Abstract
Enhanced neurogenesis in the dentate gyrus of the hippocampus following seizure activity, especially status epilepticus, is associated with ectopic residence and aberrant integration of newborn granule cells. Hilar ectopic granule cells may be detrimental to the stability of dentate circuitry by means of their electrophysiological properties and synaptic connectivity. We hypothesized that status epilepticus also increases ectopic granule cells in the molecular layer. Status epilepticus was induced in male Sprague-Dawley rats by intraperitoneal injection of pilocarpine. Immunostaining showed that many doublecortin-positive cells were present in the molecular layer and the hilus 7 days after the induction of status epilepticus. At least 10 weeks after status epilepticus, the estimated number of cells positive for both prospero homeobox protein 1 and neuron-specific nuclear protein in the hilus was significantly increased. A similar trend was also found in the molecular layer. These findings indicate that status epilepticus can increase the numbers of mature and ectopic newborn granule cells in the molecular layer.
Keywords: neural regeneration, basic research, status epilepticus, hippocampus, dentate gyrus, granule cells, molecular layer, prospero homeobox protein 1, neuron-specific nuclear protein, doublecortin, grants-supported paper, photographs-containing paper, neuroregeneration
Research Highlights
(1) The association between neurogenesis and epilepsy is an active area of research.
(2) In the hilus, the properties of ectopic granule cells following status epilepticus have been widely studied, but granule cells in the molecular layer are less well understood.
(3) We studied the distribution of granule cells in the molecular layer after pilocarpine-induced status epilepticus.
(4) A history of status epilepticus was associated with an increase in the numbers of mature and ectopic newborn granule cells in the molecular layer.
(5) The abnormal polarization of ectopic granule cells suggests that their electrophysiological properties and synaptic connections may be different from those of normally distributed granule cells.
INTRODUCTION
Temporal lobe epilepsy is commonly associated with multiple forms of histopathological alterations, such as neuronal loss, reactive gliosis, mossy fiber sprouting, granule cell dispersion, and enhanced neurogenesis in the hippocampus[1]. Following seizure activity, especially status epilepticus, there is a drastic increase in neuronal progenitor proliferation and maturation into granule cells in the dentate gyrus of the hippocampus[2,3,4,5]. In contrast to the neurogenesis that occurs under physiological conditions, enhanced neurogenesis ignited by status epilepticus is associated with abnormal migration[3,4,6,7,8,9,10], leading to ectopic residence and aberrant integration of newborn granule cells, a phenomenon that has been widely studied in the hilus[11,12]. Using prospero homeobox protein 1 as a molecular marker for granule cells, a previous study quantified hilar ectopic granule cells several months after pilocarpine-induced status epilepticus in the rat and found thousands of prospero homeobox protein 1- positive cells in the hilus[13]. Hilar ectopic granule cells born after status epilepticus are similar to normally located granule cells in several aspects[4,6,7,8,9]. For example, morphological and electrophysiological studies have shown that hilar ectopic granule cells integrate into the dentate circuitry. However, certain properties of hilar ectopic granule cells are strikingly different from those of normotopic granule cells. The most prominent difference is that at least half of hilar ectopic granule cells are capable of generating burst firing whereas normotopic granule cells are not[9,14]. In addition, approximately one quarter of hilar ectopic granule cells in rodents have basal dendrites in the hilus[7,9,15], compared with 0–4% of normotopic granule cells[7,9,15,16,17]. The existence of basal dendrites may lead hilar ectopic granule cells to receive more excitatory synapses[6,17,18]. Furthermore, hilar ectopic granule cells are more heterogeneous in terms of resting membrane potentials, firing patterns, and synaptic connectivity[7,9,19]. The unusual electrophysiological properties and synaptic connectivity of hilar ectopic granule cells would be expected to make dentate circuitry unstable, favoring epileptogenesis. Ectopic residence of newborn granule cells following status epilepticus may not be limited to the hilus, and may also exist in the molecular layer. Indeed, a study on surgically resected hippocampi found an increase in prospero homeobox protein 1-positive cells in the molecular layer of the dentate gyrus in patients with temporal lobe epilepsy[20]. Because significant granule cell dispersion also occurs in temporal lobe epilepsy, it is hard to know if these prospero homeobox protein 1- labeled granule cells in the molecular layer were mismigrated newborn granule cells or dispersed granule cells. In addition, no previous study has quantified ectopic granule cells in the molecular layer of the dentate gyrus.
