Abstract
Knowledge of the plasticity of language pathways in patients with low-grade glioma is important for neurosurgeons to achieve maximum resection while preserving neurological function. The current study sought to investigate changes in the ventral language pathways in patients with low-grade glioma located in regions likely to affect the dorsal language pathways. The results revealed no significant difference in fractional anisotropy values in the arcuate fasciculus between groups or between hemispheres. However, fractional anisotropy and lateralization index values in the left inferior longitudinal fasciculus and lateralization index values in the left inferior fronto-occpital fasciculus were higher in patients than in healthy subjects. These results indicate plasticity of language pathways in patients with low-grade glioma. The ventral language pathways may perform more functions in patients than in healthy subjects. As such, it is important to protect the ventral language pathways intraoperatively.
Keywords: neural regeneration, neuroimaging, low-grade glioma, plasticity, language pathways, diffusion tensor imaging, fractional anisotropy, lateralization index, ventral language pathway, dorsal language pathway, glioma, grants-supported paper, photographs-containing paper, neuroregeneration
Research Highlights
(1) Studies of plasticity in the neural basis of language in pathological conditions have mainly focused on cerebral cortex, while subcortical pathways have largely been ignored. Although several studies have examined changes in subcortical pathways in glioma using intraoperative electrical stimulation, this method can only examine changes in subcortical pathways at the boundary of a tumor. In the current study, we divided the left hemisphere into four regions according to the dual stream model, and examined patients with tumors in only one region. This method solves the problem that tumor in different areas may have different effects on language pathways.
2 Cases in which only the dorsal pathway was affected were included in this study. Examining changes in the ventral pathway revealed plasticity in the language pathways of patients with low-grade glioma. The results suggest that ventral language pathways may take on compensatory functions in patients compared with healthy subjects. These findings highlight the importance of intraoperatively protecting the ventral language pathways.
INTRODUCTION
Low-grade glioma (WHO II grade glioma) is a common intracranial tumor representing approximately 15–25% of all glioma, with an average incidence of around 1 per 1 000 000 people per year. Low-grade glioma often invades the ‘eloquent’ areas, such as the insular cortex, supplementary motor area, premotor cortex, Broca's area and Wernicke's area, and has a tendency to migrate along the main white matter pathways[1]. Nevertheless, most cases of low-grade glioma (> 80%) are detected by seizure activity, with little or no neurological deficit[1,2]. This phenomenon can be explained by long-term brain plasticity due to the low growth rate of low-grade glioma[3]. Increased knowledge of brain plasticity in low-grade glioma will help neurosurgeons achieve maximal resection while preserving neurologic function[4], improving the life expectancy of low-grade glioma patients[5,6]. In addition, studying low-grade glioma patients can expand our understanding of brain plasticity more generally.
A number of preoperative functional neuroimaging studies of cortical plasticity in low-grade glioma have used functional MRI, positron emission tomography, and magnetoencephalography[7,8,9,10], leading to several proposed models of cortical reorganization[11]. However, no imaging studies have examined white matter plasticity in the language pathways in low-grade glioma patients.
In diffusion tensor imaging, diffusion is measured in a series of different spatial directions, from which each pixel can be described as an ellipsoid with a different shape and orientation[12]. Thus, diffusion tensor imaging can be used to parcellate white matter within substructures, and white matter tracts can be reconstructed based on diffusion tensor imaging data simply by connecting the fiber orientation information pixel-by-pixel. Tractography performed by diffusion tensor imaging has been found to correlate well with white matter[13]. In addition, fractional anisotropy values can be used to describe certain characteristics of white matter[14,15]. Diffusion tensor imaging is currently the only way to investigate white matter in humans in vivo[16], providing a noninvasive and feasible method for evaluating changes in the main language pathways.
The current study used diffusion tensor imaging to examine changes in language pathways in patients with low-grade glioma, investigating relative changes in the arcuate fasciculus, inferior longitudinal fasciculus and inferior fronto-occipital fasciculus in patients with low-grade glioma located in regions of interest.
RESULTS
Quantitative analysis of subjects
185 glioma patients were included in this study. Ten patients were selected as a case group (Figure 1). An additional ten healthy individuals were included as controls. A total of 20 subjects were included in the final analysis.
Figure 1.
