Abstract
One of the well-defined sexually dimorphic structures in the brain is the sexually dimorphic nucleus, a cluster of cells located in the preoptic area of the hypothalamus. The rodent sexually dimorphic nucleus of the preoptic area can be delineated histologically using conventional Nissl staining or immunohistochemically using calbindin D28K immunoreactivity. There is increasing use of the bindin D28K-delineated neural cluster to define the sexually dimorphic nucleus of the preoptic area in rodents. Several mechanisms are proposed to underlie the processes that contribute to the sexual dimorphism (size difference) of the sexually dimorphic nucleus of the preoptic area. Recent evidence indicates that stem cell activity, including proliferation and migration presumably from the 3rd ventricle stem cell niche, may play a critical role in the postnatal development of the sexually dimorphic nucleus of the preoptic area and its distinguishing sexually dimorphic feature: a signifi-cantly larger volume in males. Sex hormones and estrogen-like compounds can affect the size of the sexually dimorphic nucleus of the preoptic area. Despite considerable research, it remains un-clear whether estrogen-like compounds and/or sex hormones increase size of the sexually dimor-phic nucleus of the preoptic area via an increase in stem cell activity originating from the 3rd ventricle stem cell niche.
Keywords: neural regeneration, review, sexual orientation, sexual behavior, calbindin D28K, estrogen-like compound, bisphenol A, neural stem cells, grants-supported paper, neuroregeneration
Research Highlights
(1) We describe our work concerning study of the sexually dimorphic nucleus of the preoptic area. Several developmental mechanisms underlying the sexual dimorphism of the sexually dimorphic nucleus of the preoptic area are reviewed and updated by addressing the potential role of neural stem cell activity.
(2) The sexually dimorphic nucleus is a highly tractable feature that can be studied as a model system regarding how sex differences in brain function arise and are maintained. This is highly clinically relevant for understanding the origins of sex biases in psychiatric syndromes and for iden-tifying novel clinical targets.
(3) Because there is increasing evidence that perinatal exposure to estrogen-like compounds may be associated with a host of health problems including obesity and many mental disorders such as depression and also because one of those estrogen-like compounds, bisphenol A, has been shown to alter the sexually dimorphic nucleus of the preoptic area. And exploring the mechanisms by which sex hormones or estrogen-like compounds affect sexual dimorphic structures of the brain might lead to the development of new therapeutic approaches.
INTRODUCTION
The human brain is anatomically and functionally sexually dimorphic. While specific debates on this topic have occurred for decades, sexual dimorphism is generally acknowledged with respect to brain size, cognitive function, emotional expression, and other behavior patterns. One of the most widely-accepted sexually dimorphic brain structures is the sexually dimorphic nucleus, a cluster of cells located in the preoptic area of the hypothalamus.
The sexually dimorphic nucleus has been specifically defined in the brains of human and other mammalian and non-mammalian and includes the third interstitial nucleus of the anterior hypothalamus in humans[1,2], the ovine sexually dimorphic nucleus in the medial preoptic area[3], the medial preoptic and anterior hypothalamic regions in rhesus monkeys[4], a specific area in the medial preoptic nucleus in quail[5], and the sexually dimorphic nucleus of the preoptic area in rats[6,7]. The human sexually dimorphic nucleus of the preoptic area is located in the medial part of the preoptic area, between the dorsolateral supraoptic nucleus and the rostral pole of the paraventricular nucleus[8]. The interstitial nucleus of the anterior hypothalamus 3 (INAH3) in the human is now considered as the structural equivalent of the sexually dimorphic nucleus of the preoptic area of the rat[9]. As demonstrated in laboratory animal studies, the sexually dimorphic nucleus is critically implicated in sexual behavior[10,11]. In humans, the sexually dimorphic nucleus of the preoptic area has been linked to sexual orientation[9,12]. Thus, this structure allows for the study of sex differences under the normal and pathophysiological states.
Sex hormone-like compounds can be found throughout the environment, occurring in natural and processed foods, food and drink containers, and medical devices. Many of these are capable of altering normal development and exerting pathophysiological effects on the central nervous system, most noticeably in sexually dimorphic brain structures. Hundreds of synthetic compounds are estrogen-like compounds that have at least some affinity for estrogen receptors and can affect gene transcription. There is increasing evidence that perinatal exposure to estrogen-like compounds may be associated with a host of health problems. The objective of the present study is to review the latest advances in morphological definition, developmental mechanisms, and environmental factors (i.e., estrogen-like compounds) that can influence the development of the sexually dimorphic nucleus of the preoptic area.
SEXUALLY DIMORPHIC NUCLEUS
Defining sexually dimorphic nucleus
Volume is a widely-accepted sexually dimorphic feature of the sexually dimorphic nucleus: the volume of the male rat sexually dimorphic nucleus of the preoptic area is typically 3–8 times that of the female[6,7,13]. This marked sex difference in volume is due principally to an increase in the area of higher cell and neuron density seen in adult male rats[7]. Similarly, in humans, there are many more cells in the male sexually dimorphic nucleus[8].
Determining the sexually dimorphic nucleus of the preoptic area
Initial measurements of the sexually dimorphic nucleus of the preoptic area were conducted using the Nissl method to delineate the sexually dimorphic nucleus of the preoptic area by staining the negatively charged RNA blue with thionin or cresyl violet. That method is similar to that reported by others[14,15] (Figure 1). As seen in the three-dimensional reconstruction (Figure 2), the sexually dimorphic nucleus of the preoptic area can be defined by its location relative to several anatomic landmarks including the 3rd ventricle, optic chiasm, anterior commissure, and suprachiasmatic nucleus. As is clearly evident in coronal and sagittal views, the female sexually dimorphic nucleus of the preoptic area is considerably smaller.
Figure 1.
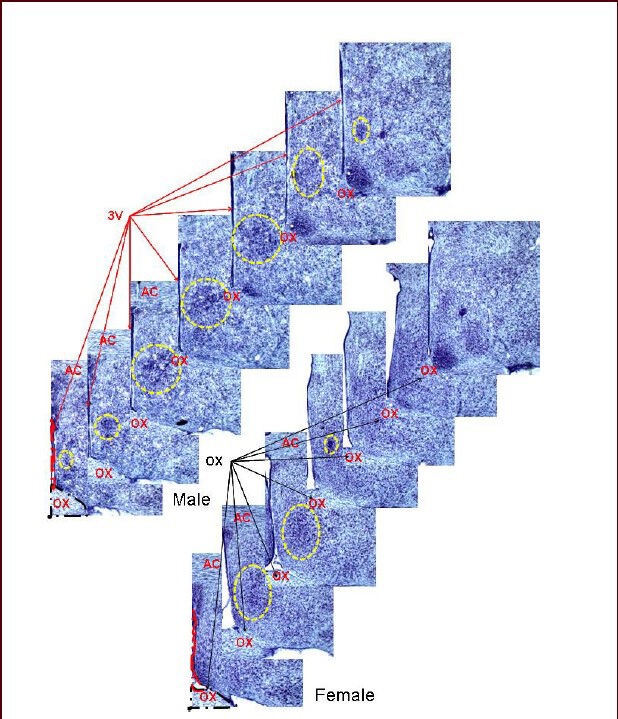
Serial brain sections: the sexually dimorphic nucleus of the preoptic area stained with thionin.