It is clear that a number of newborn cells may die through apoptosis and other pathways before reaching maturity[2,21]. Because of this, using molecular markers that can label both immature and mature granule cells to quantify newborn granule cells may artificially overestimate the number of newborn granule cells that are permanently integrated into the dentate network.
In this study, we quantified the number of mature granule cells in the molecular layer of the dentate gyrus several months following pilocarpine-induced status epilepticus in rats, using double labeling of prospero homeobox protein 1 and neuron-specific nuclear protein. The presence of newborn granule cells in the molecular layer following status epilepticus was identified by doublecortin immunostaining.
RESULTS
Quantitative analysis of experimental animals
Thirty-seven rats were randomly assigned to control (n = 14) and status epilepticus (n = 23) groups. Control and status epilepticus rats were intraperitoneally injected with normal saline and pilocarpine hydrochloride, respectively, 30 minutes after pretreatment with an intraperitoneal injection of scopolamine methyl bromide and terbutaline hemisulfate. In the status epilepticus group, five rats died within 1 hour of the pilocarpine injection, whereas 18 rats showed continuous limbic motor seizures at stage 2 or higher according to Racine's scale for more than 2 hours. Three control rats and three status epilepticus rats were sacrificed for doublecortin immunostaining 7 days after pilocarpine or saline injection. The remaining rats were used for prospero homeobox protein 1/neuron-specific nuclear protein double immunostaining. A total of 32 rats were included in the final analysis.
The presence of doublecortin-immunoreactive cells in the molecular layer 7 days after status epilepticus
In control rats, doublecortin-immunoreactive cell bodies were seen in the granule cell layer, particularly in the subgranular zone, with doublecortin-immunoreactive dendrites running into the molecular layer (Figure 1). Seven days after status epilepticus, doublecortin- immunoreactive cell bodies were found not only in the granule cell layer, but also in the molecular layer and the hilus. The polarization of doublecortin-immunoreactive cells in the molecular layer looked different from the newborn granule cells that were located in the granule cell layer (Figure 1).
Figure 1.
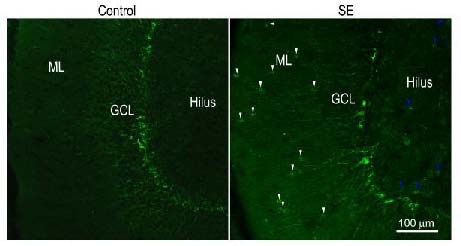
Representative confocal images showing the presence of newborn granule cells in the molecular layer 7 days after status epilepticus (SE).
Sections were immunofluorescently stained with anti-doublecortin and scanned with a Zeiss LMS 780 confocal microscope (× 10 objective lens, scale bar: 100 μm). In sections obtained from control rats (control), doublecortin-positive cells resided in the granule cell layer, in particular in the subgranule zone. In sections obtained from SE rats 7 days after pilocarpine treatment, doublecortin-immunoreactive cells were located in the hilus (blue arrows) and the molecular layer (white arrows) in addition to the granule cell layer. ML: Molecular layer; GCL: granule cell layer.