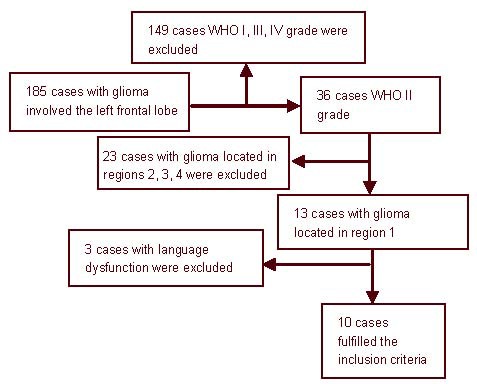
Flow chart of case inclusion.
Baseline data
Characteristics of patients are presented in Table 1. There were no significant differences between the patient and control groups in terms of sex (7 males and 3 females in each group, P = 1.00, chi-square test) or age (46 ± 5 years vs. 44 ± 4 years, P = 0.357; independent-samples t-test).
Table 1.
Clinical characteristics of patients with low-grade glioma
Tractography results
Bilateral arcuate fasciculus, inferior longitudinal fasciculus and inferior fronto-occipital fasciculus were successfully reconstructed in all patients and controls (Figures 2–4).
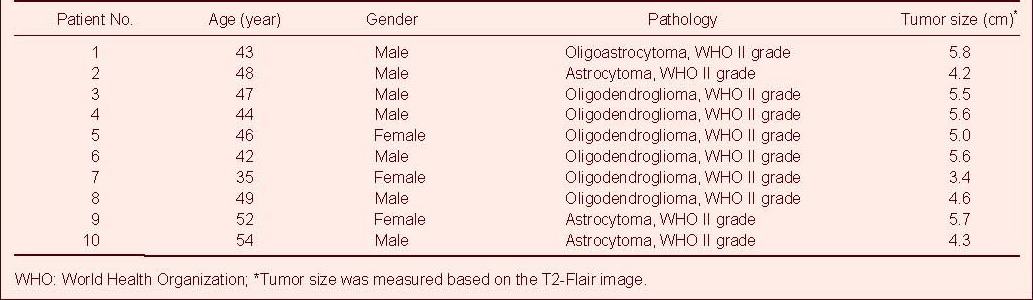
Figure 2.
Tractography of arcuate fasciculus in patients with glioma and healthy controls.
(A) The first region of interest lateral to the corona radiata on the coronal slice. (B) The second region of interest is drawn on the axial slice at the anterior commissure level lateral to the sagittal stratum. (C, D) 3D view of the arcuate fasciculus (purple) we reconstructed.
In the diffusion tensor imaging color maps, red, green, and blue colors were assigned to right-left, anterior-posterior, and superior-inferior orientations, respectively.
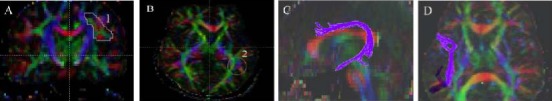
Figure 4.
Tractography of inferior fronto-occpital fasciculus in patients with glioma and healthy controls.
(A) The first region of interest is drawn on the extreme and external capsule on the coronal slice. (B) The second region of interest is drawn on the coronal slice at the occipital lobe pole level. (C, D) 3D view of the inferior fronto-occpital fasciculus (olive green) reconstructed.
In the diffusion tensor imaging color maps, red, green, and blue were assigned to right-left, anterior-posterior, and superior-inferior orientations, respectively.
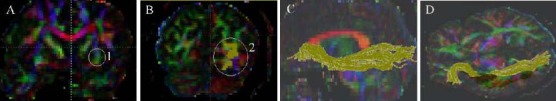
Figure 3.
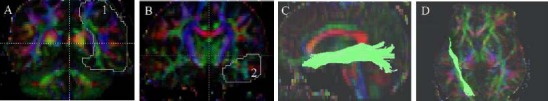
Tractography of inferior longitudinal fasciculus in patients with glioma and healthy controls.
(A) The first region of interest is drawn on the coronal slice at the level of the posterior edge of the corpus callosum. (B) The second region of interest is drawn on the coronal slice at the level of the temporal lobe pole. (C, D) 3D view of the inferior longitudinal fasciculus (green) we reconstructed.
In the diffusion tensor imaging color maps, red, green, and blue were assigned to right-left, anterior-posterior, and superior-inferior orientations, respectively.