Landmark structures that are also found throughout serial sections containing the sexually dimorphic nucleus of the preoptic area are labeled including the 3rd ventricle (3V) and the optic chiasm (OX). These structures were traced and their images used in constructing the three-dimensional images. The yellow dotted circle highlights the sexually dimorphic nucleus of the preoptic area. This figure is from our unpublished data (NCTR/US FDA Protocol P00710). Sexually dimorphic nucleus of the preoptic area: the area outlined by the yellow dashed circle; AC: anterior commissure. 10 × magnification was used.
Figure 2.
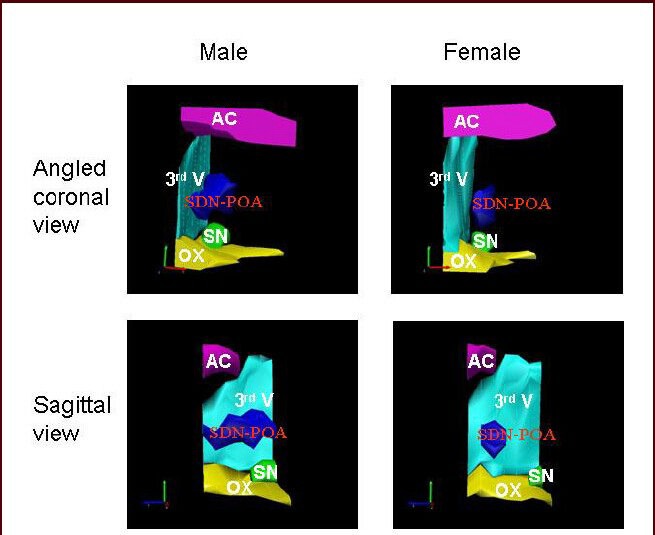
Three-dimensional view of the male and female rat sexually dimorphic nucleus of the preoptic area.
The Stereo Investigation System (MBF Bioscience, Williston, VT) was used to reconstruct these views of the sexually dimorphic nucleus of the preoptic area and its surrounding anatomical structures from seven sequential slices stained with thionin (refer to Figure 1). This figure is from our unpublished data (NCTR/US FDA Protocol P00710). AC: Anterior commissure; 3rd V: 3rd ventricle; SDN-POA: sexually dimorphic nucleus of the preoptic area; SN: suprachiasm nucleus; OX: optic chiasm.
Using serial coronal sections stained with thionin, every third slice was used in the reconstruction of the three-dimensional illustration and the sexually dimorphic nucleus of the preoptic area was identified by its anatomic location and its characteristic dense mass (Figure 1). By definition, the preoptic area is the region situated immediately below the anterior commissure, above the optic chiasm, and anterior to the hypothalamus, although it is not clearly demarcated from the hypothalamus. There are four nuclei in the preoptic area according to Terminologia Anatomica: medial, median, lateral, and paraventricular. Although the concept of the sexually dimorphic nucleus of the preoptic area is well-accepted by the scientific community, little consensus has been reached concerning its precise location. Using conventional Nissl histological methods (thionin or cresyl violet staining), the reported size of the sexually dimorphic nucleus of the preoptic area has ranged more than 7–30-fold for male and female rats, respectively[13].
Presumably, the lack of a clear-cut sexually dimorphic nucleus of the preoptic area boundary in tissue stained using conventional histological methods, including Nissl and hematoxylin and eosin, accounts for such variation. In addition, there is some disagreement as to which preoptic nuclei define the sexually dimorphic nucleus of the preoptic area. Some investigators refer to the central nucleus of the medial preoptic nucleus as the sexually dimorphic nucleus of the preoptic area[13], whereas others accept the entire medial preoptic nucleus. Using the key words “medial preoptic nucleus” and “sexually dimorphic nucleus” in a recent Medline search, more than 240 papers on this topic were retrieved. In general, the preoptic area of the hypothalamus is classified as sexually dimorphic, with differences between males and females often highlighted using immunocytochemical markers for estrogen receptor beta and the R1 subunit of the gamma-aminobutyric acid (B) receptor[16]. Further, the sexually dimorphic nucleus of the preoptic area/ central nucleus of the medial preoptic nucleus and the medial preoptic area may be functionally inseparable if the expression of Fos and/or glutamic acid decarboxylase is a defining characteristic[17].
There is increasing acceptance that the calbindin-D28K immunoreactivity-delineated nucleus-like structure located in the preoptic area can be defined as the sexually dimorphic nucleus of the preoptic area. This is probably due to the observation that calbindin-D28K immunoreactivity provides clear boundaries that are readily distinguishable from the surrounding structures (Figure 3A, B)[18]. Further, a similar sexually dimorphic nucleus of the preoptic area area in mice is not well defined using Nissl staining[19,20], but is quite evident using calbindin-D28K immunoreactivity[20,21,22,23]. Originally, the calbindin-D28K-delineated sexually dimorphic nucleus of the preoptic area was considered a subdivision of the rat sexually dimorphic nucleus of the preoptic area as determined using the Nissl method[24]. However, the calbindin-D28K-delineated area is often used now as a proxy for the sexually dimorphic nucleus of the preoptic area[25]. A recent study reported that size of the sexually dimorphic nucleus of the preoptic area, as determined by either the calbindin-D28K labeling method or the Nissl method, is similar[15]. However, data from the brains of more than 200 weanling and adult Sprague-Dawley rats collected in our laboratory supports the finding that the sexually dimorphic nucleus of the preoptic area delineated by thionin stain is much larger than that delineated by calbindin-D28K immunoreactivity (data not shown). Using a fluorescence-double/triple labeling approach, we have employed a 4’,6-diamidino-2-phenylindole (DAPI)-delineated, solid nuclear mass in the preoptic area to assist in defining the sexually dimorphic nucleus of the preoptic area (Figure 3A, B)[18,26]. Because DAPI-labeling demarcates the sexually dimorphic nucleus of the preoptic area similar to that using calbindin-D28K immunoreactivity, it serves a dual purpose: (1) anatomic landmarks delineated by the DAPI-labeling, including the anterior commissure and the optic chiasm, are provided as reference points to more accurately locate the sexually dimorphic nucleus of the preoptic area; and (2) the sexually dimorphic nucleus of the preoptic area is conceptually defined via its characteristic as a congested nuclear mass, while the DAPI-labeled nucleic mass addresses density of the cellular nuclei over there.
Figure 3.
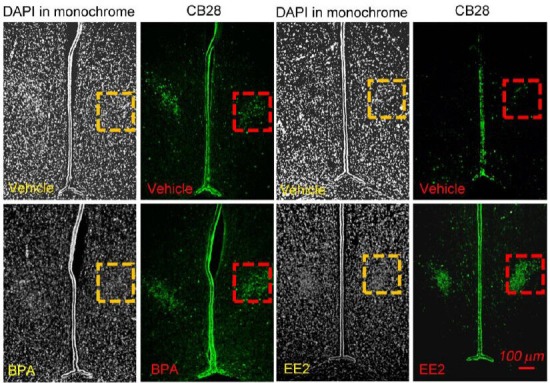
Representative images of 4’,6-diamidino-2-phenylindole (DAPI) and calbindin D28k immunoreactivity that delineate the sexually dimorphic nucleus of the preoptic area in male (A) and female rats (B).
