygous CC. Arrows indicate allele distribution.
Table 3.
Genotype distribution of the serum amyloid A –13T/C (rs11024595) polymorphism in ischemic cerebrovascular disease (ICD) and control groups
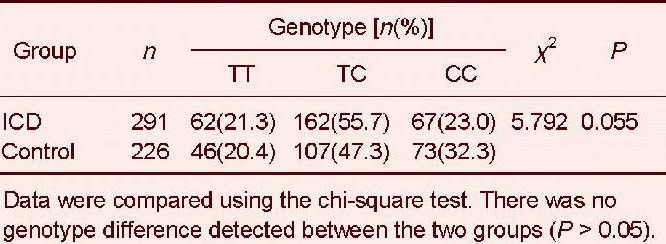
Table 4.
Allele frequency distribution of the serum amyloid A –13T/C (rs11024595) polymorphism in ischemic cerebrovascular disease (ICD) and control groups
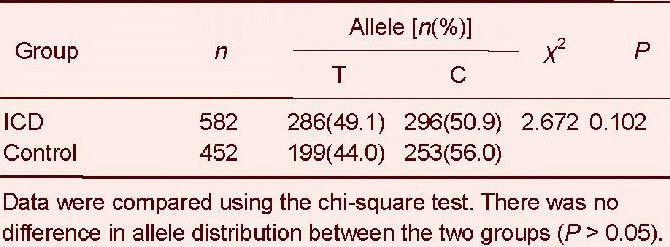
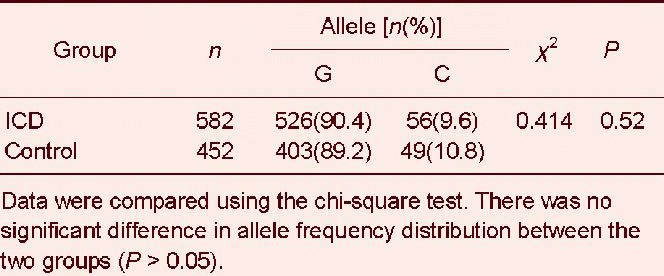
For the interleukin-18 –137G/C (rs187238) polymorphism, we found no significant difference in genotype frequency between ischemic cerebrovascular disease and control groups (χ2 = 0.668, P = 0.414; Table 5). The G allele frequency was dominant in both groups, but this was not significant (χ2 = 0.414, P = 0.52; Table 6).
Table 5.
Genotype distribution of the interleukin-18 –137G/C (rs187238) polymorphism in ischemic ce-rebrovascular disease (ICD) and control groups
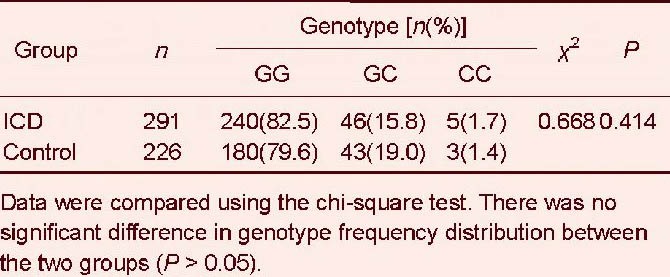
Table 6.
Allele frequency distribution of the interleukin-18 –137G/C (rs187238) polymorphism in ischemic cerebrovascular disease (ICD) and control groups
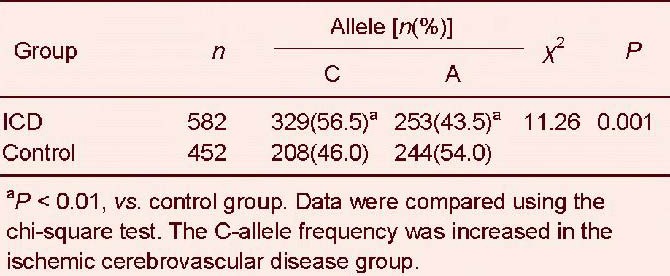
The CC genotype frequency of the interleukin-18 –607C/A (rs1946518) polymorphism was greater in ischemic cerebrovascular disease than in healthy controls (P < 0.01; Table 7). Similarly, the C allele frequency was much higher in ischemic cerebrovascular disease than in healthy controls (P < 0.01; Table 8).
Table 7.
