Abstract
Cross-training is a phenomenon related to motor learning, where motor performance of the untrained limb shows improvement in strength and skill execution following unilateral training of the homologous contralateral limb. We used functional MRI to investigate whether motor performance of the untrained limb could be improved using a serial reaction time task according to motor sequential learning of the trained limb, and whether these skill acquisitions led to changes in brain activation patterns. We recruited 20 right-handed healthy subjects, who were randomly allocated into training and control groups. The training group was trained in performance of a serial reaction time task using their non-dominant left hand, 40 minutes per day, for 10 days, over a period of 2 weeks. The control group did not receive training. Measurements of response time and percentile of response accuracy were performed twice during pre- and post-training, while brain functional MRI was scanned during performance of the serial reaction time task using the untrained right hand. In the training group, prominent changes in response time and percentile of response accuracy were observed in both the untrained right hand and the trained left hand between pre- and post-training. The control group showed no significant changes in the untrained hand between pre- and post-training. In the training group, the activated volume of the cortical areas related to motor function (i.e., primary motor cortex, premotor area, posterior parietal cortex) showed a gradual decrease, and enhanced cerebellar activation of the vermis and the newly activated ipsilateral dentate nucleus were observed during performance of the serial reaction time task using the untrained right hand, accompanied by the cross-motor learning effect. However, no significant changes were observed in the control group. Our findings indicate that motor skills learned over the 2-week training using the trained limb were transferred to the opposite homologous limb, and motor skill acquisition of the untrained limb led to changes in brain activation patterns in the cerebral cortex and cerebellum.
Keywords: neural regeneration, neuroimaging, cross-training effects, motor skill learning, cortical activation, cerebellar activation, serial reaction time task, functional MRI, response time, response accuracy, primary motor cortex, dentate nucleus, vermis, grants-supported paper, photographs-containing paper, neuroregeneration
Research Highlights
(1) We examined changes in brain activation patterns after motor sequential learning in the untrained hand after training of the opposite hand.
(2) Measurements of response time and accuracy were performed, while functional MRI was scanned during performance of a serial reaction time task.
(3) Sequential motor skill was transferred to the opposite hand, which led to changes in brain activation patterns in the cerebral and cerebellar regions.
(4) Elucidation of the cross-training effect will provide valuable therapeutic guidelines for clinicians who treat patients with unilateral limb motor dysfunction.
(5) This is the first evidence for changes in brain activity patterns in the cortical and cerebellar structures according to cross transfer of motor skill acquisition.
INTRODUCTION
Cross-training is a well-known phenomenon where training of a unilateral limb leads to movement effectiveness of the opposite untrained limb[1,2,3,4]. The potential of this effect to occur in the contralateral homologous limb is task specific. The use of unilateral limb practice affects the performance improvement of the untrained contralateral limb in terms of strength, motor skill, and endurance[5,6,7]. Numerous studies have investigated the neural mechanisms underpinning cross-training, including complex horizontal interhemispheric connections through various behavioral tasks such as figure drawing, sequential finger movements, and maze learning tasks[3,4,8,9].
Several studies have indicated that unilateral training results in an increase in strength of the contralateral untrained muscle group in strength transfer[10,11,12]. This is primarily caused by motor irradiation, which is ascribed to complex interhemispheric connections and ipsilateral corticospinal fibers from the primary motor cortex[13,14]. In addition, neuroimaging studies have demonstrated that the ipsilateral primary motor cortex, supplementary motor area, cingulate motor area, and prefrontal areas are active during unilateral training[15,16,17]. As a potential mechanism responsible for the change, the untrained limb may receive sufficient stimulus for neural adaptation.
However, despite these studies of neural contribution to motor sequence learning using the trained limb, to the best of our knowledge, the behavioral changes in the transfer effect of procedural learning after motor skill acquisition have not been reported, and the precise mechanisms underlying the contralateral training effect during learning are poorly understood.
The purpose of this study was to determine whether motor sequential learning of the untrained right hand, in terms of response time and accuracy, could be acquired through practice using the opposite unilateral limb, and whether these cross-training effects could lead to changes in brain activity patterns in the cerebral and cerebellar regions.