DAPI highlights cell nuclei throughout the slices and thus provides anatomic landmarks surrounding the sexually dimorphic nucleus of the preoptic area (SDN-POA), which is nicely highlighted using calbindin-D28K immunostaining. The red and yellow dashed squares enclose the sexually dimorphic nucleus of the preoptic area. (A) The upper panels show the SDN-POA in a vehicle-treated animal whereas the lower panels show the sexually dimorphic nucleus of the preoptic area in a postnatal day (PND) 21 rat treated with bisphenol A (BPA; 2.5 μg/kg per day). The vehicle solution or BPA was given orally via gavage from gestational days 6–21 (dams were treated orally with 5 mL/kg of the appropriate solution; no treatment occurred on the day of birth); beginning on the day after parturition (PND 1) through PND 21, pups were treated orally via gavage with the same dose as their dam had received[18,27]. (B) The upper panels show the SDN-POA in a vehicle-treated animal. The lower panels show the SDN-POA in a PND 21 rat treated with ethinyl estradiol (EE2, 10 μg/kg per day). Similar to that described for Figure 3A, the vehicle solution or EE2 was given orally via gavage on gestational days 6–21 and then directly to pups on PND 1–21[18,27]. This figure is from our unpublished data (NCTR/US FDA Protocol P00706).
However, using the immunofluorescent labeling technique alone, a sexually dimorphic nucleus of the preoptic area defined solely by the neuronal marker calbindin-D28K is preferable because of its ability to allow clear visualization of boundaries. Thus, in order to minimize confusion concerning the sexually dimorphic nucleus of the preoptic area as a specific structure, it will be defined here as a calbindin-D28K-delineated neuronal nucleus in the preoptic area in rats.
Compared to the Nissl method, calbindin-D28K-immunoreactivity demarcates the hypothalamic sexually dimorphic nucleus of the preoptic area as a smaller structure noticeable along the longitudinal axis of the brain (in a sagittal view). Using thionin staining, a male sexually dimorphic nucleus of the preoptic area can be visualized as a densely-stained blue nucleus located below the anterior commissure and above the optic chiasm. This structure has been visualized using a series of 30 μm-thick coronal slices and occupies a length of 540–630 μm along the longitudinal axis of the brain (highlighted by the dotted circle in Figure 1; left slice series) in males, while the female sexually dimorphic nucleus of the preoptic area has a length of 180–270 μm (Figure 1; right slice series). Even though scattered calbindin-D28K-positive cells can be identified in these same locations, a clear calbindin-D28K-positive cell mass is recognizable only over a length of 90–360 μm with a range of 90–270 μm in females and a range of 180–360 μm in males. Actually, the location of a DAPI-delineated nuclear mass matches the location of the calbindin-D28K-immunoreactivity-defined cell mass (Figure 3A, B)[18]. Interestingly, none of the calbindin-D28K-positive neurons in the sexually dimorphic nucleus of the preoptic area are positive for tyrosine hydroxylase (TH), yet many TH-positive axon and synapse-like structures are found there (Figure 4).
Figure 4.
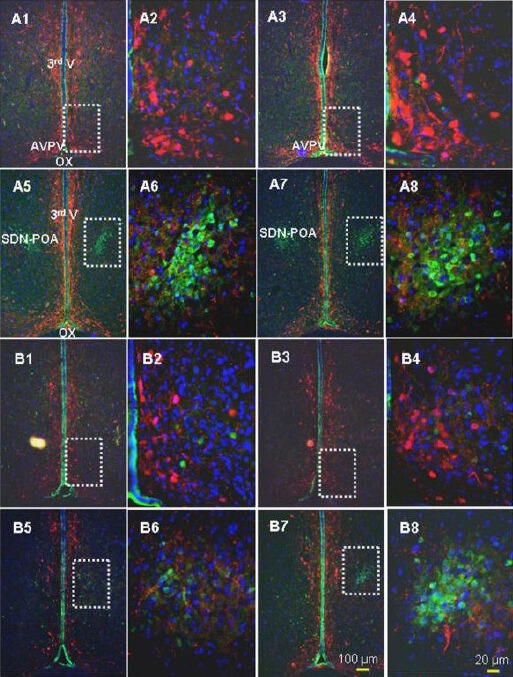
Representative images of the triple labeling approach using calbindin D28k (calbindin-D28K; green), tyrosine hydroxylase (red), and 4’,6-diamidino-2-phenylindole (DAPI; blue).
Shown are four sequential slices along the longitudinal axis of the brain with intervals of 90 μm between adjacent slices. Here, the male AVPV (A1 and A3) and male sexually dimorphic nucleus of the preoptic area (A5 and A7) and the female AVPV (B1 and B3) and female sexually dimorphic nucleus of the preoptic area (B5 and B7) are outlined by dashed rectangles. The sexually dimorphic nucleus of the preoptic area is highlighted by calbindin-D28K immunoreactivity: no TH-positive cells were found, but fine axon-like projections/synaptic structures were seen. A neuron double-labeled with calbindin-D28K and TH was rare. The white rectangles in A1, 3 and B1, 3 highlight the AVPV, which is also displayed at higher magnification in A2, 4 and B2, 4. The white, dashed rectangles in A5, 7 and B5, 7 highlight the sexually dimorphic nucleus of the preoptic area, which is also displayed at higher magnification in A6 & 8 and B6 & 8. AVPV: Anteroventral periventricular nucleus; OX: optic chiasm; 3rd V: 3rd ventricle; SDN-POA: sexually dimorphic nucleus of the preoptic area. This figure is from our unpublished data (NCTR/US FDA Protocol P00706).
In contrast, the anteroventral periventricular nucleus of the hypothalamus (AVPV) consists mainly of TH-positive neurons (Figure 4) and there is scarcely a neuron that expresses both calbindin-D28K and TH-immunoreactivity. Accordingly, a triple labeling method (calbindin-D28K-TH-DAPI) would add a contrast comparison and additional anatomic landmarks in determining the sexually dimorphic nucleus of the preoptic area.
DEVELOPMENT OF THE SEXUALLY DIMORPHIC NUCLEUS OF THE PREOPTIC AREA
Formation of the sexually dimorphic nucleus of the preoptic area
The neurons that constitute the sexually dimorphic nucleus of the preoptic area originate from the subepen-dymal lining of the 3rd ventricle, migrating upward and laterally to their final location[28]. Using the thymidine analog bromodeoxyuridine (BrdU) to tag actively proliferating cells, neurogenesis in the sexually dimorphic nucleus of the preoptic area begins on embryonic day 18[29]. In addition, it appears that the nucleus-like shape of both the sexually dimorphic nucleus of the preoptic area and the anteroventral periventricular nucleus begin to emerge on embryonic day 18 as evidenced also by BrdU-labeling.
Essentially, the sexual dimorphism of the sexually dimorphic nucleus of the preoptic area results from a difference in postnatal development: the male sexually dimorphic nucleus of the preoptic area expands continuously through to adulthood[26], whereas the female sexually dimorphic nucleus of the preoptic area remains relatively unchanged beyond weaning[24,29,30].
