Abstract
Detailed knowledge of motor outcomes enables to establish proper goals and rehabilitation strategies for stroke patients. Several previous studies have reported functional or motor outcomes in patients with a middle cerebral artery territory infarct. However, little is known about motor outcome in patients with a complete middle cerebral artery territory infarct. In this study, we investigated the motor outcomes in 23 patients with a complete middle cerebral artery territory infarct. All of these patients received comprehensive rehabilitative management, including movement therapy and neuromuscular electrical stimulation of the affected finger extensors and ankle dorsiflexors, for more than 3 months. Motor outcomes were measured at 6 months after stroke onset using the Medical Research Council, Motricity Index, the modified Brunnstrom Classification, and Functional Ambulation Category scores. The motor function of the lower extremities was found to be better than that of the upper extremities. After receiving rehabilitation treatments for 3–6 months, about 70% of these patients were able to walk independently (Functional Ambulation Category scores > 3), but no patient achieved functional hand recovery.
Keywords: neural regeneration, brain injury, cerebral infarct, motor function, stroke, middle cerebral artery, hand function, walking ability, grants-supported paper, neuroregeneration
Research Highlights
-
(1)
This study, for the first time, reported the motor outcomes of upper and lower extremities in patients with a complete middle cerebral artery territory infarct.
-
(2)
After receiving rehabilitation treatments for 3–6 months, about 70% of patients were able to walk independently, but no patient achieved functional hand recovery.
-
(3)
Results from this study will provide supporting evidence for developing rational rehabilitation strategies and establishing proper goals for stroke patients.
INTRODUCTION
Middle cerebral artery territory infarction is the most common type of cerebral vascular territory infarct, and accounts for two-thirds of all cerebral infarcts[1]. Because the middle cerebral artery supplies the largest brain territory, middle cerebral artery territory infarcts are associated with many types of neurological deficits[2]. The middle cerebral artery territory comprises the corticospinal tract, which is responsible for fine motor activity of the hands, and the corticoreticulospinal tract, which is involved in postural control and locomotor function, and therefore, motor weakness is one of the most disabling sequelae of a middle cerebral artery infarct[1,3,4,5,6,7]. Previous studies on complete middle cerebral artery territory infarct mainly focus on evaluating mortality rates and elucidating proper initial treatments[8-9]. It has been reported that all patients show motor weakness during the acute stage following a complete middle cerebral artery territory infarct[2]. Detailed knowledge about motor outcomes enables to develop rehabilitative strategies and establish proper goals for stroke patients. Several studies have been reported regarding functional or motor outcomes in patients with a middle cerebral artery territory infarct[2,10,11,12,13]. However, little is known about motor outcomes in patients with a complete middle cerebral artery territory infarct. In this study, we investigated motor outcomes and the clinical characteristics of motor deficits in patients with a complete middle cerebral artery territory infarct.
RESULTS
Medical Research Council (MRC) and Motricity Index (MI) scores
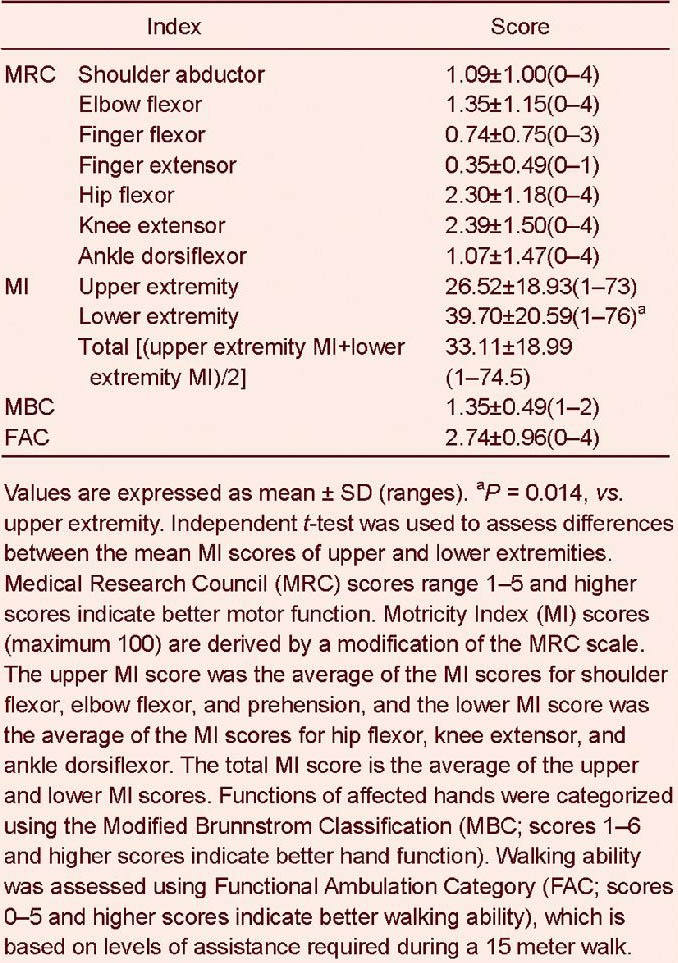
At 6 months after the onset of complete middle cerebral artery territory infarction, all patients showed motor weakness in both the upper and lower extremities. Mean MRC score was highest for knee extensors, followed by hip flexors, elbow flexors, shoulder abductors, ankle dorsiflexors, finger flexors, and lastly finger extensors (Table 1). The mean MI score of lower extremities was significantly higher than that of upper extremities (P = 0.014).