RESULTS
Quantitative analysis of subjects
Twenty right-handed healthy subjects were equally and randomly allocated into the training and control groups. The training group was trained in performance of a serial reaction time task using their non-dominant left hand, 40 minutes per day, for 10 days, within a period of 2 weeks. The control group did not receive training. All 20 subjects were included in the final analysis.
Description of demographic and behavioral data
There were no significant differences between the training and control groups in terms of age (P = 0.860), sex (P = 1.000), and the baseline variables response time (P = 0.931) and response accuracy (P = 0.379).
Table 1 shows the pre- and post-training response time and response accuracy in the serial reaction time task in the training and control groups.
Table 1.
Pre- and post-training motor performance in the serial reaction time task in the training and control groups
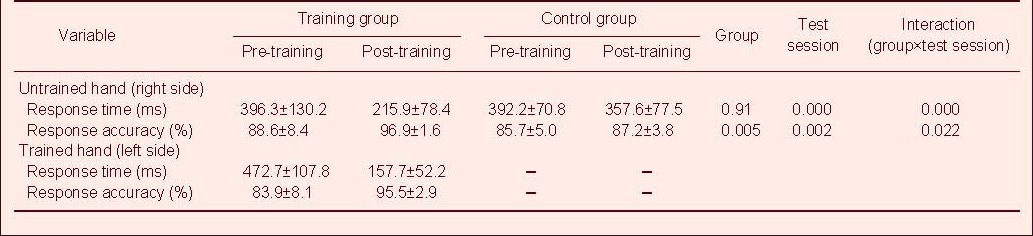
In terms of response time in the untrained right hand, two-way repeated measures analysis of variance showed a significant large main effect of test session (F(1,18) = 51.727, P < 0.001) and group-by-test session interaction (F(1,18) = 23.788, P < 0.001). However, there was no significant main effect of group (F(1,18) = 3.189, P > 0.05) in either group.
For the percentile of response accuracy of the untrained hand, two-way repeated measures analysis of variance showed a significant large main effect of group (F(1,18) = 10.234, P < 0.001), test session (F(1,18) = 12.343, P < 0.001), and group-by-test session interaction (F(1,18) = 6.271, P < 0.001), suggesting an increase in correct response and reduction in movement time in the training group compared with the control group. With all dependent variables in the trained left hand, paired t-test indicated significant differences (P < 0.001).
Blood oxygenation level-dependent signal intensity in cerebral cortices during performance of the serial reaction time task using the untrained hand
In the first functional MRI scanning, sequential movement using the right untrained hand induced cortical activations on the bilateral primary sensorimotor cortex, bilateral premotor cortex, and bilateral posterior parietal cortex in both groups.
To examine changes in blood flow across the two sets of functional MRI experiments, the blood oxygenation level-dependent signal values were analyzed for specific volumes of interest based on active regions identified in the imaging results. The averaged blood oxygenation level-dependent signal intensity in activated brain areas were as follows: in the training group (left primary sensorimotor area: 8.95 at x = –38, y = –20, z = 58; left premotor cortex: 6.78 at x = –28, y = –12, z = 62; left posterior parietal cortex: 13.58 at x = –30, y = –60, z = 50; right primary sensorimotor area: 2.01 at x = 32, y = –24, z = 55; right premotor cortex: 3.24 at x = 25, y = –17, z = 58; right posterior parietal cortex: 4.34 at x = 38, y = –63, z = 52); in the control group (left primary sensorimotor area: 18.15 at x = –33, y = –28, z = 62; left premotor cortex: 7.86 at x = –30, y = –18, z = 64; left posterior parietal cortex: 14.12 at x = –29, y = –58, z = 54; right primary sensorimotor area: 2.22 at x = 30, y = –22, z = 58; right premotor cortex: 2.98 at x = 30, y = –20, z = 62; right posterior parietal cortex: 2.18 at x = 36, y = –60, z = 54). After the 2-week training, the region of motor cortex activated during contralateral hand movement on all areas was markedly reduced, and the region of motor cortex activated during ipsilateral hand movement disappeared, as shown by initial functional MRI. A decrease in contralateral motor cortex activation and disappearance of ipsilateral motor cortex activation were observed in the control group (Figure 1). The primary sensory motor area activity was markedly decreased in the training group compared with the control group. After training, averaged blood oxygenation level-dependent signal intensity changes were observed as follows: in the training group (left primary sensorimotor area: 14.78 at x = –32, y = –26, z = 56; left premotor cortex: 5.27 at x = –31, y = –15, z = 63; left posterior parietal cortex: 3.05 at x = –28, y = –63, z = 53); in the control group (left primary sensorimotor area: 17.57 at x = –36, y = –20, z = 55; left premotor cortex: 6.21 at x = –30, y = –16, z =64; left posterior parietal cortex: 13.07 at x = –31, y = –62, z = 56).
