
Keywords: neural regeneration, brain injury, aluminum, zinc, trace elements, behavior, pathology, cerebrum, malondialdehyde, superoxide dismutase, acetylcholinesterase, dopamine, grants-supported paper, neuroregeneration
Abstract
Zinc supplementation can help maintain learning and memory function in rodents. In this study, we hypothesized that zinc supplementation could antagonize the neurotoxicity induced by aluminum in rats. Animals were fed a diet containing different doses of zinc (50, 100, 200 mg/kg) for 9 weeks, and orally administered aluminum chloride (300 mg/kg daily) from the third week for 7 consecutive weeks. Open-field behavioral test results showed that the number of rearings in the group given the 100 mg/kg zinc supplement was significantly increased compared with the group given the 50 mg/kg zinc supplement. Malondialdehyde content in the cerebrum was significantly decreased, while dopamine and 5-hydroxytryptamine levels were increased in the groups given the diet supplemented with 100 and 200 mg/kg zinc, compared with the group given the diet supplemented with 50 mg/kg zinc. The acetylcholinesterase activity in the cerebrum was significantly decreased in the group given the 100 mg/kg zinc supplement. Hematoxylin-eosin staining revealed evident pathological damage in the hippocampus of rats in the group given the diet supplemented with 50 mg/kg zinc, but the damage was attenuated in the groups given the diet supplemented with 100 and 200 mg/kg zinc. Our findings suggest that zinc is a potential neuroprotective agent against aluminum-induced neurotoxicity in rats, and the optimal dosages are 100 and 200 mg/kg.
INTRODUCTION
Aluminum is the third most common element in the earth's crust and is ubiquitous in the environment[1,2,3,4]. Despite the abundant presence and wide exposure to this metal, its biological and biochemical functions still remain unclear[5]. The major contributors to aluminum exposure are water, air, foods with aluminum-containing food additives, grain products and processed cheese[6,7].
The wide presence and use of aluminum has stimulated considerable interest in the toxicity of this metal. Daily sources of aluminum intake include water, cooking utensils, food additives, and drugs such as antacids and deodorants. AlCl3·6H2O was chosen in this study because of its widespread use as a coagulant in water treatment.
Aluminum has been associated with several neurodegenerative diseases, such as Alzheimer's disease, Parkinson's disease, dialysis encephalopathy, amyotrophic lateral sclerosis and Guam-Parkinson's dementia[8,9,10,11]. The mechanisms underlying aluminum neurotoxicity are unclear, although it appears that the metal contributes to oxidative damage[12,13,14,15], disruption of calcium homeostasis[16,17,18] and impairment of intracellular signal transduction pathways[19,20,21]. Thus the mechanisms of aluminum neurotoxicity are complex, but likely affect neurotransmission, learning and memory function, and related behavior.
Zinc, an essential trace element for growth and development, is distributed throughout the body, and concentrations are especially high in the limbic system, including the hippocampus, hypothalamus and amygdala[22,23]. Zinc plays extremely critical roles in normal growth and development, cellular homeostasis, and numerous biochemical functions, including protein synthesis and nucleic acid metabolism. Furthermore, zinc is involved in immunological, neurological, endocrine, and sensory functions, as well as gene expression[24,25,26,27,28]. The crucial role of zinc in neurogenesis, neuronal migration, synaptogenesis and neurotransmission make it closely related to learning and memory function. Liu and coworkers studied the effect of different concentrations of dietary zinc on learning and memory function, brain somatostatin levels, and zinc and calcium concentrations in Wistar rats. Their results suggested that a diet containing 100–200 mg/kg zinc is adequate for maintaining learning and memory function in rats[29].
In our previous study[30], we found that acetylcholinesterase activity was decreased and lipid peroxidation was enhanced in the cerebrum of male Wistar rats after treatment with aluminum chloride at a dose of 300 mg/kg daily for 7 weeks. In this study, we aimed to evaluate the effect of zinc supplementation on aluminum-induced neurotoxicity.
RESULTS
Quantitative analysis of experimental animals
Thirty healthy adult Wistar rats were equally and randomly divided into three groups: 50, 100 and 200 mg/kg zinc groups. Each group received a diet containing the respective concentration of zinc for 9 weeks. From the third week, rats in each group were intragastrically injected with 300 mg/kg aluminum trichloride for 7 consecutive weeks. Eight rats in each group were selected for the determination of zinc concentration and biochemical indexes in brain tissue, while the remaining two rats were used for pathological observations.
No rats exhibited signs of infection or died during the experimentation. All 30 rats were included in the final analysis, without dropout.
Effect of zinc supplementation on open- field activity in aluminum-exposed rats
On day 60 of zinc supplementation, the open-field test was carried out to evaluate the motility and spontaneous exploration of rats. Data for horizontal activity and rearings in the open field are shown in Table 1.
Table 1.
Effect of zinc supplementation on open-field activity in aluminum-exposed rats
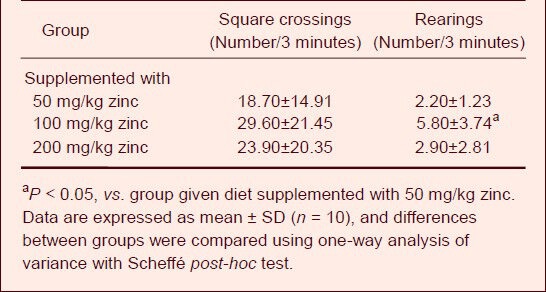
Compared with the group given the diet supplemented with 50 mg/kg zinc, the number of square crossings was increased in the groups treated with 100 and 200 mg/kg zinc, but there was no significant difference among the treatment groups. Moreover, a significant increase in the number of rearings was observed in the group given the diet supplemented with 100 mg/kg zinc compared with the group given the 50 mg/kg zinc supplement (P < 0.05).