Genotype frequency distribution of the interleukin-18 –607C/A (rs1946518) polymorphism in ischemic cerebrovascular disease (ICD) and control
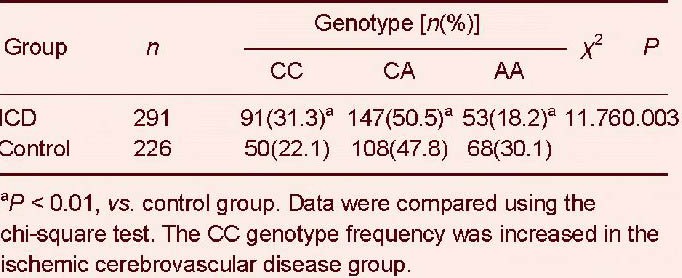
Table 8.
Allele frequency distribution of the interleukin-18 –607C/A (rs1946518) polymorphism in ischemic cerebrovascular disease (ICD) and control groups
We further examined the interleukin-18 –607C/A (rs1946518) polymorphism by multivariate logistic regression, adjusting for age, sex, smoking, hypertension, diabetes, hyperlipidemia, and family history of stroke. We found the risk for ischemic cerebrovascular disease was 2.2-fold greater in homozygous C allele carriers (CC), compared with heterozygotes (CA) or homozygote A allele carriers (AA) (OR = 2.243, 95%CI = 1.431–4.735, P = 0.002).
DISCUSSION
Serum amyloid A is a local and systemic proinflammatory cytokine[18] that can cause inflammation, lipid transfer, and atherosclerosis. In carotid atherosclerotic lesions, Meek et al[18] found serum amyloid A mRNA was expressed in most endothelial cells, certain smooth muscle cells, macrophage-derived foam cells, macrophages, and fat cells. This confirmed a role for serum amyloid A in arteriosclerosis.
Serum amyloid A can promote fat and interstitial vascular cells to produce inflammatory cytokines[19], and is not only an atherosclerosis risk factor, but also an active participant in atherogenesis[20]. Liuzzo et al[21] investigated the relationship between serum amyloid A and unstable angina, and found increased serum amyloid A levels strongly correlated with an adverse prognosis. Furthermore, serum amyloid A has been proposed as a sensitive marker for prognosis in fatal cardiovascular disease[22].
The human serum amyloid A gene is located on chromosome 11 (p15.4–p15.1), and encodes three functional genes, although only serum amyloid A1 and A2 encode acute-phase serum amyloid A. Serum amyloid A1 plays an important role during the immune response, and in chronic inflammatory disease through the stimulation of genes involved in cytokine production, phagocytosis, and anti-apoptotic pathways[23].
Both rs12218 and rs2468844, from serum amyloid A1 and A2, respectively, are associated with carotid intima-media thickness in healthy Han Chinese subjects[14]. The 5’-flanking region of the serum amyloid A1 gene has three polymorphic sites, –61C/G, –13T/C, and –2G/A[24]. We selected the –13T/C polymorphism as it has been previously associated with peripheral arterial disease[25].
We found no apparent correlation between the –13T/C polymorphism and ischemic stroke incidence. Possible reasons are: (1) Gene polymorphism distribution frequencies are affected by race, populations, environment, age, and gender. Moreover, allele shift may occur during the genetic process. (2) Different polymorphic sites examined.
Xie et al [25] investigated the correlation between serum amyloid A1/2 gene polymorphisms and carotid artery intima-media thickness in the healthy Han Chinese population using four single-nucleotide polymorphisms (SNPs), rs12218, rs2229338, rs1059559, and rs2468844. After exclusion of confounding factors, two SNPs (rs12218 and rs2468844) were found to correlate well with carotid intima-media thickness. Future research is needed to verify if these two sites are associated with ischemic stroke.
In humans, C-reactive protein is a primary, sensitive marker of the non-specific inflammatory response[26,27], and plays an important role in the progression of cerebral tissue injury[28]. Furthermore, C-reactive protein levels can predict ischemic stroke, and are an independent prognostic factor for a poorer outcome at 3 months[27,29].
In this study, serum amyloid A levels significantly increased in ischemic cerebrovascular disease, and significantly and positively correlated with C-reactive protein. Therefore, serum amyloid A may also be an important inflammatory marker in ischemic stroke.