Figure 1.
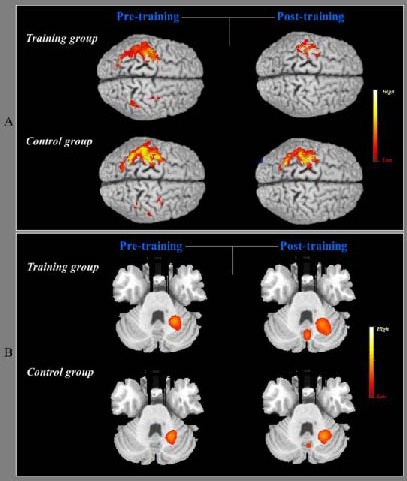
Pre- and post-training changes in cerebral cortex activation during sequential finger movement using the right untrained hand in the training and control groups.
(A) Sequential finger movement induced cortical activation on the bilateral primary sensorimotor cortex, bilateral premotor cortex, and bilateral posterior parietal cortex in the first functional MRI scans in the training and control groups. Following 2 weeks of training with the left hand, activation on the ipsilateral premotor cortex and posterior parietal cortex decreased, and contralateral activation on the primary sensorimotor area, premotor cortex, and posterior parietal cortex decreased, in the training and control groups. However, the left primary sensory motor area activity was significantly decreased in the training group compared with the control group.
(B) In the first functional MRI scans for the cerebellar regions, the ipsilateral cerebellar areas (left dentate nucleus) showed activation during rapid sequential finger movement in the training and control groups, and greater activation of the medial cerebellar area (vermis) in the training group. After 2 weeks of training with the left hand, an obvious increase in neural activation was observed in the vermis and the left dentate nucleus. In the control group, cortical activation of the left dentate nucleus was slightly increased and the vermis was newly activated. In the highlighted bar for the activated peak voxel, the yellow color indicates higher neural activation than the red color.
Blood oxygenation level-dependent signal intensity in cerebellar regions during performance of the serial reaction time task using the untrained hand
In the first functional MRI scans, activation of the ipsilateral deep cerebellar nuclei (left dentate nucleus) was observed in both the training and control groups during rapid sequential finger movement (i.e., average blood oxygenation level-dependent signal data were 8.61 at x = –24, y = –56, z = –26, and 8.23 at x = –22, y = –52, z = 26, in the training and control groups, respectively). In addition, slight activation on the medial cerebellar area (vermis) was observed in the training group (average blood oxygenation level-dependent signal intensity: 4.33 at x = –4, y = –68, z = –24). Following 2 weeks of training, a significant increase in activation was observed in the medial cerebellar area (average blood oxygenation level-dependent signal intensity: 10.78 at x = –24, y = –62, z = –23) and the ipsilateral deep cerebellar nuclei (average blood oxygenation level-dependent signal intensity: 8.12 at x = –6, y = –66, z = –26) during performance of the serial reaction time task. In the control group, activation of the ipsilateral deep cerebellar nuclei was significantly increased (average blood oxygenation level-dependent signal intensity: 8.75 at x = –20, y = –56, z = –22) compared with the initial functional MRI data, and the medial cerebellar area was newly activated (average blood oxygenation level-dependent signal intensity: 2.22 at x = –4, y = –64, z = –26). However, a greater degree of activation was observed on the ipsilateral deep cerebellar nuclei and the medial cerebellar area in the training group compared with the control group.