Effect of zinc supplementation on learning and memory function in aluminum-exposed rats
On day 61 of zinc supplementation, learning and memory function, an important aspect of cognitive function in rats, was assessed with the passive avoidance task. The retention latency in the groups given the 50, 100 and 200 mg/kg zinc supplementation was 275.80 ± 76.53, 243.80 ± 118.50 and 274.20 ± 81.59 seconds, respectively. There were no significant differences among the groups.
Effect of zinc supplementation on aluminum and zinc concentrations in the cerebrum of aluminum- exposed rats
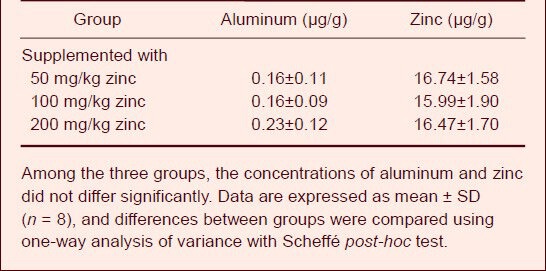
On day 63 of zinc supplementation, the aluminum and zinc concentrations in the cerebrum of animals were determined (Table 2). Among the groups, the concentrations of aluminum and zinc showed no significant differences.
Table 2.
Effect of zinc supplementation on aluminum and zinc concentrations in the cerebrum of aluminum-exposed rats
Effect of zinc supplementation on malondialdehyde content and superoxide dismutase and acetylcholinesterase activities in the cerebrum of aluminum-exposed rats
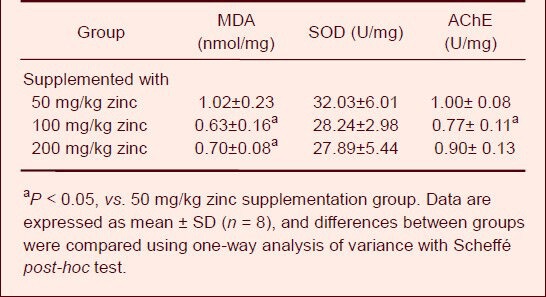
On day 63 of zinc supplementation, the malondialdehyde content, superoxide dismutase and acetylcholinesterase activities were determined. The effects of aluminum and zinc treatment on several biochemical markers in the cerebrum are shown in Table 3. The malondialdehyde content in the cerebrum was significantly increased in the groups treated with 100 and 200 mg/kg zinc compared with the group treated with 50 mg/kg zinc (P < 0.05). The acetylcholinesterase activities in cerebrum were significantly decreased in rats fed the diet containing 100 mg/kg zinc (P < 0.05). There was no significant difference in superoxide dismutase activity in the cerebrum among the three groups.
Table 3.
Effect of zinc supplementation on malondialdehyde (MDA) content, superoxide dismutase (SOD) and acetylcholinesterase (AChE) activities in the cerebrum of aluminum-exposed rats
Effect of zinc supplementation on dopamine and 5-hydroxytryptamine levels in the cerebrum of aluminum-exposed rats
On day 63 of zinc supplementation, the dopamine and 5-hydroxytryptamine concentrations in the cerebrum of rats were assayed (Table 4).
Table 4.
Effect of zinc supplementation on dopamine and 5-hydroxytryptamine levels in the cerebrum of aluminum-exposed rats
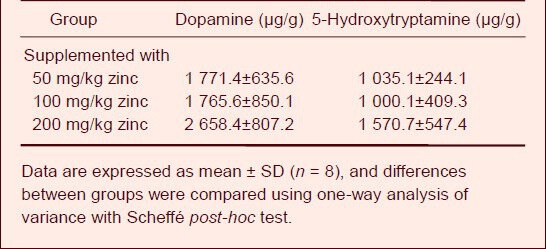
Compared with the group given the diet supplemented with 50 mg/kg zinc, dopamine and 5-hydroxytryptamine levels were increased in the groups treated with 100 and 200 mg/kg zinc, but there was no significant difference among the three groups.
Effect of zinc supplementation on histopathological changes in aluminum-exposed rats
On day 63 of zinc supplementation, the histology of the hippocampus was examined under the light microscope (Figure 1). Pyknosis is the irreversible condensation of chromatin in the nucleus of a cell undergoing programmed cell death or apoptosis. Pyknotic nuclei and prominent perineuronal spaces were observed in the hippocampus of rats in the group fed the diet supplemented with 50 mg/kg zinc. In comparison, pyramidal cells in the hippocampus displayed a relatively normal appearance in the groups treated with 100 and 200 mg/kg zinc.
Figure 1.
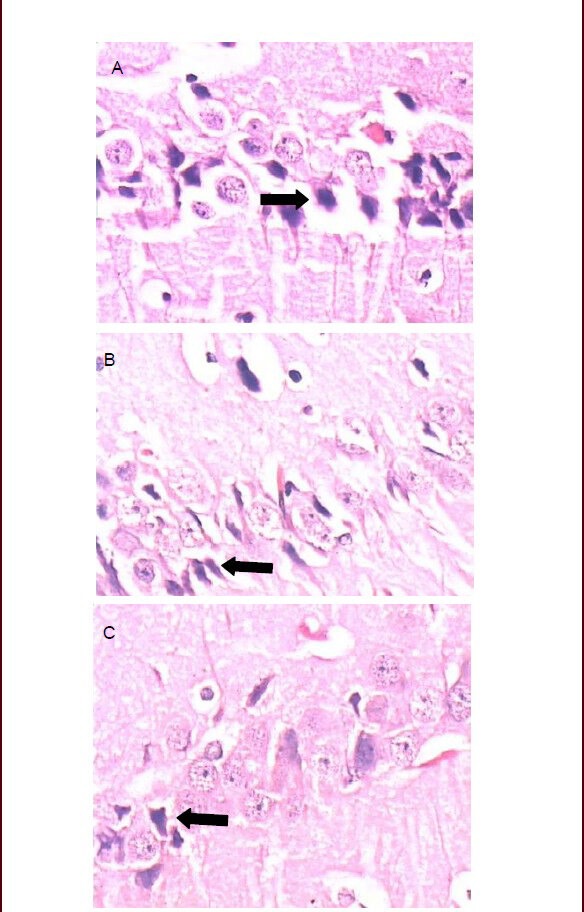
Photomicrographs showing histopathological changes in the hippocampus at 63 days after zinc supplementation in the three groups.