Interleukin-18 is a pleiotropic proinflammatory factor, and plays a key role in the inflammatory cascade effect[30,31]. Interleukin-18 is directly involved in atherosclerotic plaque progression and instability[15,32]. It is also a specific biomarker for atherosclerosis-prone patients with metabolic syndrome[33]. Serum interleukin-18 levels are significantly higher in acute myocardial infarction patients[34]. Levels of circulating interleukin-18 have been positively correlated with carotid intima-media thickness and coronary plaque area, and have been identified as important predictors of coronary events and cardiovascular mortality[35]. Interleukin-18 is also the most important independent predictor in ischemic stroke patients who had combined major adverse events within 90 days, and is related to stroke severity and degree of dysfunction[36,37].
Our study identified significantly higher serum interleukin-18 levels in ischemic cerebrovascular disease patients than in control patients. Pearson's correlation analysis showed serum interleukin-18 and C-reactive protein levels significantly correlate (r = 0.677, P < 0.01), in agreement with previous studies. These results suggest that determining serum interleukin-18 levels in ischemic stroke patients may be helpful to evaluate brain injury severity.
The gene encoding interleukin-18 is located on human chromosome 11, and is composed of six exons and five introns. The –607C/A and –137G/C polymorphisms are located upstream of exon 1, where a trans-acting factor binding site, affecting interleukin-18 transcription levels, is also found. The interleukin-18 promoter –137G/C polymorphism influences interleukin-18 levels and coronary artery disease incidence, suggesting interleukin-18 is causally involved in atherosclerosis development[38,39,40]. The –607C/A site, located in a 3’,5’-cyclic adenosine monophosphate trans-acting element binding protein-binding zone point mutation, may affect expression of interleukin-18 and interferon[38].
The –607C/A polymorphism has been associated with type 2 diabetes, with the C allele reported as a susceptibility gene for type 2 diabetes[30]. Interleukin-18 gene promoter polymorphisms may be associated with ischemic stroke pathogenesis, and the –607C allele may increase the risk of ischemic stroke in the Han Chinese population[17].
In this study, we found three genotypes of the –607C/A polymorphism, specifically CC, CA, and AA. Ischemic stroke patients have a significantly higher frequency of the CC genotype, while the AA genotype distribution frequency is significantly higher in the control group. Moreover, there were significant differences in the distribution of both alleles between the two groups.
There was no significant difference between the two groups in the allele and genotype distributions of the –137G/C polymorphism, and it showed no correlation with ischemic stroke risk. This adds credence to our finding that the –607C/A polymorphism is correlated with ischemic stroke risk, with the C allele as a potential ischemic stroke risk factor. While the AA and CA genotypes of the –607C/A polymorphism reduced ischemic stroke risk, it is likely there would be increased risk for CC carriers. This difference was still statistically significant when analyzed by logistic regression analysis.
Ischemic stroke risk in individuals who did not carry the –607 A allele (CC homozygotes) was greater by a 2.2-fold difference than individuals carrying the –607 A allele (CA heterozygote or AA homozygotes). Therefore, we propose that the A allele provides a protective effect in ischemic stroke incidence, while C allele carriers have an increased risk of ischemic stroke, consistent with the findings of Zhang et al [17].
Further research is necessary to determine if interleukin-18 variant genotypes of the –607A/C or –137G/C polymorphisms, show association with serum interleukin-18 levels, and if the –607C allele is important in transcriptional regulation of the interleukin-18 gene.
SUBJECTS AND METHODS
Design
A non-randomized, controlled study of gene polymorphisms.
Time and setting
Experiments were performed from August 2008 to May 2010 at the Central Laboratory of the Affiliated Hospital at Qingdao University Medical College, China.
Subjects
A total of 291 patients with ischemic cerebrovascular disease were recruited from the Inpatient and Outpatient Departments at the Affiliated Hospital of Qingdao University Medical College, China from August 2008 to May 2010.
Inclusion criteria
(1) All patients met diagnostic criteria of the Fourth National Cerebrovascular Disease Conference of China[40].
(2) All patients presented with large-artery atherosclerosis as determined by the Trial of Org 10172 on Acute Stroke Treatment classification. (3) Digital subtraction or CT angiography confirmed large-artery atherosclerotic stenosis-associated ischemic stroke. (4) All patients provided informed consent.
Exclusion criteria
(1) Patients with acute coronary syndrome and other heart diseases, such as dilated cardiomyopathy or rheumatic heart disease. (2) Patients with liver or kidney dysfunction, or severe heart failure. (3) Patients with infections or immune system diseases. (4) Patients with peripheral vascular disease. (5) Patients with cerebral infarction induced by arteritis, blood diseases, cancer, drugs, aneurysms, or vascular malformations.