DISCUSSION
In the current study, we investigated whether 2 weeks of training using the left hand on a serial reaction time task led to changes in motor performance of the untrained right hand in terms of response time and accuracy, and whether this cross-training effect could induce changes in brain activation patterns in the cerebral cortex and cerebellum, using functional MRI scans.
We found a significant improvement in motor performance (movement speed and accuracy) of the untrained hand following hand training. This motor improvement is likely to be caused by transitional motor skills to the homogenous contralateral hand, explained by the cross-training effect.
By contrast, compared with the training group, the control group did not show significant improvements in these motor properties. In neuroimaging data, motor skills that are cross-transferred to the untrained hand reflected changes in brain activity pattern in the cortical and cerebellar structures. These findings imply that motor skill acquisition by a cross-training effect leads to a change in motor performance capacity, accompanied by efficient and effective processing of neural networks related to motor function.
Our behavioral findings are consistent with those of numerous prior studies related to the cross-training effect, suggesting that it involves a type of motor learning associated with neural plasticity in the brain[2,4,18,19,20,21]. According to previous studies examining the effect of muscular strength by cross-transfer learning, the strength gain was achieved in the homologous muscle of the opposite untrained limb, while the same movement task was performed by the trained limb[2,6,7,11,12]. The average gain in maximal voluntary contraction using electromyography was approximately 15–20% in the untrained homologous muscle, without changes in non-homologous muscles[1,22]. In addition, previous studies have reported that electrical stimulation or mental training applied concurrently with cross-limb training result in an increase in muscle strength in the untrained limb[23,24]. Together with strength gain, behavioral effects of cross-training have been proven in a variety of motor tasks in terms of occupationally embedded exercise and ballistic motor practice[1,20,21].
These behavioral changes are well established to reflect motor skill learning in multiple brain regions in cortical and subcortical structures[25,26,27]. As we found no significant differences between the two groups at the initial functional MRI scans, our volumetric data suggest that motor sequential activity required large-scale motor networking in the premotor area, primary sensorimotor area, posterior parietal cortex, and cerebellum. These results are consistent with prior studies examining brain activity concurrent with performance of sequence movement[28,29,30]. After 2 weeks of training with the untrained hand, we found extinction of the ipsilateral hemispheric activation and decreased contralateral hemispheric activation compared with the control group during performance of sequential motor activity. In addition, blood oxygenation level-dependent signal intensity was increased in the ipsilateral dentate nucleus and the vermis of the cerebellar region. It is generally accepted that recruitment of a large-scale neural network is decreased in the automatic phase[26,28,31], as motor learning progresses from a more conscious recollection in the early learning phase to an unintentional unconscious form in the late automatic phase.
Our functional MRI findings of motor sequential learning using the untrained hand showed similar results, which are supported by previous neuroimaging studies reporting changes of cortical activation pattern accompanied by motor skill acquisition using the trained limb[29]. Possible mechanisms for this cross-training effect can be explained using the proficiency model and the cross-activation model. These models involve formation of dual engrams by transfer of a unilateral engram, which is stored contralateral to the trained limb[1,32]. In other words, the unilateral motor engram by repetitive training with the left trained hand is built on the right hemisphere, and its engram is then transferred to the opposite hemisphere according to the cross-training effect. Accordingly, we confirmed that behavioral modification by cross-training effects induced the changes in brain activity pattern similar to that induced by motor skill learning using the trained limb. Unexpectedly, the control subjects showed increased performance and slightly decreased activation in the same areas observed in the training group when compared with the initial test. Presumably, at that point, the subjects had some information on implicit learning strategies and task familiarity.
Elucidation of motor learning mechanisms is important in the field of neuroscience as it reflects a source of human behavioral modification and it is related to neuronal plasticity. In particular, understanding the cross-training effect will provide valuable therapeutic guidelines for clinicians who treat patients with motor dysfunction in the unilateral upper or lower limb, as unilateral training on the healthy limb in patients with unilateral motor dysfunction (i.e., spinal cord injury, hemiparesis and knee arthroplasty) may produce clinical motor improvement in skill transfer using cross-training methods. To our knowledge, this is the first evidence of changes in brain activity patterns in the cortical and cerebellar structures according to cross transfer of motor skill acquisition. However, we acknowledge we had a small sample size and no measurement of retention period after motor learning in our study. Thus, further studies are required to elucidate the behavioral and neural mechanisms of the cross-training effect according to full motor learning stages (i.e., initial motor skill acquisition to retention stages).