(A) Pyknotic nuclei and prominent perineuronal spaces in the hippocampus of rats are visible in the group given the 50 mg/kg zinc supplement.
(B) Fewer pyknotic nuclei are visible in the hippocampus of rats in the group given the 100 mg/kg zinc supplement.
(C) Fewer pyknotic nuclei are visible in the hippocampus of rats in the group given the 200 mg/kg zinc supplement.
Arrows indicate pyknotic nuclei (hematoxylin-eosin staining, Leica, × 400).
DISCUSSION
There are numerous investigations of aluminum neurotoxicity in experimental animals. However, the neurotoxic effects of aluminum in experimental animals have been controversial because they are dependent on the route of administration[31,32,33], type of aluminum salt[34,35,36], species of animal[37,38,39], dose of treatment[40,41,42] and time of exposure[39,40,41,42,43,44]. In most studies, the duration of aluminum exposure ranges from 8 weeks to 6 months.
The present study was performed to investigate the protective effects of zinc on aluminum-induced neurotoxicity in the cerebrum of male Wistar rats. Administration of zinc protected against histopathological changes in aluminum-exposed rats, thereby demonstrating its neuroprotective action.
In the present study, short-term memory was not significantly altered among the different groups. This result is similar to previously reported findings[45,46]. However, in many other studies, short-term memory was significantly altered after aluminum exposure[47,48]. The discrepancy in these findings may result from differences in methodology, including variations in dose, timing and route of aluminum administration.
Our previous study showed that aluminum exposure induces a significant reduction in locomotor activity in rats (data not shown). These results confirmed the findings of others using the open-field test[49,50,51]. In the present study, the impairment in locomotor activity induced by aluminum was less severe in the groups treated with 100 and 200 mg/kg zinc, compared with the group given the diet supplemented with 50 mg/kg zinc.
In this study, damage to lipids was assessed by measuring malondialdehyde content. Malondialdehyde is the end-product of lipid peroxidation[52]. Thus, malondialdehyde levels can be used to estimate the degree of lipid peroxidation and the levels of tissue oxygen radicals. Exposure to AlCl3 at 100 mg/kg per day for 8 weeks may lead to increased levels of lipid peroxidation[12]. Exposure to AlCl3 at 50 mg/kg per day for 3 months also results in enhancement of lipid peroxidation[53]. In the present study, malondialdehyde levels in the cerebrum were significantly lower in the groups treated with 100 and 200 mg/kg zinc compared with the group given the diet supplemented with 50 mg/kg zinc. This indicates that zinc may alleviate oxidative damage following aluminum exposure.
Acetylcholinesterase has received wide attention in the study of aluminum neurotoxicity[47,54]. Acetylcholines-terase is a cholinergic marker enzyme[54] and aluminum has been suggested to act as a cholinotoxin[55]. It was observed that administration of aluminum causes a significant increase in acetylcholinesterase activity in the cerebral cortex of rats[53]. Bhalla et al[47] observed a decrease in acetylcholinesterase activity after aluminum exposure in both the cerebrum and cerebellum. Similar observations were made by Ravi et al[56]. Furthermore, Kumar[57] explored the biphasic effect of aluminum on acetylcholinesterase activity in brain regions in vivo. We postulate that the disparity in findings may be due to differences in dose, duration and route of aluminum exposure in the various studies. The present study reveals that co-administration of 100 mg/kg zinc to aluminum-exposed animals is able to reduce the aluminum-induced increase in acetylcholinesterase activity in the cerebrum.
Dopamine is secreted from neurons that originate in the substantia nigra. A decrease in dopamine levels is observed after aluminum exposure in the cerebrum, cerebellum[47] and hypothalamus[58]. A significant decrease in 5-hydroxytryptamine levels is also observed after prolonged aluminum exposure in both the cerebrum and cerebellum[47], and there are region-specific variations in the levels of 5-hydroxytryptamine[47,59]. 5-Hydroxytryptamine is a neurotransmitter synthesized from tryptophan via the intermediate 5-hydroxytryptophane.
In the present study, the dopamine and 5-hydroxytryptamine levels were improved (i.e., higher) in the groups treated with 100 and 200 mg/kg zinc compared with the group treated with 50 mg/kg zinc. By microscopic observation, increased neuronal apoptosis was seen after aluminum treatment. This likely affects neurotransmission, and hence, learning and memory processes. Zinc supplementation may reduce neuronal apoptosis.
In conclusion, the present study indicates that zinc supplementation prevents aluminum-induced neurotoxicity in the cerebrum. By inhibiting oxidative stress and histopathological changes, zinc supplementation helped normalize the activity of cholinergic marker enzymes affected by aluminum. Thus, the present study shows that zinc can be used as a neuroprotectant. Zinc helps to protect against neuronal cell death and helps to maintain normal neurotransmission and behavioral functions during aluminum toxicity. Further studies are required to elucidate the mechanisms by which zinc exerts its neuroprotective effects.