In addition, 226 healthy volunteers were simultaneously selected from the Medical Examination Center at the Affiliated Hospital of Qingdao University Medical College, China, and served as the control group. The controls and patients were matched in terms of age and gender. There were 158 male and 133 female subjects, at a mean age of 66.06 ± 7.94 years.
All patients and healthy controls were from the Chinese Han population, living in northern China (north of Tsinling Mountains and Huai River). Clinical data included age, gender, smoking status, hypertension, diabetes, and hyperlipidemia. Hypertension was defined in accordance with guidelines from the World Health Organization (1999)[41] and the International League of Hypertension, specifically, systolic blood pressure ≥ 140 mmHg (18.7 kPa), and/or diastolic blood pressure ≥ 90 mmHg (12.0 kPa) with the exception of secondary hypertension[41]. Hyperlipidemia met diagnostic criteria from the Recommendations of Dyslipidemia Prevention, issued by the Chinese Society of Cardiovascular Disease[42]. Diabetes was diagnosed according to criteria from the World Health Organization[43].
According to the State Council of China, we explained the trial method and risk, and all partners provided informed consent[44].
Methods
Blood specimen collection
Within 24 hours of hospital admittance, 3 mL of elbow venous blood was collected from all candidates into ethylenediamine tetraacetic acid-anticoagulant tubes. Candidates were fasted prior to blood collection. After removal of the fat layer and exclusion of hemolyzed specimens, serum and blood cells were separated by centrifugation at 1 000 × g for 10 minutes. The resulting serum and white film (containing a small amount of serum and red blood cells) were collected into two microcentrifuge tubes. They were then immediately frozen and stored at –80°C until use. Serum was used for detection of serum levels of appropriate factors. White film was used to extract DNA for polymorphism analysis.
Determination of serum C-reactive protein, serum amyloid A, and interleukin-18 levels by immunoresonance scattering assays
Human serum C-reactive protein, serum amyloid A, and interleukin-18 levels were determined using immunoresonance scattering assays (BN-II protein analyzer; Dade Behring Company, Marburg, Germany), according to the manufacturer's instructions (Wuhan EIAab Science; Wuhan, Hubei Province, China). Serum C-reactive protein, serum amyloid A, or interleukin-18 antibody was aliquoted into 96-well microplates to prepare a solid-phase carrier. Standards and samples were added to each well, allowing serum C-reactive protein, serum amyloid A, or interleukin-18 within the samples, to interact with antibodies bound to the solid-phase carrier. Biotinylated serum C-reactive protein, serum amyloid A, or interleukin-18 antibody was then incubated, and non-adherent antibody subsequently washed away. Horseradish peroxidase-labeled avidin, and a color substrate (tetramethyl benzidine), were added for antibody visualization.
The color intensity positively correlated with serum C-reactive protein, serum amyloid A, or interleukin-18 levels. Absorbance values at 450 nm were measured using a microplate reader (Microplate Reader 550; Bio-Rad, Hercules, CA, USA), and sample concentrations were calculated. Standard concentrations were used for the vertical axis, and absorbance values for the horizontal axis.
Standard curves were plotted on a logarithmic scale (the best formula was based on a R2 value from a regression equation; a R2 value close to 1 leads to optimal results). Obtained curves were analyzed using Professional Curve Expert 1.3 software, a comprehensive curve fitting system for Windows. XY data can be modeled using a toolbox of linear regression models, nonlinear regression models, and interpolation. Sample concentrations were inferred according to absorbance values from the standard curve.
DNA extraction
DNA was extracted using a whole blood DNA extraction kit (Promega Biotech, Beijing, China). DNA (5 μL) was mixed with 195 μL of ultrapure water. Absorbances (A) at 260 and 280 nm were read using an UV spectrophotometer (DUR640; Beckman, Los Angeles, CA, USA), and the A260nm/A280nm ratio was calculated. DNA concentration (μg/μL) = A260nm × 50 × dilution factor/1 000. The A260nm/A280nm ratio determined the purity of extracted DNA, with levels between 1.7 and 2.0 indicating DNA samples were of high purity.
Determination of serum amyloid A and interleukin-18 genotype
The serum amyloid A PCR primers were designed according to three sites in the vicinity of the DNA sequence and related literature[25,45].