An increased number of mature granule cells in the molecular layer after status epilepticus
Figure 2 shows prospero homeobox protein 1 and neuron-specific nuclear protein double immunofluorescence in the dentate gyrus of representative control and status epilepticus rats. Although neuron-specific nuclear protein-positive cells appeared in all regions of the dentate gyrus, prospero homeobox protein 1-immunoreactivity was mainly located in granule cell nuclei. As prospero homeobox protein 1 staining overlapped with the neuron-specific nuclear protein, it becomes clear that prospero homeobox protein 1 is a useful molecular marker for granule cells. In control rats, prospero homeobox protein 1-immunoreactive spots could be found in both the hilus and the molecular layer; however, few of these spots were also positive for neuron-specific nuclear protein (Figure 2).
Figure 2.
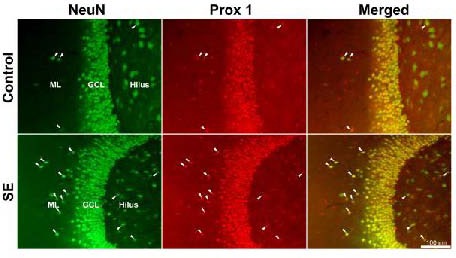
Representative images showing mature ectopic granule cells labeled by neuron-specific nuclear protein (NeuN) and prospero homeobox protein 1 (Prox 1) immunofluorescence in the dentate gyrus (scale bar: 100 μm).
Microscope images were captured with an Olympus fluorescence microscope equipped with a digital camera and a 20 × objective lens. NeuN is shown in green whereas Prox 1 is in red. White arrowheads indicate cells positive for both NeuN and Prox 1 in the molecular layer and the hilus. GCL: Granule cell layer; ML: molecular layer; SE: status epilepticus.
This indicates that not all prospero homeobox protein 1-positive spots can be considered to be mature granule cells. Following status epilepticus, the number of cells positive for both prospero homeobox protein 1 and neuron-specific nuclear protein increased in the hilus as well as in the molecular layer (Figure 2). A statistical comparison of the numbers of mature ectopic granule cells in the hilus and the molecular layer is shown in Figure 3. The estimated number of mature ectopic granule cells in the hilus and the molecular layer was not significantly different in either group. The estimated number of mature ectopic granule cells in the hilus increased from 454 ± 59 cells per hippocampus in control rats (n = 11) to 983 ± 143 cells per hippocampus in status epilepticus rats (n = 15, P < 0.01). The number of mature ectopic granule cells in the molecular layer increased by a similar degree, from 535 ± 66 cells per hippocampus in control rats (n = 11) to 1 282 ± 126 cells per hippocampus in status epilepticus rats (n = 15, P < 0.01).
Figure 3.
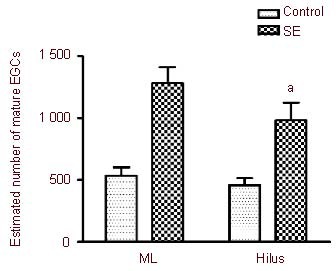
Bar graphs showing the estimated number of mature ectopic granule cells (EGCs) in the hilus and molecular layer (ML) between control and status epilepticus (SE) rats.
aP < 0.01, vs. control group. The bars show the mean ± SEM of 11 control rats and 15 SE rats. The means were compared with the Student-Newman-Keuls test following one-way analysis of variance. There was no significant difference in the numbers of mature EGCs between the hilus and ML in either group.
DISCUSSION
Beyond enhanced neurogenesis, pilocarpine-induced status epilepticus is associated with an abnormal migration of newborn granule cells[4,5,9]. This results in an ectopic distribution of newborn granule cells, a phenomenon that has been well characterized in the hilus[11,12]. Whether aberrant neurogenesis also occurs in the molecular layer, and, if so, how many newborn granule cells may become mature granule cells, remains to be addressed. In the present study, we found that following pilocarpine-induced status epilepticus, abnormal migration of newborn granule cells occurs also in the molecular layer. We also found that the number of mature granule cells in the molecular layer was approximately equal to that in the hilus.