Table 1.
Motor outcomes of patients at 6 months after complete middle cerebral artery territory infarction
Modified Brunnstrom Classification (MBC) and Functional Ambulation Category (FAC) scores
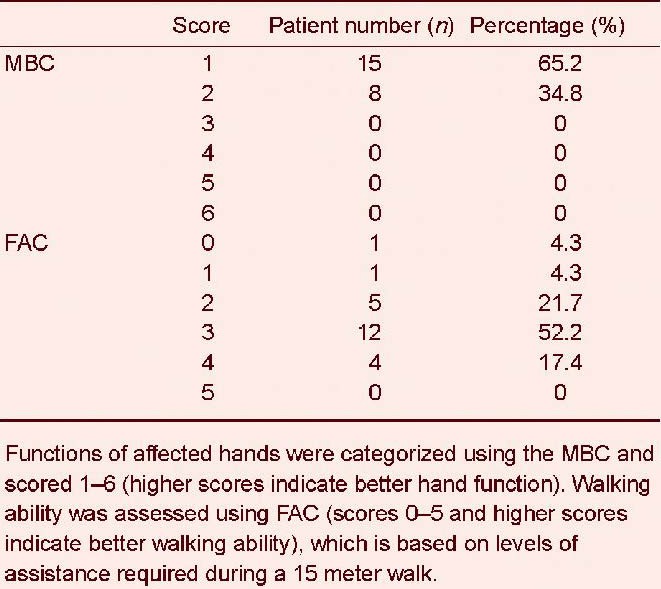
Mean MBC and FAC scores were 1.35 ± 0.49 and 2.74 ± 0.96, respectively (Table 1). All patients had a functional ability score of < 3 on the MBC, indicating that they were unable to move their fingers to a functional degree. Sixteen (70%) patients had an FAC score of ≥ 3 (Table 2), indicating that they were capable of independent walking.
Table 2.
Distribution of Modified Brunnstrom Classification (MBC) and Functional Ambulation Category (FAC) scores at 6 months after the onset of complete middle cerebral artery territory infarction
DISCUSSION
Some controversies exist concerning the definition of complete middle cerebral artery territory infarction, and currently three different definitions are applied, that is[2,12,14]: > 75% involvement of the middle cerebral artery territory, > 90% involvement of the middle cerebral artery territory, and involvement of three middle cerebral artery territories, including superficial anterior, superficial posterior, and deep territories.
In this study, we considered involvement of all three middle cerebral artery territories as a complete middle cerebral artery territory infarct. As a result, the clinical characteristics of patients, in terms of motor function and functional level, could be summarized as follows: (1) motor function: the motor functions of affected lower extremities were better than those of affected upper extremities; (2) functional level: none of the patients had a functional hand (MBC: 5–6); in contrast, about 70% of patients were able to walk independently (FAC: 3–5). It is well known that stroke patients are unable to perform fine motor activities of the hands after complete injury of the lateral corticospinal tract[15,16,17]. However, recent studies have demonstrated that stroke patients are able to walk even after complete injury of the lateral corticospinal tract, which suggests that non-pyramidal tracts, such as, the corticoreticulospinal tract or brainstem locomotor center facilitate motor recovery of the lower extremities to the extent of being able to walk[7,18,19,20,21,22,23,24]. Because complete middle cerebral artery territory infarcts involve the contralateral corticospinal and corticoreticulospinal tracts, the contribution of non-pyramidal tracts originating from the unaffected hemisphere or the brainstem locomotor center seems to be related to walking ability in our patients[7,19,20,21,22,23,24]. We believe that further studies are required to clarify this topic.