Fractional anisotropy values in the arcuate fasciculus, inferior longitudinal fasciculus and inferior fronto-occipital fasciculus between hemispheres in patients and healthy subjects
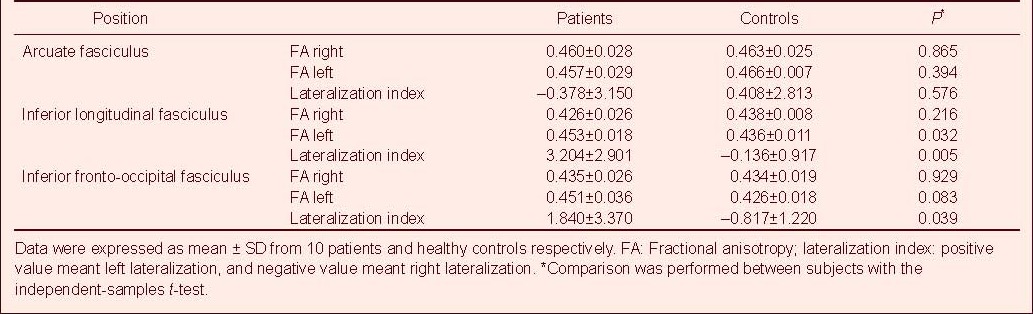
In healthy subjects, there was no significant difference in fractional anisotropy values in the arcuate fasciculus, inferior longitudinal fasciculus and inferior fronto-occipital fasciculus between hemispheres (P = 0.717, 0.684, 0.086, respectively; paired-samples t-test; Table 2).
Table 2.
Comparison of fractional anisotropy between hemispheres in patients with low-grade glioma and healthy controls
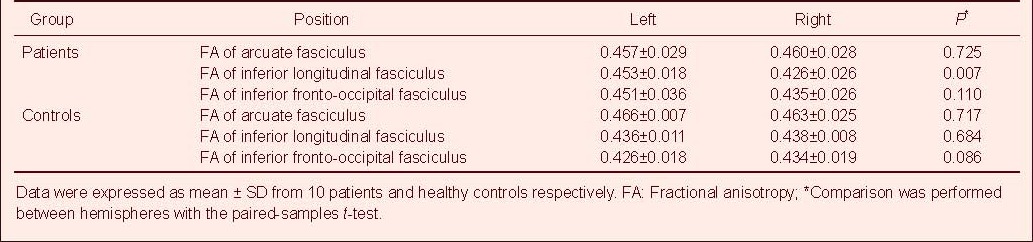
In patients, fractional anisotropy values in the inferior longitudinal fasciculus were lateralized to the left between hemispheres (P = 0.007; paired-samples t-test; Table 2), whereas fractional anisotropy values in the arcuate fasciculus and inferior fronto-occipital fasciculus were not lateralized between hemispheres (P = 0.725, 0.110 respectively; paired-samples t-test; Table 2).
Comparison of fractional anisotropy and lateralization index between patients and healthy subjects
Fractional anisotropy in the left inferior longitudinal fasciculus in patients was significantly higher than in healthy subjects (P = 0.032; independent-samples t-test). The lateralization index values of the inferior longitudinal fasciculus and inferior fronto-occipital fasciculus were significantly lateralized to the left in patients compared with healthy subjects (P = 0.005, 0.039, respectively; independent-samples t-test). There was no significant difference in fractional anisotropy and lateralization index values of the arcuate fasciculus between patients and controls (Table 3).
Table 3.
Comparison of fractional anisotropy and lateralization index between patients with low-grade glioma and healthy controls
DISCUSSION
In 1874, Wernicke first proposed that the anterior and posterior language regions were connected by white matter fiber tracts based on seminal aphasiology lesion studies. Geschwind[17] revived Wernicke's theory, proposing a model in which the left arcuate fasciculus, a large bundle running dorsally along the frontal, inferior parietal, and perisylvian temporal cortices, was the main white matter fiber tract thought to connect the classical Broca's (left inferior frontal gyrus) and Wernicke's (left posterior superior and middle temporal gyrus) language areas. Recent developments in diffusion tensor imaging and intraoperative subcortical mapping have led several researchers to propose a dual stream model for language[18,19,20,21,22]. This model consists of two streams: dorsal and ventral. The dorsal stream refers to the arcuate fasciculus, while the ventral stream is constituted by at least two pathways: (1) a direct pathway in the inferior fronto-occipital fasciculus, connecting the posterior temporal areas and orbitofrontal region; and (2) an indirect pathway in the inferior longitudinal fasciculus, which links the posterior occipitotemporal and the temporal pole, relayed by the uncinate fasciculus, which connects the temporal pole to the basifrontal areas. This ventral pathway is argued to play a key role in language comprehension[23], complementing the role of the arcuate fasciculus pathway in language production. The pathways and specialized brain areas (such as Broca's and Wernicke's areas) are thought to constitute a large-scale network for language. This study divided the left hemisphere into four regions according to this dual stream model.