MATERIALS AND METHODS
Design
A randomized, controlled animal experiment.
Time and setting
The experiment was performed at the laboratory in the Department of Nutrition, Institute of Health & Environmental Medicine, Academy of Military Medical Sciences, Tianjin, China, from September 2008 to January 2011.
Materials
A total of 30 healthy male Wistar rats weighing 217.6 ± 7.6 g were purchased from the Experimental Animal Center of the Military Medical Sciences, China (license No. SCXK-2007-004). All experimental protocols were in strict accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals, issued by the Ministry of Science and Technology of China[60].
Prior to the experiments, animals were allowed to acclimatize for 7 days. Thirty male Wistar rats were housed in polypropylene cages, under pathogen-free conditions, with a 12-hour light/dark cycle, and temperature was maintained at 22 ± 2°C. Animals were given feed and deionized water ad libitum.
All animal studies conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health.
Methods
Zinc supplementation
Aluminum was administered by gavage. Rats were randomly assigned to three groups, each group consisting of ten animals. Male Wistar rats were exposed orally to aluminum chloride, whereas zinc was given in the form of ZnSO4·7H2O (Tianjin Shentai, Tianjin, China) through diet. In all groups, rats received aluminum chloride (300 mg/kg per day; Tianjin Standard Science and Technology, Tianjin, China). Animals were administered different doses of zinc in the diet, namely 50, 100 or 200 mg/kg zinc[29]. The diets were formulated as described previously by the American Institute of Nutrition AIN-93[61]. The treatments were given over a period of 9 weeks, and all rats received aluminum chloride as of the fifteenth day. At the end of the treatment schedules, the following behavioral studies were conducted.
Behavioral tests
Open-field test for motility and spontaneous exploration: The open-field test was carried out on day 60 to evaluate the motility and spontaneous exploration of rats. An open-field apparatus was utilized, consisting of a black plastic 1 m × 1 m arena divided into 20 cm × 20 cm squares. The open field was enclosed with 50-cm-high side walls. Each animal was placed in the middle of the field, and activity was counted during 3-minute sessions. Both horizontal (square crossings) and vertical (rearings) activities were measured. The number of square crossings and rearings was recorded.
Passive avoidance test for short-term memory: Passive avoidance performance was observed on day 61 to assess short-term memory as reported previously[62]. Briefly, the test was conducted for 2 days, including one acquisition trial and one testing trial, during the same time of day. In the acquisition trial, a rat was put into the bright compartment, and it received an electric foot shock (40 V) for 3 seconds through the stainless steel grid floor if it entered the dark compartment. Then, the rat would escape back to the bright compartment.
After 24 hours, the testing trial was conducted, identical to the acquisition trial, except that no electric shock was delivered if the rat entered the dark compartment. The time the rat took to enter the dark compartment was recorded and tabulated as escape latency (seconds). Rats were allowed to stay in the bright compartment for 300 seconds in the testing trials.
Biochemical analysis
Tissue homogenate preparation: On day 63, rats were lightly anesthetized and killed by decapitation after the behavioral tests were completed. Brains were removed and weighed individually. Thereafter, cerebrums were dissected out for biochemical analysis. Homogenates of the cerebrum were prepared in 0.9% NaCl solution. The homogenate was centrifuged at 3 000 r/min, 4°C, for 15 minutes. The clear supernatant was used for the estimation of malondialdehyde content, superoxide dismutase activity and acetylcholinesterase activity.
Malondialdehyde content determination: Malondialdehyde, a reliable marker of lipid peroxidation, was determined with thiobarbituric acid according to the manufacturer's instructions (Nanjing Jiancheng Bioengineering Institute, Nanjing, Jiangsu Province, China) on day 63. Supernatants (0.1 mL) were transferred to a testing tube. A standard solution (0.1 mL) was added to each standard testing tube, and distilled water (0.1 mL) was added to another blank testing tube. A 2 mL aliquot of thiobarbituric acid was added into each of these two tubes, which were agitated several times, and then incubated at 95°C for 1 hour. Then, each specimen was cooled to room temperature and centrifuged at 3 000 r/min for 10 minutes. Finally, the supernatant in each tube was subjected to colorimetric assay at 532 nm. Total protein content in samples was analyzed using a bicinchoninic acid protein assay kit (Shanghai Sangon Biotech, Shanghai, China). The results were expressed in nmol MDA per milligram of tissue homogenate (nmol/mg protein).
Superoxide dismutase activity determination: Superoxide dismutase is a key enzyme in the dismutation of superoxide radicals, resulting from cellular oxidative metabolism, into hydrogen peroxide. On day 63, superoxide dismutase activity was determined using an assay kit (Nanjing Jiancheng Bioengineering Institute) and expressed as units per microgram of total protein (U/mg). Total protein content in samples was assessed using a bicinchoninic acid protein assay kit (Shanghai Sangon Biotech).
Acetylcholinesterase measurement: On day 63, acetylcholinesterase activity was determined using an assay kit (Nanjing Jiancheng Bioengineering Institute). The substrate used was acetylthiocholine. The mercaptan formed was reacted with DTNB, which splits into two products, one of which is 5-thio-2-nitrobenzoate, which absorbs at 412 nm. The results were expressed as units per microgram of total protein (U/mg).
Estimation of neurotransmitter levels: At 63 days after zinc supplementation, dopamine and 5-hydroxytryptamine concentrations in the cerebrum were assayed using a Waters 600 HPLC system (Waters, USA) with electrochemical detection. Frozen cerebrums were homogenized in 1 mL 0.1 mol/L HCl with an internal standard added. Homogenates were centrifuged at 12 000 r/min, 4°C, for 10 minutes in a refrigerated centrifuge. Dihydroxybenzylamine was used as an internal standard. The results were expressed as micrograms per gram (μg/g).