Several studies have investigated the clinical outcomes of middle cerebral artery territory infarction[2,10,11,12,13]. In 1998, Heinsius and colleagues[2] reported functional outcomes at 1 month after onset in 208 patients with a large middle cerebral artery territory infarct (at least 2 out of 3 middle cerebral artery sub-territories were invovled). One hundred and thirty-nine (66.8%) patients presented severe disability or death, and 62 (86.2%) of 72 patients with a complete middle cerebral artery territory infarct (more than 90% of the middle cerebral artery territory was invovled) presented severe disability or death. Moreover, they found that 99% of patients with a large middle cerebral artery territory infarct and 100% of patients with a complete middle cerebral artery territory infarct experienced motor deficits. In 1999, Miyai et al [12] compared characteristics of motor recovery according to the presence of premotor cortex lesions in 31 patients with a middle cerebral artery infarct, and found that patients with an intact premotor cortex showed better recovery in terms of mobility and motor function. In 2007, Ng et al[13] reported that functional outcomes, which were measured using the Functional Independence Measure at 1 month after onset, were poorest in patients with a middle cerebral artery infarct among 2 213 patients with different types of vascular territory infarcts. In 2009, Goto et al[11] demonstrated a negative correlation between infarct size and functional locomotion at 6 months after onset in 247 patients with a middle cerebral artery territory infarct. Recently, Balaban et al [10] evaluated functional outcomes using the Functional Independence Measure and Bathel Index at 6 months after stroke onset in 80 patients with a middle cerebral artery infarct and found that age was negatively correlated with functional outcome. As we described above, the majority of studies have focused on functional outcomes and relatively few have reported on a part of motor outcomes[2,10,11,12,13]. We believe that this is the first study to report detailed motor outcome data for patients with a complete middle cerebral artery infarct. However, direct comparisons between our results and those of previous studies are not possible because different assessment scales and inclusion criteria were used.
In conclusion, we investigated the motor outcomes of complete middle cerebral artery territory infarcts and found that motor function of the affected lower extremity is better than that of the affected upper extremity. As a result, about 70% of our patients were able to walk independently, but no patient achieved functional hand recovery. This study is limited to relatively small number of subjects used.
Moreover, because over half of the patients in this study received initial treatment for cerebral infarct in other hospitals, we were not able to show more detailed information about these patients, like infarct etiology, initial treatment, and initial states of patients. Therefore, further studies are required to compensate for these limitations. Nonetheless, we believe that this study provides useful information for clinicians regarding patients with a complete middle cerebral artery infarct.
SUBJECTS AND METHODS
Design
A retrospective study.
Time and setting
This study was performed at the Department of Physical Medicine and Rehabilitation, Yeungnam University Hospital, Republic of Korea from March 2004 to February 2012.
Subjects
This study was performed retrospectively among patients admitted for rehabilitation at Yeungnam University hospital. Twenty-three patients, 16 males and 7 females, aged 58.9 ± 11.3 (range, 40–74) years, were admitted to the Department of Physical Medicine and Rehabilitation at Yeungnam University hospital for rehabilitation treatments and recruited in this study. Fourteen patients had right hemisphere infarction. The inclusion criteria included (1) first-ever stroke; (2) aged 20–75 years; and (3) a complete middle cerebral artery territory infarct (superficial anterior, superficial posterior, and deep territories were involved)[12]. Patients with additional involvement of the anterior cerebral artery and/or posterior cerebral artery territories were excluded. Of these 23 patients, 10 underwent initial treatment for cerebral infarction at Department of Neurology in our hospital, and were then transferred to Department of Physical Medicine and Rehabilitation. The remainders received initial treatment at Department of Neurology in other hospitals, and were then transferred to our hospital for rehabilitation. The mean Barthel Index score of the patients was 49.5 ± 29.9[25]. The Mini-Mental State Examination (23.0 ± 8.3) and the Motor-Free Visual Perception Test (19.5 ± 8.5) were performed in 18 of the 23 patients[26,27].