Only the dorsal steam i.e. arcuate fasciculus was located in region 1. Neither the ventral nor dorsal steams were located in region 2. Both the ventral and dorsal streams were located in regions 3 and 4. We only included patients exhibiting low-grade glioma in region 1 in the current study, because the ventral pathway is not likely to be directly affected by low-grade glioma in these patients, and changes in the ventral pathway reflect the plasticity of language pathways.
Numerous studies of language pathways in patients with low-grade glioma have been performed using intraoperative subcortical mapping during surgery while patients were awake[24,25,26]. Intraoperative subcortical mapping can provide direct functional information about the white matter tract, but only white matter tracts adjacent to a tumor can be investigated. Changes of overall language networks cannot be delineated. Diffusion tensor tractography provides a full view of the main tracts constituting the language networks. Fractional anisotropy values calculated based on diffusion tensor imaging data indicate the degree of directional sensitivity of water diffusion within a voxel, which is generally interpreted as an indicator for fiber connectivity, and is thus considered a suitable measure for exploring possible alterations in white matter tract micro-structure[27]. The DtiStudio[28] imaging processing software used in the current study is able to reconstruct the arcuate fasciculus, the inferior longitudinal fasciculus and the inferior fronto-occipital fasciculus with high reproducibility[29]. As such, it is thought to provide a reliable tool for studying white matter.
Many factors can affect fractional anisotropy values. Tumor infiltration, edema around a tumor and demyelination of the white matter tract can decrease fractional anisotropy values, tumor compression and myelination of the white matter tract can increase it. All patients in the current study exhibited tumors in region 1, most likely affecting the left arcuate fasciculus. However, we found no significant difference in fractional anisotropy values in the arcuate fasciculus between groups or hemispheres. One possible explanation for this result is that only part of the arcuate fasciculus was affected by tumor infiltration and edema, while we calculated fractional anisotropy values over the entire tract. Alternatively, the fractional anisotropy values may have increased in some cases because of tumor compression.
Reorganization of language-specialized brain areas in patients with low-grade glioma has been reported in a number of studies using functional MRI, positron emission tomography, magnetoencephalography and intraoperative brain mapping. The language-specialized areas are thought to function like nodes in a language network. However, it remains unclear whether changes within these nodes are accompanied by changes in connectivity between these nodes (i.e. the language pathways). Yeatman et al[30] reported a case of a 12-year-old girl missing the arcuate fasciculus but with an intact ventral stream bilaterally, who exhibited normal expressive language, sentence repetition, and reading ability, even though these functions are thought to depend on signals carried by arcuate fasciculus. Yogarajah et al[31] observed increased fractional anisotropy values in the ventral stream in response to damage to the dorsal pathway in epilepsy patients. This report indicated that the ventral stream may compensate for the role of the arcuate fasciculus in pathological cases. The present study revealed that fractional anisotropy and lateralization index values in the left inferior longitudinal fasciculus, and lateralization index values in the left inferior fronto-occipital fasciculus were significantly higher in patients than in healthy subjects. These results may reflect structural reorganization in response to a slow-growing tumor, potentially providing an indicator for increased ventral stream connectivity, such that more signals are transferred through the ventral stream connecting the anterior and posterior language regions. The current results provide indirect evidence supporting the dual stream language model. The dorsal and ventral pathways both play a role in language; the current findings suggest that the ventral pathways may compensate the role of the dorsal pathway when the dorsal pathway is inhibited. In addition, our findings also highlighted the importance of protecting the ventral language pathways in low-grade glioma surgery, since its role may be different from that in healthy subjects. However, diffusion tensor imaging is only capable of providing anatomical information, and cannot examine functional changes. Combined anatomical and functional studies[32] are required to verify that ventral pathway function is enhanced in low-grade glioma, and to clarify the potential mechanisms underlying such an effect.