Estimation of aluminum and zinc concentrations
At 63 days after zinc supplementation, the aluminum and zinc concentrations in the cerebrum were estimated from each animal following wet acid digestion with concentrated nitric acid[63,64,65,66,67,68,69,70].
Digested samples were brought to a constant volume and determination of tissue aluminum concentration was performed with an Elan DRCII ICP-MS (Perkin-Elmer, Norwalk, USA). The tissue zinc concentrations were measured directly by flame atomic absorption spectrophotometry (Hitachi, Tokyo, Japan).
Histopathological examination
At 63 days after zinc supplementation, animals were sacrificed at the end of the experiment. Brain specimens were dissected out and immediately fixed in 10% formalin. Tissues were processed for preparation of paraffin blocks (paraffin method).
Sections were cut using a rotator microtome, mounted on slides, stained with hematoxylin and eosin and examined by light microscopy (Leica, Wetzlar, Germany).
Statistical analysis
Statistical analysis was performed using one-way analysis of variance with Scheffé post-hoc test, using SPSS 11.5 software (SPSS, Chicago, IL, USA). Data are expressed as mean ± SD. All P values were two-tailed, and differences were considered significant at P < 0.05.
Research background: The biological functions of zinc in neurogenesis, neuronal migration, synapse formation and neurotransmission are closely associated with learning and memory function.
Research frontiers: No existing study has explored the protective effect of zinc supplementation against aluminum-induced neurotoxicity. This study aims to investigate the mechanisms underlying the neuroprotective effect of zinc supplementation against brain dysfunction induced by aluminum exposure in rats.
Clinical significance: The experimental findings further our understanding of the mechanisms underlying the neuroprotective effect of zinc supplementation against brain dysfunction in aluminum-exposed rats. The findings should help in developing strategies for preventing and treating neurodegenerative diseases caused by aluminum exposure.
Academic terminology: Lipid peroxidation–oxidation by hydrogen peroxide or superoxide of unsaturated fatty acids to produce peroxides non-enzymatically.
Peer review: This study examined the effect of different doses of zinc on behavioral function, cerebral cortex biochemical indices and pathological abnormalities in rats exposed to aluminum, with the aim of understanding the role of zinc in the prevention and treatment of chronic aluminum exposure-induced neurotoxicity. Further study, in which a control group has received no zinc supplement, is urgently needed.
Acknowledgments
We would like to express our sincere gratitude to Sun SD and Tan L from the Department of Nutrition, Institute of Health & Environmental Medicine, Academy of Military Medical Sciences in Tianjin, China for assistance with the research.
Footnotes
Funding: This study was funded by the National Nature Science Foundation of China, No. 30872098, 30901185; the National Nature Science Foundation of Tianjin, No. 05YFJMJC 05500; the Medical Science and Technology Project of Chinese PLA, No. 13QNP069.
Conflicts of interest: None declared.
Ethical approval: This experiment was approved by the Institute of Health and Environmental Medicine Care and Use Committee of Tianjin in China.
(Reviewed by Patel B, Norman C, Tu QY, Tu HS)
(Edited by Wang J, Yang Y, Li CH, Song LP, Liu WJ, Zhao M)
REFERENCES
- [1].Domingo JL. Reproductive and developmental toxicity of aluminum: a review. Neurotoxicol Teratol. 1995;17(4):515–521. doi: 10.1016/0892-0362(95)00002-9. [DOI] [PubMed] [Google Scholar]
- [2].Shafer TJ, Mundy WR. Effects of aluminum on neuronal signal transduction: mechanisms underlying disruption of phosphoinositide hydrolysis. Gen Pharmacol. 1995;26(5):889–895. doi: 10.1016/0306-3623(94)00296-y. [DOI] [PubMed] [Google Scholar]
- [3].Yao XL, Jenkins EC, Wisniewski HM. Effect of aluminum chloride on mitogenesis, mitosis, and cell cycle in human short-term whole blood cultures: lower concentrations enhance mitosis. J Cell Biochem. 1994;54(4):473–477. doi: 10.1002/jcb.240540414. [DOI] [PubMed] [Google Scholar]
- [4].Zatta PF, Zambenedetti P, Masiero S. Effects of aluminum lactate on murine neuroblastoma cells. Neurotoxicology. 1994;15(4):789–797. [PubMed] [Google Scholar]
- [5].Yokel RA. Aluminum chelation principles and recent advances. Coord Chem Rev. 2002;228:97–113. [Google Scholar]
- [6].Pennington JA, Schoen SA. Estimates of dietary exposure to aluminium. Food Addit Contam. 1995;12(1):119–128. doi: 10.1080/02652039509374286. [DOI] [PubMed] [Google Scholar]