As a microtubule-associated protein, doublecortin is expressed in migrating neuroblasts and immature neurons[22]. doublecortin expression is considered to be specific for newly generated neurons[23]. The presence of doublecortin-immunoreactive cells in the molecular layer after status epilepticus confirms that abnormal migration indeed occurs in this area. Because some newborn granule cells die during maturation[2,21], it remains unclear whether newborn granule cells may survive to become mature cells in the molecular layer and what mechanism(s) determine their fates following pilocarpine injection.
To answer the first question raised above, we used prospero homeobox protein 1/neuron-specific nuclear protein double labeling to count mature granule cells in the molecular layer. In the hippocampus, prospero homeobox protein 1 is known as a specific molecular marker of both immature and mature granule cells, whereas neuron-specific nuclear protein is only expressed in mature granule cells[24]. Cells positive for both prospero homeobox protein 1 and neuron-specific nuclear protein are therefore considered to be mature granule cells. Stereological quantification of hilar ectopic granule cells has previously shown that thousands of prospero homeobox protein 1-positive cells reside in the hilus of control rats[13]. This number is roughly three times the estimate obtained in our study. In addition to differences in counting methods, other reasons may underlie this discrepancy. First, in contrast to the previous study, we specifically quantified mature granule cells rather than mature and immature granule cells together. This specificity was supported by the fact that there were many spots positive for prospero homeobox protein 1 but negative for neuron-specific nuclear protein in the hilus of control rats. Second, regional differences in hippocampal neurogenesis have been shown to exist[25]. Thus, if intense neurogenesis occurs only in a limited region, using the total number of cells in several sections from different parts of the dentate gyrus could lead to an underestimation of ectopic granule cells. Third, the use of horizontal sections of the hippocampus might have made the dorsal part of dentate gyrus unrecognizable, leaving a number of ectopic granule cells uncounted. Despite these shortcomings of the counting method, our results clearly show that status epilepticus induced by pilocarpine doubles the number of mature granule cells in both the hilus and the molecular layer.
The increased number of cells positive for both neuron-specific nuclear protein and prospero homeobox protein 1 in the molecular layer might have been a consequence of granule cell dispersion rather than enhanced neurogenesis coupled with abnormal migration of newborn cells. However, this is unlikely because of the following reasons. First, the presence of many doublecortin-positive cells in the molecular layer 1 week after status epilepticus gave unequivocal support to the existence of abnormal migration of newborn granule cells into the molecular layer. Second, the staining patterns of neuron-specific nuclear protein and prospero homeobox protein 1 showed that the boundary between the granule cell layer and the molecular layer remained sharp.
Although a number of newborn granule cells might have died during maturation[2,21], the increase in cells positive for both neuron-specific nuclear protein and prospero homeobox protein 1 in the molecular layer indicates that at least a fraction of mismigrated newborn cells become permanent residents in this area. Theoretically, the effect of enhanced neurogenesis after status epilepticus on the dentate network is determined by many factors, such as the location, number, intrinsic electrophysiological properties, and synaptic connectivity of individual newborn granule cells. Studies have suggested that increased neurogenesis following status epilepticus may be detrimental to the stability of the dentate network, because hilar ectopic granule cells have unique synaptic connectivity and unusual electrophysiological properties compared with normally located granule cells[3,4,7,8,9,19]. To our knowledge, no previous studies have investigated newborn granule cells in the molecular layer following status epilepticus, either morphologically or functionally. The abnormal polarization of newborn granule cells in the molecular layer strongly suggests that the synaptic connectivity of newborn ectopic granule cells in this area could be different from those of normally located cells. Obviously, further studies are needed to elucidate the possible contribution of newborn ectopic molecular granule cells to epileptogenesis.
In conclusion, status epilepticus induced by pilocarpine increases the number of mature granule cells in the molecular layer as much as it does in the hilus. Further studies are necessary to determine the morphological features, intrinsic electrophysiological properties and synaptic connectivity of newborn granule cells in the molecular layer and their influence on the dentate network.