The current study involves several limitations. First, our sample size was small, weakening the statistical analysis. As such, we were unable to analyze the effects of age, gender and pathology on the plasticity of the language pathway in patients. Second, diffusion tensor tractography can only reconstruct large-scale white matter tracts, meaning that changes in small tracts related to language function cannot be examined. A voxel-based diffusion-tensor imaging study[33] may clarify this issue. Third, the current study was cross-sectional in design. A longitudinal study including preoperative, postoperative and follow-up diffusion tensor imaging data is needed to better understand white matter changes.
In conclusion, fractional anisotropy values in the left ventral language pathways were increased in patients with low-grade glioma compared with healthy subjects. These findings indicate language pathway reorganization, possibly explaining the presence of normal preoperative language function. Further functional studies are required to confirm and extend the current findings. This study provides a basis for future research into the plasticity of language pathways in patients with low-grade glioma.
SUBJECTS AND METHODS
Design
A retrospective study in radiology.
Time and setting
Experiments were performed in the Nuclear Magnetic Resonance Laboratory, Department of Neurosurgery, Chinese PLA General Hospital, China from April 2010 to April 2012.
Subjects
We recruited 185 patients undergoing surgery for glioma (diagnosis confirmed by postoperative pathological examination) involving the left frontal lobe at the Chinese PLA General Hospital. Among the 185 patients, 10 patients fulfilled the following inclusion criteria: (1) patients underwent diffusion tensor imaging scanning in our hospital preoperatively; (2) the tumor location was confined to the left frontal region above the level of the superior insular fissure and anterior to the posterior edge of the corpus callosum (as shown in Figure 5); (3) the functional MRI and Edinburgh handedness index indicated left language dominance[34]; (4) preoperative language function was normal (aphasia quotients > 93.8)[35]; (5) the tumor diameter was more than 3 cm; (6) pathological diagnosis revealed WHO II grade glioma. Exclusion criteria: patients with disease that may affect white matter, such as multiple sclerosis, dementia and schizophrenia were excluded.
Figure 5.
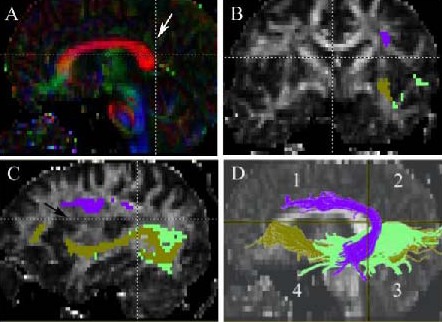
The regions we defined in the included patients.
(A) Middle sagittal view of the horizontal and vertical section. The vertical section (white arrow) we defined is at the posterior edge of the corpus callosum.
(B) Coronal view of the horizontal section we defined, arcuate fasciculus (purple) is above the section, the inferior longitudinal fasciculus (green) and the inferior fronto-occpital fasciculus (olive green) are under the section.
(C) Paramedian sagittal view of the horizontal section we defined. The horizontal section is at the level of the superior insular fissure (black arrow). The inferior longitudinal fasciculus (green) and the inferior fronto-occpital fasciculus (olive green) are under the section.
(D) The hemisphere is divided into four regions by the horizontal and vertical sections we defined. Only patients with tumors located in region 1 are included. The inferior longitudinal fasciculus (green) and the inferior fronto- occpital fasciculus (olive green) located in regions 3 and 4.
Of the 10 included patients, seven were male and three were female, with ages ranging from 35 to 54 years. A total of 10 normal right-handed controls (from the healthy check-up population) were also tested (seven males and three females, ages ranging from 38 to 49 years). Handedness was determined using the Edinburgh handedness inventory. Informed consent was provided by participants and family members, and the study was conducted in accordance with the Declaration of Helsinki.
Methods
Image acquisition
Diffusion tensor imaging was performed with a 1.5 T scanner (Siemens Espree, Erlangen, Germany). We used a single-shot spin-echo diffusion weighted echo-planar imaging sequence[36]. The sequence parameters were as follows: echo time 147 ms, repetition time 9 400 ms, matrix 128 × 128, field of view 232 mm × 232 mm, slice thickness 2.7 mm. 12 directions of diffusion-weighted imaging, b1 (high b value) 1 000 s/mm2, b0 (low b value) 0 s/mm2, voxel size 1.8 mm × 1.8 mm × 2.7 mm. Forty-five layers were continuously acquired four times to improve the signal-to-noise ratio. Co-registered magnetization-prepared rapid gradient echo images were recorded for anatomical guidance.