Timeline of formation of the sexually dimorphic nucleus of the preoptic area
Sexual dimorphism of the Nissl-stained sexually dimorphic nucleus of the preoptic area has been reported to occur as early as postnatal day 1[30]; however, a more recent study indicated that there was no detectable sex difference in the calbindin-D28K-labeled sexually dimorphic nucleus of the preoptic area at postnatal day 4[31]. By postnatal day 8, males have a significantly larger sexually dimorphic nucleus of the preoptic area whether determined using Nissl-staining or a calbindin-D28K label[24,31]. On postnatal 8, size of the male sexually dimorphic nucleus of the preoptic area is about twice that of the female when characterized using a calbindin-D28K label[24,31]. At weaning and thereafter, however, the male sexually dimorphic nucleus of the preoptic area is 3–4 times larger than that of the female[18,24,26]. Stage of development is very important when considering the role of apoptosis versus stem cell activity on sexually dimorphic nucleus of the preoptic area size differences between the sexes (discussed below).
Developmental mechanisms that shape the sexually dimorphic nucleus of the preoptic area
Migration
There is increasing evidence that perinatal cell migration is important in the development of the sexually dimorphic nucleus of the preoptic area. As mentioned earlier, the initial appearance of the sexually dimorphic nucleus of the preoptic area occurs on embryonic day 18 using BrdU labeling[29]. The principal component of the sexually dimorphic nucleus of the preoptic area (i.e., neurons) originates from the subependymal lining of the 3rd ventricle[28]. In males, cell migration is faster in a medial-lateral orientation in the preoptic area/anterior hypothalamus[32]. Neural progenitor cells exist in the ependymal layer of the 3rd cerebral ventricle in adult rats and they may migrate into the hypothalamus and differentiate into functionally normal neurons[33,34,35]. Under normal circumstances, ependymal tissue harbors stem cells that are pluripotent and thus maintain the ability to generate various cell types, including neurons and glia. In addition to two other well known neurogenic areas in the adult rat brain (i.e., the subventricular and subgranular zones), the ependymal surface and spinal cord ventricular axis structures, including the 3rd ventricle, also contain cells that exhibit features of neuronal progenitors[36]. Neuron-like cells reside on the ependymal surface of the 3rd ventricle, many of which migrate in response to trauma in the adult brain[37]. This demonstrates that the ependymal/stem cells can serve as sources for repair/replacement--at least in the case of trauma--for surrounding structures, presumably including the sexually dimorphic nucleus of the preoptic area. This process involves cellular proliferation and migration and seemingly could continue to contribute to the anatomy of the sexually dimorphic nucleus of the preoptic area, even in adult animals. However, it is not likely that the migration of cells from the 3rd ventricle ependymal tissue plays a primary role in determining the anatomic features of the sexually dimorphic nucleus of the preoptic area in adult rodents since there is a very low level of hypothalamic progenitor cell activity[34,35]. A “re-migration” hypothesis has been proposed for postnatal and juvenile male rats in which neurons drift radially from the center to the peripheral areas of the sexually dimorphic nucleus of the preoptic area. This activity is hypothesized to account for the sexual dimorphism since it does not appear to occur in the female[29].
It is not well established that migration alone serves as the major mechanism shaping the sexually dimorphic nucleus of the preoptic area and determining sexual dimorphism. The migration rate distinguishing males from females was determined on embryonic day 15[32], prior to the development of sex differences in the sexually dimorphic nucleus of the preoptic area[24,31]. The assumption of “re-migration” is also in question because the initial study[29] has not been replicated using state-of-the-art technology such as stereological assessments. The original study[29] employed cell counts/section instead of cell counts/volume (density) to develop the hypothesis of “re-migration”. On the other hand, incomplete penetration of the first or second antibody may prevent the correct determination of cell number of calbindin-D28K-positive neurons in the sexually dimorphic nucleus of the preoptic area. Unpublished data from our laboratory have demonstrated that DAPI is capable of penetrating through tissue slices as thick as 30 μm, whereas calbindin-D28K immunoreactivity is only detectable 2/3 or less of the way through that thickness, a finding that suggests non-stereological based observations may introduce bias.
Apoptosis
Apoptosis is responsible, at least in part, for shaping the anatomic features of the sexually dimorphic nucleus of the preoptic area. Sex differences in the incidence of apoptotic cells in the sexually dimorphic nucleus of the preoptic area have been observed from postnatal days 7–10[38] with female rats having more apoptotic cells than males[38,39]. Further, testosterone significantly inhibits apoptosis during postnatal days 6–10 and this testosterone effect is sexually dimorphic nucleus of the preoptic area region-specific. No sex differences in the incidence of apoptosis were observed in other (control) regions such as the lateral preoptic area[38]. There is an inverse correlation between sex differences in apoptotic cell number during development and the number of living cells in the mature animal in the sexually dimorphic nucleus[40]. A combination analysis using the proliferation marker BrdU-labeling and immunohistochemistry for single-stranded DNA (ssDNA, an apoptosis marker) indicates that the sex difference in the number of sexually dimorphic nucleus of the preoptic area neurons is at least partially caused by sex differences in postnatal apoptosis[31].
Several pathways have been proposed as key players in triggering apoptotic mechanisms. N-methyl-D-aspartate (NMDA) receptors are highly expressed in male fetuses and activation of NMDA receptors is thought to protect sexually dimorphic nucleus of the preoptic area neurons from naturally programmed neuronal death via modulating testosterone levels and/or Bcl-2 expression[41]. Several genes (Bcl-2, cytochrome oxidase subunit II, cytochrome oxidase subunit III) are regulated by NMDA receptor activation and govern neuronal growth and/or apoptosis and the important signaling pathway involving NF kappa-B activation and its target gene, Bcl-2, that inhibits neuronal apoptosis in the sexually dimorphic nucleus of the preoptic area of male rats during sexual development[42]. There are also sex differences in the levels of Bcl-2 (female < male) and Bax (female > male) in the sexually dimorphic nucleus of the preoptic area, as well as sex differences in the induction of apoptosis via caspase-3 activation (female > male)[43,44]. Postnatally, estrogen upregulates Bcl-2 expression and downregulates Bax expression in the sexually dimorphic nucleus of the preoptic area indicating that effects of estrogen on the Bcl-2 family of proteins are likely responsible for at least some of the sex differences seen in apoptosis in the sexually dimorphic nucleus of the preoptic area during this developmental period[44].
In spite of this evidence, controversy remains about the involvement of apoptosis in the development of morphological sexually dimorphic nucleus of the preoptic area sex differences. First, the sexually dimorphic nucleus of the preoptic area continues to expand into adulthood in rats (postnatal days 8–28[24]; postnatal days 8–60[40]; postnatal days 21–110[26]), whereas the sex difference in number of apoptotic cells ends at postnatal day 10[38,39], a time when the sexual dimorphism of the sexually dimorphic nucleus of the preoptic area is barely demonstrable as determined using calbindin-D28K- immunoreactivity[24,45]. Second, the time frame over which the apoptotic activity in the sexually dimorphic nucleus of the preoptic area occurs does not coincide with the time frame over which there is increased expression of the somatostatin gene in the sexually dimorphic nucleus of the preoptic area: the sex difference in somatostatin gene-expressing cells occurs over postnatal days 8–35, and both the cell count and the volume are maximal on postnatal day 15, both being higher in males than females[45], although it remains unclear what role the somatostatin gene plays in the development of the sexually dimorphic nucleus of the preoptic area. Furthermore, recent findings[26] demonstrate that the sexually dimorphic nucleus of the preoptic area continues to increase in size, most notably in male rats, between weaning and adulthood and other reports indicate that sex differences in apoptosis may contribute little to the development of the sexually dimorphic nucleus of the preoptic area[25,26,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46].