The serum amyloid A 5’-flanking region –13T/C polymorphism primers are:
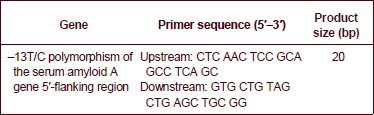
PCR amplification was performed using an Eppendorf 22331 PCR reaction device (Eppendorf, Hamburg, Germany). PCR reactions, in a total volume of 30 μL, contained 15 μL of Taq PCR Master Mix, 0.6 μL of each upstream and downstream primers, 0.8 μL of DNA template (0.04–0.08 μg/μL), and ultra-pure water. For amplification, a total of 36 cycles were run, comprising an initial cycle of 94°C predenaturation for 3 minutes, followed by 35 cycles of 94°C denaturation for 30 seconds, 60°C annealing for 30 seconds, and 72°C extension for 45 seconds, followed by a 72°C extension for 5 minutes.
Samples were electrophoresed on 2% agarose gels in 1 × Tris-acetate-ethylenediamine tetraacetic acid electrophoresis buffer at 110–120 V for 40 minutes (Power-Supply PAC300 horizontal electrophoresis apparatus; Bio-Rad) to visualize PCR amplification bands. PCR amplified fragments were sequenced by Shanghai Sangon Biotechnology (Shanghai, China) to determine T/C genotypes and allele frequencies.
The interleukin-18 PCR primers were designed according to three sites in the vicinity of the DNA sequence and related literature[46,47]. Primers and PCR product sizes for the polymorphisms at positions –137 and –607 of the interleukin-18 gene promoter are:
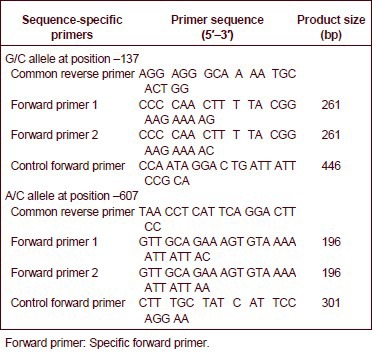
PCR amplification was performed using an Eppendorf 22331 PCR reaction device (Eppendorf). PCR reactions, in a total volume of 30 μL, contained 15 μL of Taq PCR Master Mix, 1.0 μL of DNA template (0.04–0.08 μg/μL), one sequence-specific primer, a common reverse primer, and the internal position control primer (all at a volume of 0.4 μL), and ultra-pure water. The amplification conditions for the –137G/C polymorphism were initial denaturation at 94°C for 5 minutes, then 40 cycles of denaturation at 94°C for 30 seconds, annealing at 58°C for 30 seconds, and extension at 72°C for 45 seconds, with a final extension step at 72°C for 10 minutes.
The amplification conditions for the –607C/A polymorphism were initial denaturation at 94°C for 5 minutes, then 40 cycles of denaturation at 94°C for 30 seconds, annealing at 53°C for 30 seconds, and extension at 72°C for 45 seconds, with a final extension step at 72°C for 10 minutes.
After PCR amplification, samples were electrophoresed on 2% agarose gels in 1 × Tris-acetate-ethylenediamine tetraacetic acid electrophoresis buffer at 110–120 V for 40 minutes (PowerSupply PAC300 horizontal electrophoresis apparatus; Bio-Rad) to visualize PCR amplification bands.
Statistical analysis
Data were analyzed using SPSS 13.0 software (SPSS, Chicago, IL, USA). Measurement data were expressed as mean ± SD and compared with independent sample t-tests. Quantification data were compared using chisquare tests. Pearson's correlation analysis was used to determine the correlation between serum amyloid A, interleukin-18, and C-reactive protein in ischemic cerebrovascular disease. Serum amyloid A and interleukin-18 genotype and allele frequencies were calculated using the frequency counting method. Genotype and allele frequencies between groups were compared using chi-square tests. The goodness-of-fit chi-square test was used to determine the Hardy-Weinberg genetic equilibrium of gene frequencies. Relative risk factors were expressed as 95%CI. Two-tailed tests were performed. A P < 0.05 was considered statistically significant. Confounding factors (including age, sex, smoking, hypertension, diabetes, hyperlipidemia, and a family history of stroke) were adjusted using multivariate logistic regression to determine the correlation between the interleukin-18 C allele of the –607 promoter polymorphism and ischemic cerebrovascular disease risk.
Footnotes
Conflicts of interest: None declared.
Ethical approval: The study was approved by the Medical Ethics Committee, Affiliated Hospital of Qingdao University Medical College in China.