MATERIALS AND METHODS
Design
A comparative functional MRI experiment with repeated measurements.
Time and setting
This study was performed at functional MRI room, Medical Center, Yeungnam University, Republic of Korea, from June to August 2012.
Subjects
Twenty healthy volunteers participated in this study by recruitment notice using the following inclusive criteria: (1) right-handed as confirmed by the modified Edinburg Handedness Inventory[33]; (2) no previous history of neurological or psychiatric illness; (3) no previous participation in experiments regarding motor sequencing learning with serial reaction time tasks. All subjects were randomly divided into training (6 males, mean age: 24.7 ± 2.4 years) or control groups (6 males, mean age: 24.9 ± 2.6 years). To control the known effects of the serial reaction time task, age and sex were matched in the two groups (P > 0.05). The subjects understood the purpose of this study and provided written informed consent prior to participation of this study, in accordance with the ethical standards of the Declaration of Helsinki[34].
Methods
Motor task paradigm and training procedure
Subjects were seated in a comfortable chair and placed 50 cm in front of a 17-inch monitor. The square-shaped response buttons were arranged horizontally on a response box connected to a computer. The motor task paradigm was designed using SuperLab Pro 4.0 software (Cedrus Co., San Pedro, CA, USA). Each button was labeled with a number representing the finger of the left hand to be used (1, 2, 3, and 4 represented the index, middle, ring, and little fingers, respectively). Motor sequence was composed of a combination of 1, 2, 3, and 4, which were randomly presented on the center of the computer monitor, with equal probabilities of 20%. A total of 200 stimuli per session were provided, and there were 50 trials for each number. When one of the numbers was presented on the screen, the subject was instructed to press the corresponding response button as accurately and quickly as possible using the dominant (right) hand. Stimuli were presented for a period of 1 000 ms, and the interval time between each stimulus was 500 ms for preparation of the next stimuli. Finally, one training session lasting 8 minutes consisted a 5-minute run phase and a 3-minute resting phase. The non-dominant left hand in the training group performed five repetitions of each training session for 40 minutes per day. The training group received 5 days of motor training per week for 2 consecutive weeks, whereas the control group did not receive any training. Figure 2 indicates the motor task and the functional MRI paradigm in the total experimental procedure.
Figure 2.
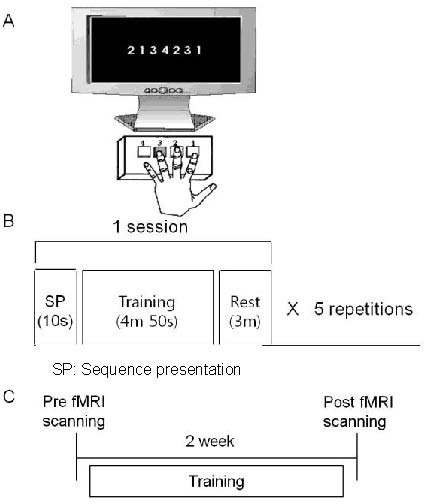
Schematic of the experiment.
(A) Example of training setting. Subjects were instructed to repeatedly push the four numbered response buttons as accurately and quickly as possible using the non-dominant (left) hand during the task block.
(B) Composition of the training paradigms. One training session was composed of display for 10 seconds, training for 4 minutes 50 seconds, and rest for 3 minutes, repeated five times.
(C) Timetable of motor training and functional MRI (fMRI) experiments in the training group.
Behavioral and functional MRI measurements during the pre- and post-training
Functional MRI scanning was performed on all participants in a supine position. The head, trunk, and arms were restrained to prevent motion artifacts during functional MRI experiments. The serial reaction time task for functional MRI scanning was presented by SuperLab Pro 4.0 software. Through a tilted mirror on the head coil of the MRI equipment, a rear-projection screen outside of the scanner was visible to the participants. According to the visual stimulation presented on the screen, participants pressed the buttons (LU440-LINE, Cedrus Co.), which were arranged horizontally on a response box connected to a computer. For the functional MRI paradigm, the activation paradigm was designed as four repeating blocks, which consisted of the serial reaction time task phase and the resting phase (each for 63 seconds). A ready period of 3 seconds was provided to allow the participants to prepare for task performance. Finally, to test region-specific condition effects for each of the phases, we subtracted the four resting phases from the four serial reaction time task phases.