Image processing
Diffusion tensor imaging datasets were transferred to a PC running Windows. The DICOM files were converted to the 4D NifTI image format for analysis using dcm2nii software (University of South Carolina, Columbia, NJ, USA, www.mricro.com). We used diffusion tensor imaging studio (Hangyi Jiang and Susumu Mori, Johns Hopkins University and Kennedy Krieger institute, http://godzilla.kennedykrieger.org or http://lbam.med.jhmi.edu) analysis software to process the data. Images were first realigned using the Automatic Image Registration command to remove any potential small bulk motions that occurred during the scans. All diffusion-weighted images were then visually inspected by the researchers for apparent artifacts due to subject motion and instrument malfunction. The six elements of the diffusion tensor were calculated for each pixel using multivariate linear fitting[29]. After tensor diagonalization, three eigenvalues and eigenvectors were obtained and fractional anisotropy maps were calculated. The eigenvector associated with the largest eigenvalue was used as an indicator for fiber orientation. In the diffusion tensor imaging color maps, red, green, and blue colors were assigned to right-left, anterior-posterior, and superior-inferior orientations, respectively.
Tractography
The arcuate fasciculus, inferior longitudinal fasciculus and inferior fronto-occipital fasciculus were reconstructed. Fiber assignment was performed with a continuous tracking algorithm[37] in this study with a fractional anisotropy threshold of 0.2 and an inner product threshold of 0.70. Fiber tracking was performed using diffusion tensor imaging studio (Johns Hopkins University and Kennedy Krieger institute, Baltimore, MD, USA). A multi-region of interest approach was used to reconstruct tracts of interest, exploiting existing anatomical knowledge of tract trajectories. Tracking was performed from all pixels inside the brain (brute-force approach) and results penetrating the manually defined regions of interest were assigned to the specific tracts associated with the regions of interest. We defined the regions of interest according to previously reported protocols[27]. After tracts were reconstructed, mean fractional anisotropy was calculated over the entire tract. The lateralization index was used to express relative change of fractional anisotropy values between hemispheres. The lateralization index was calculated from the following equations[38]:
Lateralization index of fractional anisotropy = 100 × (left fractional anisotropy – right fractional anisotropy)/(left fractional anisotropy + right fractional anisotropy)
Statistical analysis
All data were analyzed using SPSS 17.0 software (SPSS, Chicago, IL, USA). Fractional anisotropy and lateralization index data were presented as mean ± SD. The independent-samples t-test and chi-square test were used for comparisons between groups. Paired-samples t-tests were used for comparison between hemispheres. A P-value less than 0.05 (two-sided) was considered statistically significant.
Acknowledgments
We would like to thank Xinguang Yu, Jun Zhang, Zhenghui Sun, Jinli Jiang, Xiaodong Ma, Bo Bu and Ruyuan Zhu from Department of Neurosurgery, Chinese PLA General Hospital for their collaborative support.
Footnotes
Funding: This work was supported by the National Natural Science Foundation of China, No. 31040039; the Natural Science Foundation of Beijing, No. 7102145; and the Military Clinical High-Tech Foundation, No. 2010gxjso94.
Conflicts of interest: None declared.
Ethical approval: The pilot project was approved by the Medical Ethics Committee of Chinese PLA General Hospital, China.