(Reviewed by James R, Norman C, Yang KB, Kang ZC, Wang F)
(Edited by Wang J, Yang Y, Li CH, Song LP, Liu WJ, Zhao M)
REFERENCES
- [1].Pruissen DM, Kappelle LJ, Rosendaal FR, et al. Prothrombotic genetic variants and atherosclerosis in patients with cerebral ischemia of arterial origin. Atherosclerosis. 2009;204(1):191–195. doi: 10.1016/j.atherosclerosis.2008.08.033. [DOI] [PubMed] [Google Scholar]
- [2].Yuan C, Zhang SX, Polissar NL, et al. Identification of fibrous cap rupture with magnetic resonance imaging is highly associated with recent transient ischemic attack or stroke. Circulation. 2002;105(2):181–185. doi: 10.1161/hc0202.102121. [DOI] [PubMed] [Google Scholar]
- [3].Altaf N, Daniels L, Morgan PS, et al. Cerebral white matter hyperintense lesions are associated with unstable carotid plaques. Eur J Vasc Endovasc Surg. 2006;31(1):8–13. doi: 10.1016/j.ejvs.2005.08.026. [DOI] [PubMed] [Google Scholar]
- [4].Zaman AG, Helft G, Worthley SG, et al. The role of plaque rupture and thrombosis in coronary artery disease. Atherosclerosis. 2000;149(2):251–266. doi: 10.1016/s0021-9150(99)00479-7. [DOI] [PubMed] [Google Scholar]
- [5].Man SF, Van Eeden S, Sin DD. Vascular risk in chronic obstructive pulmonary disease: role of inflammation and other mediators. Can J Cardiol. 2012;28(6):653–661. doi: 10.1016/j.cjca.2012.06.013. [DOI] [PubMed] [Google Scholar]
- [6].Zakynthinos E, Pappa N. Inflammatory biomarkers in coronary artery disease. J Cardiol. 2009;53(3):317–333. doi: 10.1016/j.jjcc.2008.12.007. [DOI] [PubMed] [Google Scholar]
- [7].Hojo Y, Ikeda U, Takahashi M, et al. Increased levels of monocyte-related cytokines in patients with unstable angina. Atherosclerosis. 2002;161(2):403–408. doi: 10.1016/s0021-9150(01)00636-0. [DOI] [PubMed] [Google Scholar]
- [8].Libby P. Current concepts of the pathogenesis of the acute coronary syndromes. Circulation. 2001;104(3):365–372. doi: 10.1161/01.cir.104.3.365. [DOI] [PubMed] [Google Scholar]
- [9].Rao DS, Goldin JG, Fishbein MC. Determinants of plaque instability in atherosclerotic vascular disease. Cardiovasc Pathol. 2005;14(6):285–293. doi: 10.1016/j.carpath.2005.07.003. [DOI] [PubMed] [Google Scholar]
- [10].Ikonomidis I, Michalakeas CA, Parissis J, et al. Inflammatory markers in coronary artery disease. Biofactors. 2012;38(5):320–328. doi: 10.1002/biof.1024. [DOI] [PubMed] [Google Scholar]
- [11].Verma S, Szmitko PE, Ridker PM. C-reactive protein comes of age. Nat Clin Pract Cardiovase Med. 2005;2(1):29–36. doi: 10.1038/ncpcardio0074. [DOI] [PubMed] [Google Scholar]
- [12].Jousilahti P, Salomaa V, Rasi V, et al. The association of c-reactive protein,serum amyloid a and fibrinogen with prevalent coronary heart disease-baseline findings of the PAIS project. Atherosclerosis. 2001;156(2):451–456. doi: 10.1016/s0021-9150(00)00681-x. [DOI] [PubMed] [Google Scholar]
- [13].Hu Y, Xu W, Pan JJ, et al. Relationship between acute-phase serum amyloid A and atherosclerosis in type 2 diabetes. Linchuang Neike Zazhi. 2008;25(10):684–686. [Google Scholar]
- [14].Xie X, Ma YT, Yang YN, et al. Polymorphisms in the SAA1/2 gene are associated with carotid intima media thickness in healthy Han Chinese subjects: the cardiovascular risk survey. PLoS One. 2010;5(11):e13997. doi: 10.1371/journal.pone.0013997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Leung BP, Culshaw S, Gracie JA, et al. A role for IL-18 in neutrophil activation. J Immunol. 2001;167(5):2879–2886. doi: 10.4049/jimmunol.167.5.2879. [DOI] [PubMed] [Google Scholar]