To measure the changes in motor response and cortical activation pattern according to the cross-training effect, functional MRI scanning was performed twice during pre- and post-training, while the subjects performed the serial reaction time task with their dominant right hand using the same paradigm of the training procedure. The examiner continuously monitored the situation of the task performance through a video camera outside of the functional MRI scanner. Before performance of the serial reaction time task, all subjects took a practice block for accommodation with the task and the experimental procedure. In the behavioral function of the reaction time at the two tests, response time and percentile of correct response were measured using SuperLab Pro 4.0 software.
Functional MRI analysis
The blood oxygenation level-dependent functional MRI measurement, which employs the echo planar imaging technique, was performed using a 1.5-T Philips Gyroscan Intera scanner (Hoffman-LaRoche, Ltd., Best, The Netherlands) with a standard head coil. Foam padding was used to secure and limit the head motion of each participant within the coil. Twenty-eight slices were acquired using a single shot echo planar imaging sequence (time of repetition/echo time = 3 000/50 ms, flip angle 70°, field of view 210 mm, matrix 64 × 64, slice thickness 5 mm), and each functional run consisted of 85 images (including 5 dummies). Repetitive alternating phases of control and stimulation with serial reaction time task were performed as the stimulation task. Each “control and stimulation” task was repeated four times.
SPM8 software (Wellcome Department of Cognitive Neurology, London, UK) was used for analysis of all functional images. A slice timing correction and motion realignment were used for preprocessing of all images. Next, for multiple-subject comparisons, we performed spatial normalization to the Montreal Neurological Institute echo planar imaging template supplied with SPM8 software, and smoothing with an 8 mm the full width at half maximum Gaussian kernel. Differences in brain activation between the training and control groups were compared by a random effect group analysis (corrected P < 0.001). Quantitative comparisons between the training and control groups were obtained in terms of the changes of blood oxygenation level-dependent signal in selected regions of interest, focusing on the hand area of the primary sensorimotor area, premotor area, supplementary motor area, posterior parietal lobes, and cerebellum. For blood oxygenation level-dependent signal analysis, spherical volumes of interest (5 mm radius) were defined using the Talairach coordinates of the highest t values in the regions of interest of individual data. Averaged map changes of blood oxygenation level-dependent signal intensities by group analysis were extracted for each volume of interest using the differences of signal changes between the activation and recall conditions of each functional MRI experiment.
Statistical analysis
An independent t-test was used for the comparison of differences between the training and control groups in terms of the baseline data for response time, percentile of correct response, and age. All data were evaluated through separate univariate analyses of variance, using two-way repeated measures analysis of variance (groups: training group, control group) × 2 (test sessions: pre-training, post-training) on the two dependent variables of response time and correct response. PASW 18.0 (SPSS, Chicago, IL, USA) was used for all statistical analyses. P < 0.05 was considered statistically significant.
Footnotes
Funding: This study was supported by the Yeungnam College of Science & Technology Research Grants in 2012.
Conflicts of interest: None declared.
Ethical approval: The study protocol was approved by the Ethics Committee, Institutional Review Board of Yeungnam University Hospital, Republic of Korea.