MATERIALS AND METHODS
Design
A randomized, controlled animal study.
Time and setting
The experiments were performed at the Institute of Physiology at Shandong University School of Medicine, China from July 2010 to March 2012.
Materials
A total of 37 adult male Sprague-Dawley rats (140–170 g, 6–7 weeks old) were purchased from the Animal Center of Shandong University of Traditional Chinese Medicine in China (license No. SCXK (Lu) 20110003). Animals were allowed to habituate to the university facility for at least 1 week before experiments began. The experiments were performed according to the Guidance Instructions for the Care and Use of Laboratory Animals, issued by the Ministry of Science and Technology of China[26].
Methods
Status epilepticus induction
Status epilepticus was induced by intraperitoneal injection of pilocarpine as described previously[9] with modifications. Rats were injected intraperitoneally with the first dose of pilocarpine hydrochloride (360 mg/kg) 30 minutes after pretreatments with an intraperitoneal injection of scopolamine methyl bromide and terbutaline hemisulfate (both 2 mg/kg). If the first dose of pilocarpine did not induce continuous limbic motor seizures, a second dose of 100 mg/kg was injected 45 minutes later. Animals were accepted for further studies if the continuous limbic motor seizure corresponded to stage 2 or higher according to Racine's scale and lasted for more than 2 hours (n = 15)[27]. Status epilepticus was allowed to self-terminate after 6 hours. Control rats (n = 11) were treated with intraperitoneal injection of normal saline following pretreatment with scopolamine methyl bromide and terbutaline hemisulfate (both 2 mg/kg). Pilocarpine hydrochloride, scopolamine methyl bromide, and terbutaline hemisulfate were purchased from Sigma (St. Louis, MO, USA).
Transcardial perfusion and brain tissue preparation
Seven days or at least 10 weeks after the induction of status epilepticus, individual animals were deeply anesthetized with an intraperitoneal injection of sodium pentobarbital (60 mg/kg). After complete paralysis, the animals were perfused through the left ventricle with heparinized saline (20–30 mL), followed by 200–300 mL of 4% paraformaldehyde in 0.1 M PBS (pH 6.8) over a period of 45 minutes. The fixed brain was extracted, the cerebellum and brainstem were trimmed off, and the tissue was post-fixed in the same fixative at 4°C overnight. Thereafter, the tissue block was sequentially transferred into 10% sucrose in 0.1 M PBS for 4 hours, 15% sucrose in 0.1 M PBS for 8 hours, and finally 20% sucrose in 0.1 M PBS at 4 °C overnight. The tissue block was embedded in a medium composed of 30% (w/v) chicken egg albumin (Sigma), 0.5% (w/v) gelatin and 0.9% (v/v) glutaraldehyde in 0.1 M PBS, and serial horizontal slices (60 μm thick) were cut through the hippocampi using a Vibratome 1000 (Vibratome Co., St. Louis, MO, USA). A previous study applying stereological counting methods has shown that the number of granule cells in the hilus is the same in the two hemispheres[14], so hippocampal sections from the left hemisphere were collected into 0.1 M PBS. Horizontal sections containing the ventral hippocampus were used for quantitative comparison of ectopic granule cells, because the ventral hippocampus is more seizure-prone than the dorsal hippocampus[28,29].