(Edited by Tu QY, Bai H/Qiu Y/Song LP)
REFERENCES
- [1].Duffau H. New concepts in surgery of WHO grade II gliomas: functional brain mapping, connectionism and plasticity&a review. J Neurooncol. 2006;79(1):77–115. doi: 10.1007/s11060-005-9109-6. [DOI] [PubMed] [Google Scholar]
- [2].Sanai N, Chang S, Berger MS. Low-grade gliomas in adults. J Neurosurg. 2011;115(5):948–965. doi: 10.3171/2011.7.JNS101238. [DOI] [PubMed] [Google Scholar]
- [3].Mandonnet E, Delattre JY, Tanguy ML, et al. Continuous growth of mean tumor diameter in a subset of grade II gliomas. Ann Neurol. 2003;53(4):524–528. doi: 10.1002/ana.10528. [DOI] [PubMed] [Google Scholar]
- [4].Duffau H. The challenge to remove diffuse low-grade gliomas while preserving brain functions. Acta Neurochir (Wien) 2012;154(4):569–574. doi: 10.1007/s00701-012-1275-7. [DOI] [PubMed] [Google Scholar]
- [5].Sanai N, Berger MS. Extent of resection influences outcomes for patients with gliomas. Rev Neurol (Paris) 2011;167(10):648–654. doi: 10.1016/j.neurol.2011.07.004. [DOI] [PubMed] [Google Scholar]
- [6].Sanai N, Berger MS. Operative techniques for gliomas and the value of extent of resection. Neurotherapeutics. 2009;6(3):478–486. doi: 10.1016/j.nurt.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Heiss WD, Thiel A, Kessler J, et al. Disturbance and recovery of language function: correlates in PET activation studies. Neuroimage. 2003;20(Suppl 1):S42–49. doi: 10.1016/j.neuroimage.2003.09.005. [DOI] [PubMed] [Google Scholar]
- [8].Thiel A, Herholz K, Koyuncu A, et al. Plasticity of language networks in patients with brain tumors: a positron emission tomography activation study. Ann Neurol. 2001;50(5):620–629. doi: 10.1002/ana.1253. [DOI] [PubMed] [Google Scholar]
- [9].Muller RA, Rothermel RD, Behen ME, et al. Language organization in patients with early and late left-hemisphere lesion: a PET study. Neuropsychologia. 1999;37(5):545–557. doi: 10.1016/s0028-3932(98)00109-2. [DOI] [PubMed] [Google Scholar]
- [10].Thiel A, Habedank B, Winhuisen L, et al. Essential language function of the right hemisphere in brain tumor patients. Ann Neurol. 2005;57(1):128–131. doi: 10.1002/ana.20342. [DOI] [PubMed] [Google Scholar]
- [11].Desmurget M, Bonnetblanc F, Duffau H. Contrasting acute and slow-growing lesions: a new door to brain plasticity. Brain. 2007;130(Pt 4):898–914. doi: 10.1093/brain/awl300. [DOI] [PubMed] [Google Scholar]
- [12].Basser PJ, Mattiello J, LeBihan D. Estimation of the effective self-diffusion tensor from the NMR spin echo. J Magn Reson B. 1994;103(3):247–254. doi: 10.1006/jmrb.1994.1037. [DOI] [PubMed] [Google Scholar]
- [13].Berman JI, Berger MS, Chung SW, et al. Accuracy of diffusion tensor magnetic resonance imaging tractography assessed using intraoperative subcortical stimulation mapping and magnetic source imaging. J Neurosurg. 2007;107(3):488–494. doi: 10.3171/JNS-07/09/0488. [DOI] [PubMed] [Google Scholar]
- [14].Park HJ. Quantification of white matter using diffusion-tensor imaging. Int Rev Neurobiol. 2005;66:167–212. doi: 10.1016/S0074-7742(05)66006-0. [DOI] [PubMed] [Google Scholar]
- [15].Powell HW, Parker GJ, Alexander DC, et al. Imaging language pathways predicts postoperative naming deficits. J Neurol Neurosurg Psychiatry. 2008;79(3):327–330. doi: 10.1136/jnnp.2007.126078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Catani M, Howard RJ, Pajevic S, et al. Virtual in vivo interactive dissection of white matter fasciculi in the human brain. Neuroimage. 2002;17(1):77–94. doi: 10.1006/nimg.2002.1136. [DOI] [PubMed] [Google Scholar]
- [17].Geschwind N. The organization of language and the brain. Science. 1970;170(3961):940–944. doi: 10.1126/science.170.3961.940. [DOI] [PubMed] [Google Scholar]
- [18].Griffiths JD, Marslen-Wilson WD, Stamatakis EA, et al. Functional organization of the neural language system: dorsal and ventral pathways are critical for syntax. Cereb Cortex. doi: 10.1093/cercor/bhr386. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Parker GJ, Luzzi S, Alexander DC, et al. Lateralization of ventral and dorsal auditory-language pathways in the human brain. Neuroimage. 2005;24(3):656–666. doi: 10.1016/j.neuroimage.2004.08.047. [DOI] [PubMed] [Google Scholar]