- [7].Kumar V, Gill KD. Aluminium neurotoxicity: neurobehavioural and oxidative aspects. Arch Toxicol. 2009;83(11):965–978. doi: 10.1007/s00204-009-0455-6. [DOI] [PubMed] [Google Scholar]
- [8].Kawahara M. Effects of aluminum on the nervous system and its possible link with neurodegenerative diseases. J Alzheimers Dis. 2005;8(2):171–182. doi: 10.3233/jad-2005-8210. discussion 209-215. [DOI] [PubMed] [Google Scholar]
- [9].Bondy SC. The neurotoxicity of environmental aluminum is still an issue. Neurotoxicology. 2010;31(5):575–581. doi: 10.1016/j.neuro.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Walton JR. Bioavailable Aluminum: Its Effects on Human Health. Encyclopedia of Environmental Health. 2011:331–342. [Google Scholar]
- [11].Crisponi G, Nurchi VM, Bertolasi V, et al. Chelating agents for human diseases related to aluminium overload. Coord Chem Rev. 2012;256:89–104. [Google Scholar]
- [12].Nehru B, Anand P. Oxidative damage following chronic aluminium exposure in adult and pup rat brains. J Trace Elem Med Biol. 2005;19(2-3):203–208. doi: 10.1016/j.jtemb.2005.09.004. [DOI] [PubMed] [Google Scholar]
- [13].Kumar V, Bal A, Gill KD. Aluminium-induced oxidative DNA damage recognition and cell-cycle disruption in different regions of rat brain. Toxicology. 2009;264(3):137–144. doi: 10.1016/j.tox.2009.05.011. [DOI] [PubMed] [Google Scholar]
- [14].Kumar V, Bal A, Gill KD. Susceptibility of mitochondrial superoxide dismutase to aluminium induced oxidative damage. Toxicology. 2009;255(3):117–123. doi: 10.1016/j.tox.2008.10.009. [DOI] [PubMed] [Google Scholar]
- [15].Kumar A, Dogra S, Prakash A. Protective effect of curcumin (Curcuma longa), against aluminium toxicity: Possible behavioral and biochemical alterations in rats. Behav Brain Res. 2009;205(2):384–390. doi: 10.1016/j.bbr.2009.07.012. [DOI] [PubMed] [Google Scholar]
- [16].Kaur A, Gill KD. Disruption of neuronal calcium homeostasis after chronic aluminium toxicity in rats. Basic Clin Pharmacol Toxicol. 2005;96(2):118–122. doi: 10.1111/j.1742-7843.2005.pto960205.x. [DOI] [PubMed] [Google Scholar]
- [17].Julka D, Gill KD. Altered calcium homeostasis: a possible mechanisms of aluminium-induced neurotoxicity. Biochim Biophys Acta. 1996;1315(1):47–54. doi: 10.1016/0925-4439(95)00100-x. [DOI] [PubMed] [Google Scholar]
- [18].Guo GW, Liang YX. Aluminum-induced apoptosis in cultured astrocytes and its effect on calcium homeostasis. Brain Res. 2001;888(2):221–226. doi: 10.1016/s0006-8993(00)03057-2. [DOI] [PubMed] [Google Scholar]
- [19].Shafer TJ, Mundy WR. Effects of aluminum on neuronal signal transduction: mechanisms underlying disruption of phosphoinositide hydrolysis. Gen Pharmacol. 1995;26(5):889–895. doi: 10.1016/0306-3623(94)00296-y. [DOI] [PubMed] [Google Scholar]
- [20].Haug A, Shi B, Vitorello V. Aluminum interaction with phosphoinositide-associated signal transduction. Arch Toxicol. 1994;68(1):1–7. doi: 10.1007/s002040050023. [DOI] [PubMed] [Google Scholar]
- [21].Shi B, Chou K, Haug A. Aluminium impacts elements of the phosphoinositide signalling pathway in neuroblastoma cells. Mol Cell Biochem. 1993;121(2):109–118. doi: 10.1007/BF00925969. [DOI] [PubMed] [Google Scholar]
- [22].Vallee BL, Auld DS. Zinc coordination, function, and structure on zinc enzymes and other proteins. Biochemistry. 1990;29(24):5647–5659. doi: 10.1021/bi00476a001. [DOI] [PubMed] [Google Scholar]
- [23].Takeda A. Movement of zinc and its functional significance in the brain. Brain Res Rev. 2000;34(3):137–148. doi: 10.1016/s0165-0173(00)00044-8. [DOI] [PubMed] [Google Scholar]
- [24].Mocchegiani E, Giacconi R, Muzzioli M, et al. Zinc, infections and immunosenescence. Mech Ageing Dev. 2000;121(1-3):21–35. doi: 10.1016/S0047-6374(00)00194-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Cuajungco MP, Lees GJ. Zinc and Alzheimer's disease: is there a direct link? Brain Res Brain Res Rev. 1997;23(3):219–236. doi: 10.1016/s0165-0173(97)00002-7. [DOI] [PubMed] [Google Scholar]
- [26].Saha AR, Hadden EM, Hadden JW. Zinc induces thymulin secretion from human thymic epithelial cells in vitro and augments splenocyte and thymocyte responses in vivo. Int J Immunopharmacol. 1995;17(9):729–733. doi: 10.1016/0192-0561(95)00061-6. [DOI] [PubMed] [Google Scholar]
- [27].Dreosti IE. Zinc and the gene. Mutat Res. 2001;475(1-2):161–167. doi: 10.1016/s0027-5107(01)00067-7. [DOI] [PubMed] [Google Scholar]
- [28].Tamura T, Goldenberg RL. Zinc nutriture and pregnancy outcome. Nutr Res. 1996;16:139–181. [Google Scholar]
- [29].Liu YQ, Gu JF, Li ST, et al. Study on zinc supplementation dosage for maintaining best learning and memory function in rat. Yingyang Xuebao. 2000;22(4):289–293. [Google Scholar]