(Edited by Dushanova J/Song LP)
REFERENCES
- [1].Hortobágyi T. Cross education and the human central nervous system. IEEE Eng Med Biol Mag. 2005;24(1):22–28. doi: 10.1109/memb.2005.1384096. [DOI] [PubMed] [Google Scholar]
- [2].Farthing JP. Cross-education of strength depends on limb dominance: implications for theory and application. Exerc Sport Sci Rev. 2009;37(4):179–187. doi: 10.1097/JES.0b013e3181b7e882. [DOI] [PubMed] [Google Scholar]
- [3].Kirsch W, Hoffmann J. Asymmetrical intermanual transfer of learning in a sensorimotor task. Exp Brain Res. 2010;202(4):927–934. doi: 10.1007/s00221-010-2184-8. [DOI] [PubMed] [Google Scholar]
- [4].Stöckel T, Wang J. Transfer of short-term motor learning across the lower limbs as a function of task conception and practice order. Brain Cogn. 2011;77(2):271–279. doi: 10.1016/j.bandc.2011.07.010. [DOI] [PubMed] [Google Scholar]
- [5].Camus M, Ragert P, Vandermeeren Y, et al. Mechanisms controlling motor output to a transfer hand after learning a sequential pinch force skill with the opposite hand. Clin Neurophysiol. 2009;120(10):1859–1865. doi: 10.1016/j.clinph.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Pearce AJ, Hendy A, Bowen WA, et al. Corticospinal adaptations and strength maintenance in the immobilized arm following 3 weeks unilateral strength training. Scand J Med Sci Sports. doi: 10.1111/j.1600-0838.2012.01453.x. in press. [DOI] [PubMed] [Google Scholar]
- [7].Yuza N, Ishida K, Miyamura M. Cross transfer effects of muscular endurance during training and detraining. J Sports Med Phys Fitness. 2000;40(2):110–117. [PubMed] [Google Scholar]
- [8].van Mier HI, Petersen SE. Intermanual transfer effects in sequential tactuomotor learning: evidence for effector independent coding. Neuropsychologia. 2006;44(6):939–949. doi: 10.1016/j.neuropsychologia.2005.08.010. [DOI] [PubMed] [Google Scholar]
- [9].Halsband U. Left hemisphere preponderance in trajectorial learning. Neuroreport. 1992;3(5):397–400. doi: 10.1097/00001756-199205000-00005. [DOI] [PubMed] [Google Scholar]
- [10].Lee M, Gandevia SC, Carroll TJ. Unilateral strength training increases voluntary activation of the opposite untrained limb. Clin Neurophysiol. 2009;120(4):802–808. doi: 10.1016/j.clinph.2009.01.002. [DOI] [PubMed] [Google Scholar]
- [11].Folland JP, Williams AG. The adaptations to strength training: morphological and neurological contributions to increased strength. Sports Med. 2007;37(2):145–168. doi: 10.2165/00007256-200737020-00004. [DOI] [PubMed] [Google Scholar]
- [12].Farthing JP, Chilibeck PD, Binsted G. Cross-education of arm muscular strength is unidirectional in right-handed individuals. Med Sci Sports Exerc. 2005;37(9):1594–600. doi: 10.1249/01.mss.0000177588.74448.75. [DOI] [PubMed] [Google Scholar]
- [13].Hortobágyi T, Richardson SP, Lomarev M, et al. Interhemispheric plasticity in humans. Med Sci Sports Exerc. 2011;43(7):1188–1199. doi: 10.1249/MSS.0b013e31820a94b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hendy AM, Spittle M, Kidgell DJ. Cross education and immobilisation: mechanisms and implications for injury rehabilitation. J Sci Med Sport. 2012;15(2):94–101. doi: 10.1016/j.jsams.2011.07.007. [DOI] [PubMed] [Google Scholar]
- [15].Anguera JA, Russell CA, Noll DC, et al. Neural correlates associated with intermanual transfer of sensorimotor adaptation. Brain Res. 2007;1185:136–151. doi: 10.1016/j.brainres.2007.09.088. [DOI] [PubMed] [Google Scholar]
- [16].Seidler RD, Noll DC, Chintalapati P. Bilateral basal ganglia activation associated with sensorimotor adaptation. Exp Brain Res. 2006;175(3):544–555. doi: 10.1007/s00221-006-0571-y. [DOI] [PubMed] [Google Scholar]
- [17].Farthing JP, Krentz JR, Magnus CR, et al. Changes in functional magnetic resonance imaging cortical activation with cross education to an immobilized limb. Med Sci Sports Exerc. 2011;43(8):1394–1405. doi: 10.1249/MSS.0b013e318210783c. [DOI] [PubMed] [Google Scholar]