Neurogenesis
Neural stem cell activity has been identified in the hypothalamus[33,47,48,49,50], a territory which includes the sexually dimorphic nucleus of the preoptic area. Neural progenitor cells from the ependymal layer of the 3rd ventricle migrate and differentiate into neurons in the hypothalamus[34,35,51]. Our recent report indicated that stem cell activity accounts for some of the continuous postweaning development of the sexually dimorphic nucleus of the preoptic area[26]. This interpretation was based on the following findings: sexually dimorphic nucleus of the preoptic area volume increased 43% from weaning to adulthood in male Sprague-Dawley rats; the number of Ki67-positive (proliferating) cells in the sexually dimorphic nucleus of the preoptic area and the hypothalamus, respectively, was significantly higher (3.3 and 3.5 fold in the male) at weaning than in adulthood; a subset of the Ki67-positive cells in the sexually dimorphic nucleus of the preoptic area exhibited morphology indicative of dividing cells; and finally, nestin-immunoreactivity (a marker for neural stem cells)[34,52,53], delineated a tub-like structure 360 μm in length starting at the rostral end of the 3rd ventricle and extending along the longitudinal axis of the brain in both young and adult rats. We have named this tube-like structure the macroscopic 3rd ventricle stem cell niche (3VSCN)[26]. Interestingly, volume of the female sexually dimorphic nucleus of the preoptic area also enlarged from weaning to adulthood, although this difference was not statistically significant. In addition, the number of the Ki67-positive cells in the sexually dimorphic nucleus of the preoptic area appears higher (1.7 fold) at weaning than adulthood in females, but again this difference was not statistically significant. The number of Ki67-positive cells in the hypothalamus of postnatal day 21 males was significantly higher than the same age females and adult males and females[26]. Nevertheless, it is not known whether the Ki67-positive cells in the sexually dimorphic nucleus of the preoptic area are neural stem/progenitor cells that originated and migrated from the 3rd ventricle stem cell niche. Also unknown is whether those cells that may have migrated into the sexually dimorphic nucleus of the preoptic area from the 3rd ventricle stem cell niche are still capable of returning to a “silent” status or proliferating into daughter stem cells/committed progenitor cells, which then differentiate into mature cells such as neurons, particularly in response to specific stimuli such as sex hormones or estrogen-like compounds.
SEXUALLY DIMORPHIC NUCLEUS OF THE PREOPTIC AREA AND EFFECTS OF SEX HORMONES AND ELCS
Sexually dimorphic nucleus of the preoptic area size is sensitive to exogenous sex hormone or estrogen-like compounds treatment during development. For example, estrogen agonists such as diethylstilbestrol increase the sexually dimorphic nucleus of the preoptic area volume in female rats[54,55,56,57,58,59]. On the other hand, estrogen antagonists such as tamoxifen decrease the male sexually dimorphic nucleus of the preoptic area volume[60,61,62]. Lifetime dietary exposure to the estrogen-like compound genistein (5–500 ppm) or nonylphenol (25–750 ppm) increased sexually dimorphic nucleus of the preoptic area volume of adult male, but not female, rats[63]. More limited exposures to genistein or nonylphenol (gestational day 15-postnatal day 10) did not alter the sexually dimorphic nucleus of the preoptic area volume of either sex[60,61]. Very high doses of genistein given from postnatal days 1–10 increased sexually dimorphic nucleus of the preoptic area volume of females[55].
A recent study in our laboratory demonstrated that gestational treatment of the pregnant dam followed by direct treatment of the pups after birth with low doses of the putative estrogen-like compound, bisphenol A, significantly increased sexually dimorphic nucleus of the preoptic area volume of postnatal day 21 male rats, but had no effect in same-age females[18]. As expected, the reference estrogen, ethinyl estradiol (EE2), increased sexually dimorphic nucleus of the preoptic area volume of postnatal day 21 females and the higher ethinyl estradiol dose of 10.0 μg/kg per day also increased sexually dimorphic nucleus of the preoptic area volume of postnatal day 21 males. As shown in Figure 3, a bisphenol A-treated male rat or an ethinyl estradiol treated female rat appeared to display a higher density of the nucleic mass (indicating a higher cell number) in the sexually dimorphic nucleus of the preoptic area in addition to increased volume as compared with their same-sex controls (male: the upper row vs. the lower row, Figure 3A; female: the upper row vs. the lower row, Figure 3B).
Data from those postnatal day 21 rats, reared under strictly controlled environmental conditions which minimized background levels of estrogen-like compounds[18,27], were comparable to those from the original report[24] in which the calbindin-D28K labeling method was described: sexually dimorphic nucleus of the preoptic area volumes were 2.7 and 1.0 × 10−3mm3 on postnatal day 12 and 5.5 and 2.0 × 10−3mm3 on postnatal day 26 in male and female Sprague-Dawley rats, respectively. Interestingly, sexually dimorphic nucleus of the preoptic area volumes of male and female postnatal day 21 rats raised under standard laboratory conditions were slightly larger after exposure to potential estrogen-like compounds than when raised under conditions minimizing background estrogen-like compound levels (4.91 ± 0.48 × 10−3mm3 vs. 4.02 ± 2.31 × 10−3 mm3 in males and 1.71 ± 0.20 × 10−3 mm3 vs. 1.03 ± 0.29 × 10−3 mm3 in females)[26]. These data indicate that exposure to estrogen-like compounds at background levels found in the typical vivarium or diets containing phytoestrogens may affect the development of the sexually dimorphic nucleus of the preoptic area.
The mechanism(s) underlying the ability of sex hormones and/or estrogen-like compounds to increase sexually dimorphic nucleus of the preoptic area volume are not clear. If the sexually dimorphic nucleus of the preoptic area volume is determined using calbindin-D28K-immunoreactivity, it is conceivable that increases in cell number, cell body size and/or cellular structures such as dendrites and axons, and extracellular space could lead to increases in the size of the sexually dimorphic nucleus of the preoptic area. Theoretically, direct interaction of sex hormones or estrogen-like compounds with nuclear estrogen receptors could be an important step in the processes responsible for the sexually dimorphic nucleus of the preoptic area volumetric sexual dimorphism. The effect of oral bisphenol A treatment (2.5 and 25.0 μg/kg per day) to increase the volume of the sexually dimorphic nucleus of the preoptic area was male-specific[18] and an understanding of the mechanisms underlying this effect would be most enlightening. Accordingly, since age-related levels of 5-HT[64], the protein NELL2[65], aromatase[66,67], calcium binding proteins such as calbindin-D28K[68,69], and progesterone receptors[70] are sexually dimorphic, bisphenol A treatment could have affected the sexually dimorphic nucleus of the preoptic area volume via mechanism(s) mediated by one or more of those components. Both in vitro and in vivo bisphenol A treatments have been shown to increase the expression of aromatase protein in the hippocampus as well as in testicular Leydig cells[71,72], but the effect of bisphenol A treatment on aromatase levels in the sexually dimorphic nucleus of the preoptic area has not been described. There is clearly a knowledge gap concerning how exposure to sex hormones and/or estrogen-like compounds may be linked to cell number, cell body size, cellular structures, or intercellular space in the sexually dimorphic nucleus of the preoptic area. Our recent report[26] demonstrated an age-related increase in the volume of the sexually dimorphic nucleus of the preoptic area and suggested a potential role for neural stem cells in that process; thus, offering a mechanism to potentially bridge the knowledge gap between the effects of sex hormones or estrogen-like compounds and mechanisms by which they might increase sexually dimorphic nucleus of the preoptic area volume.