Methods
MRI protocol
MRI information was obtained within 1 week of onset (Figure 1). MRI was performed using a sensitivity-encoding head coil on a 1.5-T Philips Gyroscan Intera (Hoffman-LaRoche, Ltd., Best, the Netherlands) with single-shot echo-planar imaging and navigator echo. Diffusion-weighted MR images (matrix = 176 × 172, field of view = 220 × 220 mm2, echo time = 83.3 ms, repetition time = 3 431.0 ms, b = 1 000 mm2/s, number of excitations = 4.0, with a 5 mm slice thickness) and T2-weighted MR images (matrix = 300 × 250, field of view = 210 × 210 mm2, echo time = 100.0 ms, repetition time = 4 202.0 ms, number of excitations = 3.0, with a 5.0 mm slice thickness) were acquired parallel to the bicommissure line of the anterior commissure-posterior commissure.
Figure 1.
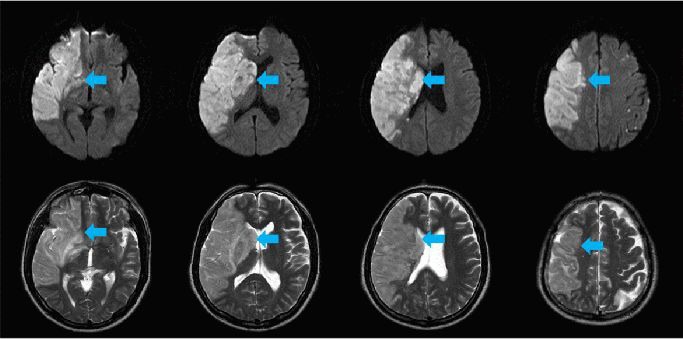
Diffusion-weighted (upper panel) and T2-weighted (lower panel) magnetic resonance images of a 55-year-old male patient with a complete middle cerebral artery territory infarct (blue arrows).
Clinical evaluations
Motor recovery usually reaches a plateau at 6 months after stroke[28,29]; thus, we evaluated motor outcome at or around 6 months after onset. Motor outcomes were evaluated by physiatrists using the MRC, MI, MBC, and FAC scores. MRC scores were determined as follows: 0, no contraction; 1, palpable contraction but no visible movement; 2, movement without gravity; 3, movement against gravity; 4, movement against a resistance lower than the resistance overcome by the healthy side; and 5, movement against a resistance equal to the maximum resistance overcome by the healthy side. MI scores (maximum score 100) are derived using a modification of the MRC scale. The MI score, except for prehension, is as follows: 0; no movement, 28; palpable contraction, but no movement, 42; movement, but not full range or against gravity, 56; movement, full range against gravity, not against resistance, 74; movement against resistance, weaker than the contralateral side, 100; normal strength. The MI score for prehension is as follows: 0; no movement, 33; beginning of prehension, 56; grips cube, without gravity, 65; holds cube, against gravity, 77; grips against pull, but weaker than the other side, 100; normal. The upper MI score is the average of the MI scores for shoulder flexor, elbow flexor, and prehension, and the lower MI score is the average of the MI scores for hip flexor, knee extensor, and ankle dorsiflexor. The total MI score is the average of the upper and lower MI score.
Functions of affected hands were categorized using the MBC and scored as follows; 1, unable to move fingers voluntarily; 2, able to move fingers voluntarily; 3, able to close the affected hand voluntarily, but unable to open the hand; 4, able to grasp a card between thumb and the medial side of the index finger, and able to extend fingers slightly; 5, able to pick up and hold a glass and extend fingers; and 6, able to catch and throw a ball in a near-normal fashion, and able to button and unbutton a shirt. Walking ability was assessed using FAC, which is based on levels of assistance required during a 15 meter walk. The six categories are described as follows; 0, non-ambulatory; 1, a need for continuous support from one person; 2, a need for intermittent support from one person; 3, a requirement for verbal supervision only; 4, help required on stairs and uneven surfaces; and 5, able to walk independently anywhere. The reliabilities and validities of the MRC, MI, MBC, and FAC have been well established[30,31,32,33].
Statistical analysis
Data were analyzed using SPSS15.0 software (SPSS, Chicago, IL, USA). Independent t-test was used to assess differences between mean MI scores of the upper and lower extremities. P < 0.05 was considered statistically significant.
Footnotes
Funding: This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology, No. 2012R1A1A4A01001873.
Conflicts of interest: None declared.