Immunofluorescence and image acquisition
To count ectopic granule cells, the 4th, 12th, 20th, 28th, 36th, 44th, 52nd and 60th (if it existed) hippocampal horizontal slices were chosen for prospero homeobox protein 1 and neuron-specific nuclear protein double immunofluorescent staining of free-floating sections. After a thorough wash in 0.1 M PBS, the 7–8 sections were incubated in a blocking buffer consisting of 5% normal goat serum, 2.5% bovine serum albumin and 0.2% Triton X-100 in 0.1 M PBS for 2.5 hours at 4°C to minimize non-specific reactions. Thereafter, the sections were incubated with a cocktail of mouse anti-neuron-specific nuclear protein (1:200) and rabbit anti-prospero homeobox protein 1 (1:6 000) at 4°C overnight. The primary antibodies, both purchased from Millipore (Temecula, CA, USA), were diluted in the blocking buffer. After three 15-minute washes in 0.1 M PBS, the sections were incubated for 2.5 hours at 4°C with a mixture of Alexa Fluor 488-conjugated goat anti-mouse IgG and Alexa Flour 568-conjugated goat anti-rabbit IgG (Invitrogen, San Diego, CA, USA) diluted in the blocking buffer. The final concentration of both secondary antibodies was 1.6 μg/mL. After washing (three times, 15 minutes each), the sections were mounted on standard glass slides and cover-slipped with 75% glycerol in 0.1 M PBS. Sections were visualized with an Olympus fluorescence microscope. Images were captured with a digital camera using 10 × and 20 × objectives.
Doublecortin immunostaining performed 7 days after pilocarpine or normal saline injection was used to determine the ectopic distribution of newborn granule cells. The procedures for transcardial perfusion, tissue preparation and immunofluorescence were identical to those described above. Goat anti-doublecortin (Santa Cruz Biotechnology, Santa Cruz, CA, USA) was used as the primary antibody (1:600), whereas Alexa Fluor 488-conjugated donkey anti-goat IgG (Invitrogen) was used as a secondary antibody (1:600). Donkey serum was used to minimize non-specific reactions. Stained sections were mounted on glass slides and scanned using a Carl Zeiss confocal microscope (LSM 780, Jena, Thuringia, Germany).
Cell counting
Paired fluorescent images for prospero homeobox protein 1 and neuron-specific nuclear protein were acquired using the 10 × objective and merged in Adobe Photoshop 7 (Adobe Systems Inc., San Jose, CA, USA). Cells positive for both neuron-specific nuclear protein and prospero homeobox protein 1 were counted by a blinded examiner in the hilus and molecular layer, as defined by the contours illustrated in Figure 4. Granule cells in the hilus were considered to be ectopic when they were located at least two granule cell body widths away from the inner border of the granule cell layer[6,10]. Ectopic granule cells in the molecular layer were considered ectopic when they were at least three granule cell body widths away from the outer border of the granule cell layer. The number of cells qualified as ectopic granule cells in the hilus or the molecular layer of each section was summed. This number was multiplied by the number of sections (7–8) to approximate the total number of ectopically distributed mature granule cells in the hilus and the molecular layer.
Figure 4.
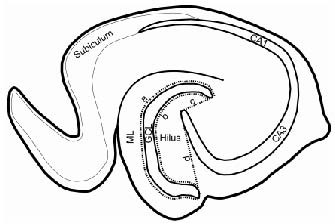
Schematic drawing of the hippocampal subregions in a horizontal rat brain section, showing the hilus and molecular layer (ML) where ectopic granule cells (EGCs) were counted.
Granule cells in the area between the dotted line “a” and the outer border of the ML were counted as EGCs in the ML. The dotted line “a” is located three granule cell body widths from the outer border of the granule cell layer (GCL). The dotted line “b” indicates two granule cell body widths away from the inner border of the GCL whereas the dotted lines “c” and “d” connect the beginning of the CA3 pyramidal cell layer with the two ends of the GCL. Granule cells in the area enclosed by the dotted lines “b”, “c”, and “d” were counted as hilar EGCs.
Statistical analysis
Quantitative data are presented as mean ± SEM and statistically compared by Student-Newman-Keuls tests following one-way analysis of variance GraphPad Prism (version 4.0, San Diego, CA, USA). A P-value less than or equal to 0.05 was considered statistically significant.
Acknowledgments
The authors would like to thank Prof. Aijun Hao, Department of Histology and Embryology of Shandong University School of Medicine, for help with epifluorescence microscopy.