- [18].Lee M, Carroll TJ. Cross education: possible mechanisms for the contralateral effects of unilateral resistance training. Sports Med. 2007;37(1):1–14. doi: 10.2165/00007256-200737010-00001. [DOI] [PubMed] [Google Scholar]
- [19].Kasuga S, Nozaki D. Cross talk in implicit assignment of error information during bimanual visuomotor learning. J Neurophysiol. 2011;106(3):1218–1226. doi: 10.1152/jn.00278.2011. [DOI] [PubMed] [Google Scholar]
- [20].Nagel MJ, Rice MS. Cross-transfer effects in the upper extremity during an occupationally embedded exercise. Am J Occup Ther. 2001;55(3):317–323. doi: 10.5014/ajot.55.3.317. [DOI] [PubMed] [Google Scholar]
- [21].Farthing JP, Borowsky R, Chilibeck PD, et al. Neurophysiological adaptations associated with cross-education of strength. Brain Topogr. 2007;20(2):77–88. doi: 10.1007/s10548-007-0033-2. [DOI] [PubMed] [Google Scholar]
- [22].Kristeva R, Cheyne D, Deecke L. Neuromagnetic fields accompanying unilateral and bilateral voluntary movements: topography and analysis of cortical sources. Electroencephalogr Clin Neurophysiol. 1991;81(4):284–298. doi: 10.1016/0168-5597(91)90015-p. [DOI] [PubMed] [Google Scholar]
- [23].Hortobágyi T, Lambert NJ, Hill JP. Greater cross education following training with muscle lengthening than shortening. Med Sci Sports Exerc. 1997;29(1):107–112. doi: 10.1097/00005768-199701000-00015. [DOI] [PubMed] [Google Scholar]
- [24].Yue G, Cole KJ. Strength increases from the motor program: comparison of training with maximal voluntary and imagined muscle contractions. J Neurophysiol. 1992;67(5):1114–1123. doi: 10.1152/jn.1992.67.5.1114. [DOI] [PubMed] [Google Scholar]
- [25].Bédard P, Sanes JN. On a basal ganglia role in learning and rehearsing visual-motor associations. Neuroimage. 2009;47(4):1701–1710. doi: 10.1016/j.neuroimage.2009.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Bapi RS, Miyapuram KP, Graydon FX, et al. fMRI investigation of cortical and subcortical networks in the learning of abstract and effector-specific representations of motor sequences. Neuroimage. 2006;32(2):714–727. doi: 10.1016/j.neuroimage.2006.04.205. [DOI] [PubMed] [Google Scholar]
- [27].Krutky MA, Perreault EJ. Motor cortical measures of use-dependent plasticity are graded from distal to proximal in the human upper limb. J Neurophysiol. 2007;98(6):3230–3241. doi: 10.1152/jn.00750.2007. [DOI] [PubMed] [Google Scholar]
- [28].Park JW, Kim YH, Jang SH, et al. Dynamic changes in the cortico-subcortical network during early motor learning. NeuroRehabilitation. 2010;26(2):95–103. doi: 10.3233/NRE-2010-0540. [DOI] [PubMed] [Google Scholar]
- [29].Park JW, Kwon YH, Lee MY, et al. Brain activation pattern according to exercise complexity: a functional MRI study. NeuroRehabilitation. 2008;23:283–288. [PubMed] [Google Scholar]
- [30].Kwon YH, Nam KS, Park JW. Identification of cortical activation and white matter architecture according to short-term motor learning in the human brain: functional MRI and diffusion tensor tractography study. Neurosci Lett. 2012;520(1):11–15. doi: 10.1016/j.neulet.2012.05.005. [DOI] [PubMed] [Google Scholar]
- [31].Han Y, Yang H, Lv YT, et al. Gray matter density and white matter integrity in pianists’ brain: a combined structural and diffusion tensor MRI study. Neurosci Lett. 2009;459(1):3–6. doi: 10.1016/j.neulet.2008.07.056. [DOI] [PubMed] [Google Scholar]
- [32].Thut G, Cook ND, Regard M, et al. Intermanual transfer of proximal and distal motor engrams in humans. Exp Brain Res. 1996;108(2):321–327. doi: 10.1007/BF00228105. [DOI] [PubMed] [Google Scholar]
- [33].Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- [34].World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. J Postgrad Med. 2002;48(3):206–208. [PubMed] [Google Scholar]