- [30].Li J, Lu H, Yang HP, et al. Effects of sub-chronic aluminium exposure on learning and memory functions and antioxidative capacity in rats. Xiandai Yufang Yixue. 2011;38(11):2015–2017. [Google Scholar]
- [31].Sharma P, Mishra KP. Aluminum-induced maternal and developmental toxicity and oxidative stress in rat brain: response to combined administration of Tiron and glutathione. Reprod Toxicol. 2006;21(3):313–321. doi: 10.1016/j.reprotox.2005.06.004. [DOI] [PubMed] [Google Scholar]
- [32].Erazi H, Ahboucha S, Gamrani H. Chronic exposure to aluminum reduces tyrosine hydroxylase expression in the substantia nigra and locomotor performance in rats. Neurosci Lett. 2011;487(1):8–11. doi: 10.1016/j.neulet.2010.09.053. [DOI] [PubMed] [Google Scholar]
- [33].Struys-Ponsar C, Kerkhofs A, Gauthier A, et al. Effects of aluminum exposure on behavioral parameters in the rat. Pharmacol Biochem Behav. 1997;56(4):643–648. doi: 10.1016/s0091-3057(96)00515-1. [DOI] [PubMed] [Google Scholar]
- [34].Yellamma K, Saraswathamma S, Kumari BN. Cholinergic system under aluminium toxicity in rat brain. Toxicol Int. 2010;17(2):106–112. doi: 10.4103/0971-6580.72682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].El-Rahman SS. Neuropathology of aluminum toxicity in rats (glutamate and GABA impairment) Pharmacol Res. 2003;47(3):189–194. doi: 10.1016/s1043-6618(02)00336-5. [DOI] [PubMed] [Google Scholar]
- [36].Newairy AS, Salama AF, Hussien HM, et al. Propolis alleviates aluminium-induced lipid peroxidation and biochemical parameters in male rats. Food Chem Toxicol. 2009;47(6):1093–1098. doi: 10.1016/j.fct.2009.01.032. [DOI] [PubMed] [Google Scholar]
- [37].Yousef MI. Aluminium-induced changes in hemato-bio- chemical parameters, lipid peroxidation and enzyme activities of male rabbits: protective role of ascorbic acid. Toxicology. 2004;199(1):47–57. doi: 10.1016/j.tox.2004.02.014. [DOI] [PubMed] [Google Scholar]
- [38].Golub MS, Keen CL, Gershwin ME. Neurodevelopmental effect of aluminum in mice: fostering studies. Neurotoxicol Teratol. 1992;14(3):177–182. doi: 10.1016/0892-0362(92)90013-z. [DOI] [PubMed] [Google Scholar]
- [39].Flora SJ, Mehta A, Satsangi K, et al. Aluminum-induced oxidative stress in rat brain: response to combined ad-ministration of citric acid and HEDTA. Comp Biochem Physiol C Toxicol Pharmacol. 2003;134(3):319–328. doi: 10.1016/s1532-0456(02)00269-7. [DOI] [PubMed] [Google Scholar]
- [40].Baydar T, Nagymajtényi L, Isimer A, et al. Effect of folic acid supplementation on aluminum accumulation in rats. Nutrition. 2005;21(3):406–410. doi: 10.1016/j.nut.2004.07.008. [DOI] [PubMed] [Google Scholar]
- [41].Yousef MI, Salama AF. Propolis protection from reproductive toxicity caused by aluminium chloride in male rats. Food Chem Toxicol. 2009;47(6):1168–1175. doi: 10.1016/j.fct.2009.02.006. [DOI] [PubMed] [Google Scholar]
- [42].Kumar V, Bal A, Gill KD. Aluminium-induced oxidative DNA damage recognition and cell-cycle disruption in different regions of rat brain. Toxicology. 2009;264(3):137–144. doi: 10.1016/j.tox.2009.05.011. [DOI] [PubMed] [Google Scholar]
- [43].Sood PK, Nahar U, Nehru B. Curcumin attenuates aluminum-induced oxidative stress and mitochondrial dysfunction in rat brain. Neurotox Res. 2011;20(4):351–361. doi: 10.1007/s12640-011-9249-8. [DOI] [PubMed] [Google Scholar]
- [44].Kaur A, Joshi K, Minz RW, et al. Neurofilament phosphorylation and disruption: a possible mechanism of chronic aluminium toxicity in Wistar rats. Toxicology. 2006;219(1-3):1–10. doi: 10.1016/j.tox.2005.09.015. [DOI] [PubMed] [Google Scholar]
- [45].Colomina MT, Roig JL, Sánchez DJ, et al. Influence of age on aluminum-induced neurobehavioral effects and morphological changes in rat brain. Neurotoxicology. 2002;23(6):775–781. doi: 10.1016/S0161-813X(02)00008-6. [DOI] [PubMed] [Google Scholar]
- [46].Domingo JL, Llorens J, Sa´nchez DJ, et al. Age-related effects of aluminum ingestion on brain aluminum accumulation and behavior in rats. Life Sci. 1996;58(3):1387–1395. doi: 10.1016/0024-3205(96)00108-7. [DOI] [PubMed] [Google Scholar]
- [47].Bhalla P, Garg ML, Dhawan DK. Protective role of lithium during aluminium-induced neurotoxicity. Neurochem Int. 2010;56(2):256–262. doi: 10.1016/j.neuint.2009.10.009. [DOI] [PubMed] [Google Scholar]
- [48].Zhang ZJ, Qian YH, Hu HT, et al. The herbal medicine Dipsacus asper wall extract reduces the cognitive deficits and overexpression of beta-amyloid protein induced by aluminum exposure. Life Sci. 2003;73(19):2443–2454. doi: 10.1016/s0024-3205(03)00649-0. [DOI] [PubMed] [Google Scholar]
- [49].Erazi H, Sansar W, Ahboucha S, et al. Aluminum affects glial system and behavior of rats. C R Biol. 2010;333(1):23–27. doi: 10.1016/j.crvi.2009.09.016. [DOI] [PubMed] [Google Scholar]