(Reviewed by Sharma VK, Zhang N, Wang LS)
(Edited by Li CH, Song LP, Liu WJ, Zhao M)
REFERENCES
- [1].Bogousslavsky J, Caplan LR. 2nd ed. New York: Cambridge University Press; 2001. Stroke Syndromes: Superficial Middle Cerebral Artery Syndromes. [Google Scholar]
- [2].Heinsius T, Bogousslavsky J, Van Melle G. Large infarcts in the middle cerebral artery territory. Etiology and outcome patterns. Neurology. 1998;50:341–350. doi: 10.1212/wnl.50.2.341. [DOI] [PubMed] [Google Scholar]
- [3].Hong JH, Jang SH. Motor recovery by anterior choroidal artery teriitory in a patient with middle cerebral artery infarct. Neural Regen Res. 2010;5:1357–1360. [Google Scholar]
- [4].Jang SH. Isolated corticospinal tract in a patient with intracerebral hemorrhage. Neural Regen Res. 2011;6:558–560. doi: 10.4103/1673-5374.187066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Jang SH, Cho SH, Lee MY, et al. Age-related changes of the corticospinal tract in the human brain. Neural Regen Res. 2011;6:283–287. [Google Scholar]
- [6].Jang SH, Choi BY, Chang CM, et al. Prediction of motor outcome based on diffusion tensor tractography findings in thalamic hemorrhage. Int J Neurosci. 2013;123:233–239. doi: 10.3109/00207454.2012.752364. [DOI] [PubMed] [Google Scholar]
- [7].Yeo SS, Chang MC, Kown YH, et al. Corticoreticular pathway in the human brain: Diffusion tensor tractography study. Neurosci Lett. 2012;508:9–12. doi: 10.1016/j.neulet.2011.11.030. [DOI] [PubMed] [Google Scholar]
- [8].Chen WH, Bai CH, Huang SJ, et al. Outcome of large hemispheric infarcts: an experience of 50 patients in Taiwan. Surg Neurol. 2007;68(Suppl 1):S68–73. doi: 10.1016/j.surneu.2007.07.086. [DOI] [PubMed] [Google Scholar]
- [9].Schwab S, Steiner T, Aschoff A, et al. Early hemicraniectomy in patients with complete middle cerebral artery infarction. Stroke. 1998;29:1888–1893. doi: 10.1161/01.str.29.9.1888. [DOI] [PubMed] [Google Scholar]
- [10].Balaban B, Tok F, Yavuz F, et al. Early rehabilitation outcome in patients with middle cerebral artery stroke. Neurosci Lett. 2011;498:204–207. doi: 10.1016/j.neulet.2011.05.009. [DOI] [PubMed] [Google Scholar]
- [11].Goto A, Okuda S, Ito S, et al. Locomotion outcome in hemiplegic patients with middle cerebral artery infarction: the difference between right- and left-sided lesions. J Stroke Cerebrovasc Dis. 2009;18:60–67. doi: 10.1016/j.jstrokecerebrovasdis.2008.09.003. [DOI] [PubMed] [Google Scholar]
- [12].Miyai I, Suzuki T, Kang J, et al. Middle cerebral artery stroke that includes the premotor cortex reduces mobility outcome. Stroke. 1999;30:1380–1383. doi: 10.1161/01.str.30.7.1380. [DOI] [PubMed] [Google Scholar]
- [13].Ng YS, Stein J, Ning M, et al. Comparison of clinical characteristics and functional outcomes of ischemic stroke in different vascular territories. Stroke. 2007;38:2309–2314. doi: 10.1161/STROKEAHA.106.475483. [DOI] [PubMed] [Google Scholar]
- [14].Al-Buhairi AR, Phillips SJ, Llewellyn G, et al. Prediction of infarct topography using the Oxfordshire Community Stroke Project classification of stroke subtypes. J Stroke Cerebrovasc Dis. 1998;7:339–343. doi: 10.1016/s1052-3057(98)80052-9. [DOI] [PubMed] [Google Scholar]
- [15].Binkofski F, Seitz RJ, Arnold S, et al. Thalamic metbolism and corticospinal tract integrity determine motor recovery in stroke. Ann Neurol. 1996;39:460–470. doi: 10.1002/ana.410390408. [DOI] [PubMed] [Google Scholar]
- [16].Jo HM, Choi BY, Chang SH, et al. The clinicl chracteristics of motor function in chronic hemiparetic stroke patients with complete corticospinal tract injury. NeuroRehabilitation. 2012;31:207–213. doi: 10.3233/NRE-2012-0790. [DOI] [PubMed] [Google Scholar]