- [16].Puren AJ, Fantuzzi G, Gu Y, et al. Interleukin-18 (IFN-gamma-inducing factor) induces IL-8 and IL-1 beta via TNF-alpha production from non-CD14+ human blood mononuclear cells. J Clin Invest. 1998;101(3):711–721. doi: 10.1172/JCI1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Zhang N, Yu JT, Yu NN, et al. Interleukin-18 promoter polymorphisms and risk of ischemic stroke. Brain Res Bull. 2010;81(6):590–594. doi: 10.1016/j.brainresbull.2010.01.008. [DOI] [PubMed] [Google Scholar]
- [18].Meek RL, Urieli-Shoval S, Benditt EP. Expression of apolipoprotein serum amyloid A mRNA in human atherosclerotic lesions and cultured vascular cells: implications for serum amyloid A function. Proc Natl Acad Sci U S A. 1994;91(8):3186–3190. doi: 10.1073/pnas.91.8.3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Yang RZ, Lee MJ, Hu H, et al. Acute-phase serum amyloid A: an inflammatory adipokine and potential link between obesity and its metabolic complications. PLoS Med. 2006;3(6):0884–0893. doi: 10.1371/journal.pmed.0030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Dong Z, Wu T, Qin W, et al. Serum amyloid A directly accelerates the progression of atherosclerosis in apol ipoprotein E-deficient mice. Mol Med. 2011;17(11-12):1357–1364. doi: 10.2119/molmed.2011.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Liuzzo G, Buffon A, Biasucci LM, et al. Enhanced inflammatory response to coronary angioplasty in patients with severe unstable angina. Circulation. 1998;98(22):2370–2376. doi: 10.1161/01.cir.98.22.2370. [DOI] [PubMed] [Google Scholar]
- [22].Thorn CF, Lu ZY, Whitehead AS. Regulation of the human acute phase serum amyloid A genes by tumour necrosis factor-alpha, interleukin-6 and glucocorticoids in hepatic and epithelial cell lines. Scand J Immunol. 2004;59(2):152–158. doi: 10.1111/j.0300-9475.2004.01369.x. [DOI] [PubMed] [Google Scholar]
- [23].Leow KY, Goh WW, Heng CK. Effect of serum amyloid A1 treatment on global gene expression in THP-1-derived macrophages. Inflamm Res. 2012;61(4):391–398. doi: 10.1007/s00011-011-0424-4. [DOI] [PubMed] [Google Scholar]
- [24].Masato M, Chihiro T, Hirotaka K, et al. A novel single-nucleotide polymorphism at the 5'-flanking region of SAA1 associated with risk of type AA amyloidosis secondary to rheumatoid arthritis. Arthritis Rheum. 2001;44(6):1266–1272. doi: 10.1002/1529-0131(200106)44:6<1266::AID-ART218>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- [25].Xie X, Ma YT, Yang YN, et al. Polymorphisms in the SAA1 gene are associated with ankle-to-brachial index in Han Chinese healthy subjects. Blood Press. 2011;20(4):232–238. doi: 10.3109/08037051.2011.566244. [DOI] [PubMed] [Google Scholar]
- [26].Winbeck K, Poppert H, Etgen T, et al. Prognostic relevance of early serial C-reactive protein measurements after first ischemic stroke. Stroke. 2002;33(10):2459–2464. doi: 10.1161/01.str.0000029828.51413.82. [DOI] [PubMed] [Google Scholar]
- [27].Rajeshwar K, Kaul S, Al-Hazzani A, et al. C-reactive protein and nitric oxide levels in ischemic stroke and its subtypes: correlation with clinical outcome. Inflammation. 2012;35(3):978–984. doi: 10.1007/s10753-011-9401-x. [DOI] [PubMed] [Google Scholar]
- [28].Ormstad H, Aass HC, Lund-Sørensen N, et al. Serum levels of cytokines and C-reactive protein in acute ischemic stroke patients, and their relationship to stroke lateralization, type, and infarct volume. J Neurol. 2011;258(4):677–685. doi: 10.1007/s00415-011-6006-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Makita S, Nakamura M, Satoh K, et al. Serum C-reactive protein levels can be used to predict future ischemic stroke and mortality in Japanese men from the general population. Atherosclerosis. 2009;204(1):234–238. doi: 10.1016/j.atherosclerosis.2008.07.040. [DOI] [PubMed] [Google Scholar]
- [30].Wyman TH, Dinarello CA, Banerjee A, et al. Physiological levels of interleukin-18 stimulate multiple neutrophil functions through p38 MAP kinase activation. J Leukoc Biol. 2002;72:401–409. [PubMed] [Google Scholar]