- [50].Abdel-Aal RA, Assi AA, Kostandy BB. Rivastigmine reverses aluminum-induced behavioral changes in rats. Eur J Pharmacol. 2011;659(2-3):169–176. doi: 10.1016/j.ejphar.2011.03.011. [DOI] [PubMed] [Google Scholar]
- [51].Sethi P, Jyoti A, Singh R, et al. Aluminium-induced electrophysiological, biochemical and cognitive modifications in the hippocampus of aging rats. Neurotoxicology. 2008;29(6):1069–1079. doi: 10.1016/j.neuro.2008.08.005. [DOI] [PubMed] [Google Scholar]
- [52].Requena JR, Fu MX, Ahmed MU, et al. Lipoxidation products as biomarkers of oxidative damage to proteins during lipid peroxidation reactions. Nephrol Dial Transplant. 1996;11(Suppl 5):48–53. doi: 10.1093/ndt/11.supp5.48. [DOI] [PubMed] [Google Scholar]
- [53].Bihaqi SW, Sharma M, Singh AP, et al. Neuroprotective role of Convolvulus pluricaulis on aluminium induced neurotoxicity in rat brain. J Ethnopharmacol. 2009;124(3):409–415. doi: 10.1016/j.jep.2009.05.038. [DOI] [PubMed] [Google Scholar]
- [54].Mahadik SP, Laev H, Korenovsky A, et al. Haloperidol alters rat CNS cholinergic system: enzymatic and morphological analyses. Biol Psychiatry. 1988;24(2):199–217. doi: 10.1016/0006-3223(88)90275-2. [DOI] [PubMed] [Google Scholar]
- [55].Gulya K, Rakonczay Z, Kása P. Cholinotoxic effects of aluminium in rat brain. J Neurochem. 1990;54:1020–1026. doi: 10.1111/j.1471-4159.1990.tb02352.x. [DOI] [PubMed] [Google Scholar]
- [56].Ravi SM, Prabhu BM, Raju TR, et al. Long-term effects of postnatal aluminium exposure on acetylcholinesterase activity and biogenic amine neurotransmitters in rat brain. Indian J Physiol Pharmacol. 2000;44(4):473–478. [PubMed] [Google Scholar]
- [57].Kumar S. Biphasic effect of aluminium on cholinergic enzyme of rat brain. Neurosci Lett. 1998;248(2):121–123. doi: 10.1016/s0304-3940(98)00267-5. [DOI] [PubMed] [Google Scholar]
- [58].Tsunoda M, Sharma RP. Altered dopamine turnover in murine hypothalamus after low-dose continuous oral administration of aluminum. J Trace Elem Med Biol. 1999;13(4):224–231. doi: 10.1016/S0946-672X(99)80040-6. [DOI] [PubMed] [Google Scholar]
- [59].Kumar S. Aluminium-induced changes in the rat brain serotonin system. Food Chem Toxicol. 2002;40(12):1875–1880. doi: 10.1016/s0278-6915(02)00180-1. [DOI] [PubMed] [Google Scholar]
- [60].The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006 Sep 30; [Google Scholar]
- [61].Reeves PG, Nielsen FH, Fahey GC. AIN-93 purified diets for laboratory rodents: Final report of the American Institute of Nutrition and ad hoc writing committee on the reformulation of the AIN-75 rodent diet. J Nutr. 1993;123(11):1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- [62].Liu J, Jiang YG, Huang CY, et al. Proteomic analysis reveals changes in the hippocampus protein pattern of rats exposed to dietary zinc deficiency. Electrophoresis. 2010;31(8):1302–1310. doi: 10.1002/elps.200900733. [DOI] [PubMed] [Google Scholar]
- [63].Yasuda H, Yasuda Y, Tsutsui T, et al. Estimation of autistic children by metallomics analysis. Sci Rep. 2013;3:1199. doi: 10.1038/srep01199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Wong CM, Rabl A, Thach TQ, et al. Impact of the 1990 Hong Kong legislation for restriction on sulfur content in fuel. Res Rep Health Eff Inst. 2012;170:5–91. [PubMed] [Google Scholar]
- [65].Cao H, Qiao L, Zhang H, et al. Exposure and risk assessment for aluminium and heavy metals in Puerh tea. Sci Total Environ. 2010;408(14):2777–2784. doi: 10.1016/j.scitotenv.2010.03.019. [DOI] [PubMed] [Google Scholar]
- [66].Shashikiran ND, Subba Reddy VV, Hiremath MC. Estimation of trace elements in sound and carious enamel of primary and permanent teeth by atomic absorption spectrophotometry: an in vitro study. Indian J Dent Res. 2007;18(4):157–162. doi: 10.4103/0970-9290.35824. [DOI] [PubMed] [Google Scholar]
- [67].Schintu M, Cogoni A, Durante L, et al. Moss (Bryum radiculosum) as a bioindicator of trace metal deposition around an industrialised area in Sardinia (Italy) Chemosphere. 2005;60(5):610–618. doi: 10.1016/j.chemosphere.2005.01.050. [DOI] [PubMed] [Google Scholar]
- [68].Ba T, Zheng M, Zhang B, et al. Estimation and congener-specific characterization of polychlorinated naphthalene emissions from secondary nonferrous metallurgical facilities in China. Environ Sci Technol. 2010;44(7):2441–2446. doi: 10.1021/es9033342. [DOI] [PubMed] [Google Scholar]
- [69].Sarkar M, Sarkar AR, Goswami JL. Mathematical modeling for the evaluation of zinc removal efficiency on clay sorbent. J Hazard Mater. 2007;149(3):666–674. doi: 10.1016/j.jhazmat.2007.04.027. [DOI] [PubMed] [Google Scholar]
- [70].Urbaniak B, Kokot ZJ. Spectroscopic investigations of fluoroquinolones metal ion complexes. Acta Pol Pharm. 2013;70(4):621–629. [PubMed] [Google Scholar]