Footnotes
Funding: This study was supported by grants from the Self-innovation Programs of Shandong University, No. 1000069961016; and the National Natural Science Foundation of China, No. 81171231.
Conflicts of interest: None declared.
Ethical approval: Animal experiments were approved by the Animal Ethics Committee of Shandong University School of Medicine in China.
(Edited by Yuan B, Yang DG/Su LL/Song LP)
REFERENCES
- [1].Dichter MA. Emerging concepts in the pathogenesis of epilepsy and epileptogenesis. Arch Neurol. 2009;66(4):443–447. doi: 10.1001/archneurol.2009.10. [DOI] [PubMed] [Google Scholar]
- [2].Bengzon J, Kokaia Z, Elmer E, et al. Apoptosis and proliferation of dentate gyrus neurons after single and intermittent limbic seizures. Proc Natl Acad Sci U S A. 1997;94(19):10432–10437. doi: 10.1073/pnas.94.19.10432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Parent JM, Yu TW, Leibowitz RT, et al. Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. J Neurosci. 1997;17(10):3727–3738. doi: 10.1523/JNEUROSCI.17-10-03727.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Scharfman HE, Goodman JH, Sollas AL. Granule-like neurons at the hilar/CA3 border after status epilepticus and their synchrony with area CA3 pyramidal cells: functional implications of seizure-induced neurogenesis. J Neurosci. 2000;20(16):6144–6158. doi: 10.1523/JNEUROSCI.20-16-06144.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Muramatsu R, Ikegaya Y, Matsuki N, et al. Early-life status epilepticus induces ectopic granule cells in adult mice dentate gyrus. Exp Neurol. 2008;211(2):503–510. doi: 10.1016/j.expneurol.2008.02.026. [DOI] [PubMed] [Google Scholar]
- [6].Jessberger S, Zhao C, Toni N, et al. Seizure-associated, aberrant neurogenesis in adult rats characterized with retrovirus mediated cell labeling. J Neurosci. 2007;27(35):9400–9407. doi: 10.1523/JNEUROSCI.2002-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Cameron MC, Zhan RZ, Nadler JV. Morphologic integration of hilar ectopic granule cells into dentate gyrus circuitry in the pilocarpine model of temporal lobe epilepsy. J Comp Neurol. 2011;519(11):2175–2192. doi: 10.1002/cne.22623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Parent JM, Elliott RC, Pleasure SJ, et al. Aberrant seizure induced aberrant neurogenesis in experimental temporal lobe epilepsy. Ann Neurol. 2006;59(1):81–91. doi: 10.1002/ana.20699. [DOI] [PubMed] [Google Scholar]
- [9].Zhan RZ, Nadler JV. Enhanced tonic GABA current in normotopic and hilar ectopic dentate granule cells after pilocarpine-induced status epilepticus. J Neurophysiol. 2009;102(2):670–681. doi: 10.1152/jn.00147.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kron M, Zhang H, Parent JM. The developmental stage of dentate granule cells dictates their contributions to seizure-induced plasticity. J Neurosci. 2010;30(6):2051–2059. doi: 10.1523/JNEUROSCI.5655-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Scharfman HE, Goodman JH, McCloskey DP. Ectopic granule cells of the rat dentate gyrus. Dev Neurosci. 2007;29(1-2):14–27. doi: 10.1159/000096208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kokaia M. Seizure induced neurogenesis in the adult brain. Euro J Neurosci. 2011;33(6):1133–1138. doi: 10.1111/j.1460-9568.2011.07612.x. [DOI] [PubMed] [Google Scholar]
- [13].McCloskey DP, Hintz TM, Pierce JP, et al. Stereological methods reveal the robust size and stability of ectopic hilar granule cells after pilocarpine-induced status epilepticus in the adult rat. Eur J Neurosci. 2006;24(8):2203–2210. doi: 10.1111/j.1460-9568.2006.05101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Scharfman HE, Sollas AL, Berger RE, et al. Perforant path activation of ectopic granue cells that are born after pilocarpine-induced status epilepticus. Neuroscience. 2003;121(4):1017–1029. doi: 10.1016/s0306-4522(03)00481-0. [DOI] [PubMed] [Google Scholar]