- [20].Hickok G, Poeppel D. The cortical organization of speech processing. Nat Rev Neurosci. 2007;8(5):393–402. doi: 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- [21].Gow DW., Jr The cortical organization of lexical knowledge: A dual lexicon model of spoken language processing. Brain Lang. 2012;121(3):273–288. doi: 10.1016/j.bandl.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Rolheiser T, Stamatakis EA, Tyler LK. Dynamic processing in the human language system: synergy between the arcuate fascicle and extreme capsule. J Neurosci. 2011;31(47):16949–16957. doi: 10.1523/JNEUROSCI.2725-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Saur D, Kreher BW, Schnell S, et al. Ventral and dorsal pathways for language. Proc Natl Acad Sci U S A. 2008;105(46):18035–18040. doi: 10.1073/pnas.0805234105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Mandonnet E, Nouet A, Gatignol P, et al. Does the left inferior longitudinal fasciculus play a role in language? A brain stimulation study. Brain. 2007;130(Pt 3):623–629. doi: 10.1093/brain/awl361. [DOI] [PubMed] [Google Scholar]
- [25].Henry RG, Berman JI, Nagarajan SS, et al. Subcortical pathways serving cortical language sites: initial experience with diffusion tensor imaging fiber tracking combined with intraoperative language mapping. Neuroimage. 2004;21(2):616–622. doi: 10.1016/j.neuroimage.2003.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Duffau H. Does post-lesional subcortical plasticity exist in the human brain? Neurosci Res. 2009;65(2):131–135. doi: 10.1016/j.neures.2009.07.002. [DOI] [PubMed] [Google Scholar]
- [27].Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion- tensor MRI. J Magn Reson B. 1996;111(3):209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- [28].Jiang H, van Zijl PC, Kim J, et al. DtiStudio: resource program for diffusion tensor computation and fiber bundle tracking. Comput Methods Programs Biomed. 2006;81(2):106–116. doi: 10.1016/j.cmpb.2005.08.004. [DOI] [PubMed] [Google Scholar]
- [29].Wakana S, Caprihan A, Panzenboeck MM, et al. Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage. 2007;36(3):630–644. doi: 10.1016/j.neuroimage.2007.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Yeatman JD, Feldman HM. Neural plasticity after pre-linguistic injury to the arcuate and superior longitudinal fasciculi. Cortex. doi: 10.1016/j.cortex.2011.08.006. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Yogarajah M, Focke NK, Bonelli SB, et al. The structural plasticity of white matter networks following anterior temporal lobe resection. Brain. 2010;133(Pt 8):2348–2364. doi: 10.1093/brain/awq175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Saur D, Schelter B, Schnell S, et al. Combining functional and anatomical connectivity reveals brain networks for auditory language comprehension. Neuroimage. 2010;49(4):3187–3197. doi: 10.1016/j.neuroimage.2009.11.009. [DOI] [PubMed] [Google Scholar]
- [33].Lee B, Park JY, Jung WH, et al. White matter neuroplastic changes in long-term trained players of the game of “Baduk” (GO): a voxel-based diffusion-tensor imaging study. Neuroimage. 2010;52(1):9–19. doi: 10.1016/j.neuroimage.2010.04.014. [DOI] [PubMed] [Google Scholar]
- [34].Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- [35].Shewan CM, Kertesz A. Reliability and validity characteristics of the Western Aphasia Battery (WAB) J Speech Hear Disord. 1980;45(3):308–324. doi: 10.1044/jshd.4503.308. [DOI] [PubMed] [Google Scholar]
- [36].Lv X, Chen X, Xu B, et al. Magnetic resonance diffusion tensor imaging-based evaluation of optic-radiation shape and position in meningioma. Neural Regen Res. 2012;7(9):686–691. doi: 10.3969/j.issn.1673-5374.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Mori S, Crain BJ, Chacko VP, et al. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol. 1999;45(2):265–269. doi: 10.1002/1531-8249(199902)45:2<265::aid-ana21>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- [38].Kim CH, Chung CK, Koo BB, et al. Changes in language pathways in patients with temporal lobe epilepsy: diffusion tensor imaging analysis of the uncinate and arcuate fasciculi. World Neurosurg. 2011;75(3-4):509–516. doi: 10.1016/j.wneu.2010.11.006. [DOI] [PubMed] [Google Scholar]