- [31].Nakanishi K, Yoshimoto T, Tsutsui H, et al. Interleukin-18 is a unique cytokine that stimulates both Th1 and Th2 responses depending on its cytokine milieu. Cytokine Growth Factor Rev. 2001;12:53–72. doi: 10.1016/s1359-6101(00)00015-0. [DOI] [PubMed] [Google Scholar]
- [32].Puren AJ, Fantuzzi G, Gu Y, et al. Interleukin-18 (IFN-gamma-inducing factor) induces IL-8 and IL-1beta via TNF-alpha production from non-CD14+ human blood mononuclear cells. J Clin Invest. 1998;101:711–721. doi: 10.1172/JCI1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Yamaoka-Tojo M, Tojo T, Wakaume K, et al. Circulating interleukin-18: a specific biomarker for atherosclerosis-prone patients with metabolic syndrome. 2011;8:3. doi: 10.1186/1743-7075-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Hedtjärn M, Leverin AL, Eriksson K, et al. Interleukin-18 involvement in hypoxic-ischemic brain injury. J Neurosci. 2002;22(14):5910–5919. doi: 10.1523/JNEUROSCI.22-14-05910.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Chen BF, Xu X, Deng Y. Relationship between helicobacter pylori infection and serum interleukin-18 in patients with carotid atherosclerosis. Helicobacter. 2013;18(2):124–128. doi: 10.1111/hel.12014. [DOI] [PubMed] [Google Scholar]
- [36].Zaremba J, Losy J. Interleukin-18 in acute ischaemic stroke patients. Neurol Sci. 2003;24(3):117–124. doi: 10.1007/s10072-003-0096-0. [DOI] [PubMed] [Google Scholar]
- [37].Liu W, Tang Q, Jiang H. Promoter polymorphism of interleukin-18 in angiographically proven coronary artery disease. Angiology. 2009;60(2):180–185. doi: 10.1177/0003319708319939. [DOI] [PubMed] [Google Scholar]
- [38].Giedraitis V, He B, Huang WX, et al. Cloning and mutation analysis of the human IL-18 promoter: a possible role of polymorphisms in expression regulation. J Neuroimmunol. 2001;112:146–152. doi: 10.1016/s0165-5728(00)00407-0. [DOI] [PubMed] [Google Scholar]
- [39].Shi J, Ding XR, Tang FM, et al. Association of -607 C/A polymorphism in IL-18 promoter gene with type 2 diabetes mellitus. Zhongguo Shiyan Zhenduanxue. 2009;13(12):1734–1736. [Google Scholar]
- [40].The 4th National Cerebrovascular Conference. The diagnosis elements of various types of cerebrovascular disease. Zhonghua Shenjing Ke Zazhi. 1996;29(6):380. [Google Scholar]
- [41].Lin JX, Wu KG. The treatment guidelines for hypertension in 1999’ the World Health Organization/International League of hypertension. Gaoxueya Zazhi. 1999;7(2):97–100. [Google Scholar]
- [42].The Chinese Society of Cardiovascular Disease. The recommendations of dyslipidemia prevention. Zhonghua Xinxueguan Bing Zazhi. 1997;25(3):169–173. [Google Scholar]
- [43].The diagnostic criteria for diabetes. Zhongguo Manxing Jibing Yufang yu Kongzhi. 2009 [Google Scholar]
- [44].State Council of the People's Republic of China. Administrative Regulations on Medical Institution. 1994 Sep 01; [Google Scholar]
- [45].Li H, Zhao Y, Zhou S, et al. SAA activates peroxisome proliferator-activated receptor γ through extracellular-regulated kinase 1/2 and COX-2 expression in hepatocytes. Biochemistry. 2010;49(44):9508–9517. doi: 10.1021/bi100645m. [DOI] [PubMed] [Google Scholar]
- [46].Yang Y, Qiao J, Li MZ. Association of polymorphisms of interleukin-18 gene promoter region with polycystic ovary syndrome in chinese population. Reprod Biol Endocrinol. 2010;8:125. doi: 10.1186/1477-7827-8-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Kim HL, Cho SO, Kim SY, et al. Association of interleukin-18 gene polymorphism with body mass index in women. Reprod Biol Endocrinol. 2012;10:31. doi: 10.1186/1477-7827-10-31. [DOI] [PMC free article] [PubMed] [Google Scholar]