CONCLUSION
Our work has been described concerning methods to study the sexually dimorphic nucleus of the preoptic area and highlighting calbindin-D28K immunoreactivity as the preferable method. Here, several developmental mechanisms which might establish the sexual dimorphism of the sexually dimorphic nucleus of the preoptic area have been reviewed and updated by addressing the potential role of neural stem cell activity. Because there is increasing evidence that perinatal exposure to estrogen-like compounds may be associated with a host of health problems and also because one of those estrogen-like compounds, bisphenol A, has been shown to alter the sexually dimorphic nucleus of the preoptic area, future studies should examine connections between the sexual dimorphic structures of the brain, including the sexually dimorphic nucleus of the preoptic area, and potential health problems. Exploring the mechanisms by which sex hormones or estrogen-like compounds affect sexual dimorphic structures of the brain might warrant new therapeutic measures against those health problems.
Research background: One well-defined sexually dimorphic structure in the brain is the sexually dimorphic nucleus, a cluster of cells located in the preoptic area of the hypothalamus. As demonstrated in laboratory animal studies, the sexually dimorphic nucleus is implicated in sexual behavior and in humans, the sexually dimorphic nucleus of the preoptic area has been linked to sexual orientation.
Research frontiers: The objective of the present study is to review the latest advances in morphological definition, developmental mechanisms and the influence of environmental factors (i.e., estrogen-like compounds) on the sexually dimorphic nucleus of the preoptic area.
Clinical significance: The article discusses mechanisms by which sex hormones or estrogen-like compounds may affect sexually dimorphic structures of the brain and thus providing insight into potential targets for new therapeutic measures against estrogen-like compounds-related health problems.
Academic terminology: (1) Sexually dimorphic nucleus of the preoptic area: The well-defined sexually dimorphic structures in the brain is the sexually dimorphic nucleus, a cluster of cells located in the preoptic area of the hypothalamus. (2) Sexual dimorphism of the sexually dimorphic nucleus in the preoptic area: the term refers to the fact that that there is a sex difference in cell number and size of the sexually dimorphic nucleus of the preoptic area: the sexually dimorphic nucleus of the preoptic area in males is larger than in females.
Peer review: This is a review of the development of the sexually dimorphic nucleus of the preoptic area with an emphasis on the authors’ contribution, showing that postnatal stem cell activity may be involved in establishing this sexual dimorphism.
Acknowledgments
We wish to thank Drs. John J. Chelonis (NCTR/FDA), Amy L. Inselman (NCTR/FDA), and Vijayalakshmi Varma (NCTR/FDA) for their careful review of this manuscript.
Footnotes
Funding: This study was supported by the National Center for Toxicological Research/FDA (Protocol P00710 to He Z and Protocol P00706 to Ferguson SA). Cui L was supported by UAMS Hornick Award to her and NIH Grant R01-NS049389 and UAMS institutional funds to Greenfield LJ.
Conflicts of interest: None declared.
(Reviewed by Roselli C, Zhang N, Wang LS)
(Edited by Li CH, Song LP, Liu WJ, Zhao M)
REFERENCES
- [1].Allen LS, Hines M, Shryne JE, et al. Two sexually dimorphic cell groups in the human brain. J Neurosci. 1989;9:497–506. doi: 10.1523/JNEUROSCI.09-02-00497.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Allen LS, Gorski RA. Sex differences in the bed nucleus of the stria terminalis of the human brain. J Comp Neurol. 1990;302:697–706. doi: 10.1002/cne.903020402. [DOI] [PubMed] [Google Scholar]
- [3].Roselli CE, Larkin K, Resko JA, et al. The volume of a sexually dimorphic nucleus in the ovine medial preoptic area/anterior hypothalamus varies with sexual partner preference. Endocrinology. 2004;145:478–483. doi: 10.1210/en.2003-1098. [DOI] [PubMed] [Google Scholar]
- [4].Byne W. The medial preoptic and anterior hypothalamic regions of the rhesus monkey: cytoarchitectonic comparison with the human and evidence for sexual dimorphism. Brain Res. 1998;793:346–350. doi: 10.1016/s0006-8993(98)00275-3. [DOI] [PubMed] [Google Scholar]
- [5].Viglietti-Panzica C, Panzica GC, Fiori MG, et al. A sexually dimorphic nucleus in the quail preoptic area. Neurosci Lett. 1986;64:129–134. doi: 10.1016/0304-3940(86)90087-x. [DOI] [PubMed] [Google Scholar]
- [6].Gorski RA, Gordon JH, Shryne JE, et al. Evidence for a morphological sex difference in the medial preoptic area of the rat brain. Brain Res. 1978;148:333–346. doi: 10.1016/0006-8993(78)90723-0. [DOI] [PubMed] [Google Scholar]
- [7].Gorski RA, Harlan RE, Jacobson CD, et al. Evidence for the existence of a sexually dimorphic nucleus in the preoptic area of the rat. J Comp Neurol. 1980;193(2):529–539. doi: 10.1002/cne.901930214. [DOI] [PubMed] [Google Scholar]
- [8].Hofman MA, Swaab DF. The sexually dimorphic nucleus of the preoptic area in the human brain: a comparative morphometric study. J Anat. 1989;164:55–72. [PMC free article] [PubMed] [Google Scholar]
- [9].Garcia-Falgueras A, Swaab DF. A sex difference in the hypothalamic uncinate nucleus: relationship to gender identity. Brain. 2008;131:3132–3146. doi: 10.1093/brain/awn276. [DOI] [PubMed] [Google Scholar]
- [10].Arendash GW, Gorski RA. Effects of discrete lesions of the sexually dimorphic nucleus of the preoptic area or other medial preoptic regions on the sexual behavior of male rats. Brain Res Bull. 1983;10:147–154. doi: 10.1016/0361-9230(83)90086-2. [DOI] [PubMed] [Google Scholar]
- [11].Rayen I, Steinbusch HW, Charlier TD, et al. Developmental fluoxetine exposure and prenatal stress alter sexual differentiation of the brain and reproductive behavior in male rat offspring. Psychoneuroendocrinology. 2013 Feb 8; doi: 10.1016/j.psyneuen.2013.01.007. pii: S0306-4530(13)00012-7 doi: 10.1016/j.psyneuen.2013.01.007. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- [12].LeVay S. A difference in hypothalamic structure between heterosexual and homosexual men. Science. 1991;253:1034–1037. doi: 10.1126/science.1887219. [DOI] [PubMed] [Google Scholar]