- [17].Jang SH. The role of the corticospinal tract in motor recovery in patients with a stroke: a review. NeuroRehabilitation. 2009;24:285–290. doi: 10.3233/NRE-2009-0480. [DOI] [PubMed] [Google Scholar]
- [18].Ahn YH, Ahn SH, Kim H, et al. Can stroke patients walk after complete lateral corticospinal tract injury of the affected hemisphere? Neuroreport. 2006;17:987–990. doi: 10.1097/01.wnr.0000220128.01597.e0. [DOI] [PubMed] [Google Scholar]
- [19].Jang SH. The recovery of walking in stroke patients: a review. Int J Rehabil Res. 2010;33:285–289. doi: 10.1097/MRR.0b013e32833f0500. [DOI] [PubMed] [Google Scholar]
- [20].Kown HG, Lee DG, Son SM, et al. Identification of the anterior corticospinal tract in the human brain using diffusion tensor imaging. Neurosci Lett. 2011;505:238–241. doi: 10.1016/j.neulet.2011.10.020. [DOI] [PubMed] [Google Scholar]
- [21].Matsuyama K, Mori F, Nakajima K, et al. Locomotor role of the corticoreticular-reticulospinal-spinal interneuronal system. Prog Brain Res. 2004;143:239–249. doi: 10.1016/S0079-6123(03)43024-0. [DOI] [PubMed] [Google Scholar]
- [22].Yang HS, Kown HG, Hong JH, et al. The rubrospinal tract in the human brain: diffusion tensor imaging study. Neu-rosci Lett. 2011;504:45–48. doi: 10.1016/j.neulet.2011.08.054. [DOI] [PubMed] [Google Scholar]
- [23].Yeo SS, Ahn SH, Choi BY, et al. Contribution of the pedunculopontine nucleus on walking in stroke patients. Eur Neurol. 2011;65:332–337. doi: 10.1159/000324152. [DOI] [PubMed] [Google Scholar]
- [24].York DH. Review of descending motor pathways involved with transcranial stimulation. Neurosurgery. 1987;20:70–73. doi: 10.1097/00006123-198701000-00021. [DOI] [PubMed] [Google Scholar]
- [25].van der Putten JJ, Hobart JC, Freeman JA, et al. Measuring change in disability after inpatient rehabilitation: comparison of the responsiveness of the Barthel index and the Functional Independence Measure. J Neurol Neurosurg Psyshiatry. 1999;66:480–484. doi: 10.1136/jnnp.66.4.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Colarusso RP, Hammill DD. 3rd ed. Novato, CA, USA: Academic Therapy Publications; 2003. Motor Free Visual Perception Test. [Google Scholar]
- [27].Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”.A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- [28].Lidberg P, Schmitz C, Forssberg H, et al. Effects of passive-active movement training on upper limb motor function and cortical activation in chronic patient with strok: a pilot study. J Rehabil Med. 2004;36:117–123. [PubMed] [Google Scholar]
- [29].Page SJ, Gater DR, Bach-Y-Rita P, et al. Reconsidering the motor recovery plateau in stroke rehabilitation. Arch Phys Med Rehabil. 2004;85:1377–1381. doi: 10.1016/j.apmr.2003.12.031. [DOI] [PubMed] [Google Scholar]
- [30].Brunnstrom S. Motor testing procedures in hemiplegia: based on sequential recovery stages. Phys Ther. 1966;46:357. doi: 10.1093/ptj/46.4.357. [DOI] [PubMed] [Google Scholar]
- [31].Cunha IT, Lim PA, Henson H, et al. Performance-based gait tests for acute stroke patients. Am J Phys Med Rehabil. 2002;81:848–856. doi: 10.1097/00002060-200211000-00008. [DOI] [PubMed] [Google Scholar]
- [32].Demeurisse G, Demol O, Robaye E. Motor evaluation in vascular hemiplegia. Eur Neurol. 1980;19:382–389. doi: 10.1159/000115178. [DOI] [PubMed] [Google Scholar]
- [33].Fujii Y, Nakada T. Cortical reorganization in patients with subcortical hemiparesis: neural mechanisms of functional recovery and prognostic implication. J Neurosurg. 2003;98:64–73. doi: 10.3171/jns.2003.98.1.0064. [DOI] [PubMed] [Google Scholar]