- [15].Murphy BL, Pun RYK, Yin H, et al. Heterogeneous integration of adult-generated granule cells into the epileptic brain. J Neurosci. 2010;31(1):105–117. doi: 10.1523/JNEUROSCI.2728-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ribak CE, Tran PH, Spigelman I, et al. Status epilepticus- induced hilar basal dendrites on rodent granule cells contribute to recurrent excitatory circuitry. J Comp Neurol. 2000;428(2):240–253. doi: 10.1002/1096-9861(20001211)428:2<240::aid-cne4>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- [17].Thind KK, Ribak CE, Buckmaster PS. Synaptic input to dentate granule cell basal dendrites in a rat model of temporal lobe epilepsy. J Comp Neurol. 2008;509(2):190–202. doi: 10.1002/cne.21745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Pierce JP, Melton J, Punsoni M, et al. Mossy fibers are the primary source of afferent input to ectopic granule cells that are born after pilocarpine-induced seizures. Exp Neurol. 2005;196(2):316–331. doi: 10.1016/j.expneurol.2005.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Zhan R-Z, Timofeeva O, Nadler JV. High ratio of synaptic excitation to synaptic inhibition in hilar ectopic granule cells of pilocarpine-treated rats. J Neurophysiol. 2010;104(6):3293–3304. doi: 10.1152/jn.00663.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Blümcke I, Kistner I, Clusmann H, et al. Towards a clinico-pathological classification of granule cell dispersion in human mesial temporal lobe epilepsies. Acta Neuropathol. 2009;117(5):535–544. doi: 10.1007/s00401-009-0512-5. [DOI] [PubMed] [Google Scholar]
- [21].Ekdahl CT, Zhu C, Bonde S, et al. Death mechanisms in status epilepticus-generated neurons and effects of additional seizures on their survival. Neurobiol Dis. 2003;14(3):513–523. doi: 10.1016/j.nbd.2003.08.022. [DOI] [PubMed] [Google Scholar]
- [22].Gleeson JG, Lin PT, Flanagan LA, et al. Doublecortin is a microtubule-associated protein and is expressed widely by migrating neurons. Neuron. 1999;23(2):257–271. doi: 10.1016/s0896-6273(00)80778-3. [DOI] [PubMed] [Google Scholar]
- [23].von Bohlen Und Halbach O. Immunohistological markers for staging neurogenesis in adult hippocampus. Cell Tissue Res. 2007;329(3):409–420. doi: 10.1007/s00441-007-0432-4. [DOI] [PubMed] [Google Scholar]
- [24].Ming GL, Song H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron. 2011;70(4):687–702. doi: 10.1016/j.neuron.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Jinno S. Topographic differences in adult neurogenesis in the mouse hippocampus: a stereology-based study using endogenous markers. Hippocampus. 2011;21(5):467–480. doi: 10.1002/hipo.20762. [DOI] [PubMed] [Google Scholar]
- [26].The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals 2006-09-30 [Google Scholar]
- [27].Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32(3):281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- [28].Racine RJ, Rose PA, Burnham WM. Afterdischarge thresholds and kindling rates in dorsal and ventral hippocampus and dentate gyrus. Can J Neurol Sci. 1977;4(4):273–278. doi: 10.1017/s0317167100025117. [DOI] [PubMed] [Google Scholar]
- [29].Ekstrand JJ, Pouliot W, Scheerlinck P, et al. Lithium pilocarpine-induced status epilepticus in postnatal day 20 rats results in greater neuronal injury in ventral versus dorsal hippocampus. Neuroscience. 2011;192(29):699–707. doi: 10.1016/j.neuroscience.2011.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]