- [13].Meredith JM, Bennett C, Scallet AC. A practical three- dimensional reconstruction method to measure the volume of the sexually-dimorphic central nucleus of the medial preoptic area (MPOC) of the rat hypothalamus. J Neurosci Methods. 2001;104:113–121. doi: 10.1016/s0165-0270(00)00331-9. [DOI] [PubMed] [Google Scholar]
- [14].Roselli CE, Stadelman H, Reeve R, et al. The ovine sexually dimorphic nucleus of the medial preoptic area is organized prenatally by testosterone. Endocrinology. 2007;148:4450–4457. doi: 10.1210/en.2007-0454. [DOI] [PubMed] [Google Scholar]
- [15].Patisaul HB, Fortino AE, Polston EK. Differential disruption of nuclear volume and neuronal phenotype in the preoptic area by neonatal exposure to genistein and bisphenol-A. Neurotoxicology. 2007;28:1–12. doi: 10.1016/j.neuro.2006.10.001. [DOI] [PubMed] [Google Scholar]
- [16].Wolfe CA, Van Doren M, Walker HJ, et al. Sex differences in the location of immunochemically defined cell populations in the mouse preoptic area/anterior hypothalamus. Brain Res Dev Brain Res. 2005;157:34–41. doi: 10.1016/j.devbrainres.2005.03.001. [DOI] [PubMed] [Google Scholar]
- [17].Yahr P. Sex difference and response to testosterone in gabaergic cells of the medial preoptic nucleus and ventral bed nuclei of the stria terminalis in gerbils. Horm Behav. 2011;59:473–476. doi: 10.1016/j.yhbeh.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].He Z, Paule MG, Sherry SA. Low oral doses of Bisphenol A increase volume of the sexually dimorphic nucleus of the preoptic area in male, but not female, rats at weaning. Neurotoxicol Teratol. 2012;34:331–337. doi: 10.1016/j.ntt.2012.03.004. [DOI] [PubMed] [Google Scholar]
- [19].Young JK. A comparison of hypothalami of rats and mice: lack of gross sexual dimorphism in the mouse. Brain Res. 1982;239:233–239. doi: 10.1016/0006-8993(82)90844-7. [DOI] [PubMed] [Google Scholar]
- [20].Orikasa C, Sakuma Y. Estrogen configures sexual dimorphism in the preoptic area of C57BL/6J and ddN strains of mice. J Comp Neurol. 2010;518:3618–3629. doi: 10.1002/cne.22419. [DOI] [PubMed] [Google Scholar]
- [21].Edelmann M, Wolfe C, Scordalakes EM, et al. Neuronal nitric oxide synthase and calbindin delineate sex differences in the developing hypothalamus and preoptic area. Dev Neurobiol. 2007;67:1371–1381. doi: 10.1002/dneu.20507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Büdefeld T, Grgurevic N, Tobet SA, et al. Sex differences in brain developing in the presence or absence of gonads. Dev Neurobiol. 2008;68:981–995. doi: 10.1002/dneu.20638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Bodo C, Rissman EF. The androgen receptor is selectively involved in organization of sexually dimorphic social behaviors in mice. Endocrinology. 2008;149:4142–4150. doi: 10.1210/en.2008-0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Sickel MJ, McCarthy MM. Calbindin-D28k immunoreactivity is a marker for a subdivision of the sexually dimorphic nucleus of the preoptic area of the rat: developmental profile and gonadal steroid modulation. J Neuroendocrinol. 2000;12:397–402. doi: 10.1046/j.1365-2826.2000.00474.x. [DOI] [PubMed] [Google Scholar]
- [25].Gilmore RF, Varnum MM, Forger NG. Effects of blocking developmental cell death on sexually dimorphic calbindin cell groups in the preoptic area and bed nucleus of the stria terminalis. Biol Sex Differ. 2012;3:5. doi: 10.1186/2042-6410-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].He Z, Ferguson SA, Cui L, et al. Stem cell activity may partially account for postweaning development of the sexually dimorphic nucleus of the preoptic area in rats. PLoS One. 2013. Jan 30, http://dx.plos.org/101371/journal.pone.0054927 . [DOI] [PMC free article] [PubMed]
- [27].Ferguson SA, Law CD, Jr, Abshire JS. Developmental treatment with bisphenol A or ethinyl estradiol causes few alterations on early preweaning measures. Toxicol Sci. 2011;124:149–160. doi: 10.1093/toxsci/kfr201. [DOI] [PubMed] [Google Scholar]
- [28].Jacobson CD, Davis FC, Gorski RA. Formation of the sexually dimorphic nucleus of the preoptic area: neuronal growth, migration and changes in cell number. Brain Res. 1985;353:7–18. doi: 10.1016/0165-3806(85)90019-7. [DOI] [PubMed] [Google Scholar]
- [29].Orikasa C, Kondo Y, Usui S, et al. Similar numbers of neurons are generated in the male and female rat preoptic area in utero. Neurosci Res. 2010;68:9–14. doi: 10.1016/j.neures.2010.05.008. [DOI] [PubMed] [Google Scholar]
- [30].Gorski RA. Structural sex differences in the brain: their origin and significance. In: Laroski JM, Perez-Polo JR, Rassin DK, editors. Neural Control of Reproductive Function Neurology and Neurobiology. Vol. 50. New York: Alan R Liss Inc; 1989. [Google Scholar]
- [31].Kato Y, Nakashima S, Maekawa F, et al. Involvement of postnatal apoptosis on sex difference in number of cells generated during late fetal period in the sexually dimorphic nucleus of the preoptic area in rats. Neurosci Lett. 2012;516:290–295. doi: 10.1016/j.neulet.2012.04.017. [DOI] [PubMed] [Google Scholar]
- [32].Henderson RG, Brown AE, Tobet SA. Sex differences in cell migration in the preoptic area/anterior hypothalamus of mice. J Neurobiol. 1999;41:252–266. doi: 10.1002/(sici)1097-4695(19991105)41:2<252::aid-neu8>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- [33].Dahiya S, Lee da Y, Gutmann DH. Comparative characterization of the human and mouse third ventricle germinal zones. J Neuropathol Exp Neurol. 2011;70:622–633. doi: 10.1097/NEN.0b013e31822200aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Ernst C, Christie BR. Nestin-expressing cells and their relationship to mitotically active cells in the subventricular zones of the adult rat. Eur J Neurosci. 2005;22:3059–3066. doi: 10.1111/j.1460-9568.2005.04499.x. [DOI] [PubMed] [Google Scholar]
- [35].Xu Y, Tamamaki N, Noda T, et al. Neurogenesis in the ependymal layer of the adult rat 3rd ventricle. Exp Neurol. 2005;192:251–264. doi: 10.1016/j.expneurol.2004.12.021. [DOI] [PubMed] [Google Scholar]
- [36].Alonso G. Neuronal progenitor-like cells expressing polysialylated neural cell adhesion molecule are present on the ventricular surface of the adult rat brain and spinal cord. J Comp Neurol. 1999;414:149–166. doi: 10.1002/(sici)1096-9861(19991115)414:2<149::aid-cne2>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- [37].Scott DE. Post-traumatic migration and emergence of a novel cell line upon the ependymal surface of the third cerebral ventricle in the adult mammalian brain. Anat Rec. 1999;256:233–241. doi: 10.1002/(SICI)1097-0185(19991101)256:3<233::AID-AR3>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- [38].Davis EC, Popper P, Gorski RA. The role of apoptosis in sexual differentiation of the rat sexually dimorphic nucleus of the preoptic area. Brain Res. 1996;734:10–18. [PubMed] [Google Scholar]
- [39].Chung WC, Swaab DF, De Vries GJ. Apoptosis during sexual differentiation of the bed nucleus of the stria terminalis in the rat brain. J Neurobiol. 2000;43:234–243. [PubMed] [Google Scholar]
- [40].Tsukahara S. Sex differences and the roles of sex steroids in apoptosis of sexually dimorphic nuclei of the preoptic area in postnatal rats. J Neuroendocrinol. 2009;21:370–376. doi: 10.1111/j.1365-2826.2009.01855.x. [DOI] [PubMed] [Google Scholar]
- [41].Hsu C, Hsieh YL, Yang RC, et al. Blockage of N-methyl- D-aspartate receptors decreases testosterone levels and enhances postnatal neuronal apoptosis in the preoptic area of male rats. Neuroendocrinology. 2000;71:301–307. doi: 10.1159/000054550. [DOI] [PubMed] [Google Scholar]
- [42].Hsu HK, Shao PL, Tsai KL, et al. Gene regulation by NMDA receptor activation in the SDN-POA neurons of male rats during sexual development. J Mol Endocrinol. 2005;34:433–445. doi: 10.1677/jme.1.01601. [DOI] [PubMed] [Google Scholar]
- [43].Tsukahara S, Kakeyama M, Toyofuku Y. Sex differences in the level of Bcl-2 family proteins and caspase-3 activation in the sexually dimorphic nuclei of the preoptic area in postnatal rats. J Neurobiol. 2006;66:1411–1419. doi: 10.1002/neu.20276. [DOI] [PubMed] [Google Scholar]
- [44].Tsukahara S, Hojo R, Kuroda Y, et al. Estrogen modulates Bcl-2 family protein expression in the sexually dimorphic nucleus of the preoptic area of postnatal rats. Neurosci Lett. 2008;432:58–63. doi: 10.1016/j.neulet.2007.12.006. [DOI] [PubMed] [Google Scholar]
- [45].Orikasa C, Kondo Y, Sakuma Y. Transient transcription of the somatostatin gene at the time of estrogen-dependent organization of the sexually dimorphic nucleus of the rat preoptic area. Endocrinology. 2007;148:1144–1149. doi: 10.1210/en.2006-1214. [DOI] [PubMed] [Google Scholar]
- [46].Sakuma Y. Gonadal steroid action and brain sex differentiation in the rat. J Neuroendocrinol. 2009;21:410–414. doi: 10.1111/j.1365-2826.2009.01856.x. [DOI] [PubMed] [Google Scholar]
- [47].Ahmed EI, Zehr JL, Schulz KM, et al. Pubertal hormones modulate the addition of new cells to sexually dimorphic brain regions. Nat Neurosci. 2008;11:995–997. doi: 10.1038/nn.2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Kokoeva MV, Yin H, Flier JS. Neurogenesis in the hypothalamus of adult mice: potential role in energy balance. Science. 2005;310:679–683. doi: 10.1126/science.1115360. [DOI] [PubMed] [Google Scholar]
- [49].Kokoeva MV, Yin H, Flier JS. Evidence for constitutive neural cell proliferation in the adult murine hypothalamus. J Comp Neurol. 2007;505:209–220. doi: 10.1002/cne.21492. [DOI] [PubMed] [Google Scholar]
- [50].Pérez-Martín M, Cifuentes M, Grondona JM, et al. IGF-I stimulates neurogenesis in the hypothalamus of adult rats. Eur J Neurosci. 2010;31:1533–1548. doi: 10.1111/j.1460-9568.2010.07220.x. [DOI] [PubMed] [Google Scholar]
- [51].Park JJ, Baum MJ, Paredes RG, et al. Neurogenesis and cell migration into the sexually dimorphic preoptic area/ anterior hypothalamus of the fetal ferret. J Neurobiol. 1996;30:315–328. doi: 10.1002/(SICI)1097-4695(199607)30:3<315::AID-NEU1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- [52].He Z, Cui L, Wu SS, et al. Increased severity of acute cerebral ischemic injury correlates with enhanced stem cell induction as well as with predictive behavioral profiling. Curr Neurovasc Res. 2004;1:399–409. doi: 10.2174/1567202043361893. [DOI] [PubMed] [Google Scholar]
- [53].He Z, Cui L, Meschia JF, et al. Hippocampal progenitor cells express nestin following cerebral ischemia in rats. Neuroreport. 2005;16:1541–1544. doi: 10.1097/01.wnr.0000179074.32035.46. [DOI] [PubMed] [Google Scholar]
- [54].Faber KA, Ayyash L, Dixon S, et al. Effect of neonatal diethylstilbestrol exposure on volume of the sexually dimorphic nucleus of the preoptic area of the hypothalamus and pituitary responsiveness to gonadotropin-releasing hormone in female rats of known anogenital distance at birth. Biol Reprod. 1993;48:947–951. doi: 10.1095/biolreprod48.5.947. [DOI] [PubMed] [Google Scholar]
- [55].Faber KA, Hughes CL., Jr Dose-response characteristics of neonatal exposure to genistein on pituitary responsiveness to gonadotropin releasing hormone and volume of the sexually dimorphic nucleus of the preoptic area (SDN-POA) in postpubertal castrated female rats. Reprod Toxicol. 1993;7:35–39. doi: 10.1016/0890-6238(93)90007-t. [DOI] [PubMed] [Google Scholar]
- [56].Kwon S, Stedman DB, Elswick BA, et al. Pubertal development and reproductive functions of Crl:CD BR Sprague-Dawley rats exposed to bisphenol A during prenatal and postnatal development. Toxicol Sci. 2000;55:399–406. doi: 10.1093/toxsci/55.2.399. [DOI] [PubMed] [Google Scholar]
- [57].Levy JR, Faber KA, Ayyash L, et al. The effect of prenatal exposure to the phytoestrogen genistein on sexual differentiation in rats. Proc Soc Exp Biol Med. 1995;208:60–66. doi: 10.3181/00379727-208-43832. [DOI] [PubMed] [Google Scholar]
- [58].Lewis RW, Brooks N, Milburn GM, et al. The effects of the phytoestrogen genistein on the postnatal development of the rat. Toxicol Sci. 2003;71:74–83. doi: 10.1093/toxsci/71.1.74. [DOI] [PubMed] [Google Scholar]
- [59].Yamamoto M, Shirai M, Tamura A, et al. Effects of maternal exposure to a low dose of diethylstilbestrol on sexual dimorphic nucleus volume and male reproductive system in rat offspring. J Toxicol Sci. 2005;30:7–18. doi: 10.2131/jts.30.7. [DOI] [PubMed] [Google Scholar]
- [60].Dohler KD, Srivastava SS, Shryne JE, et al. Differentiation of the sexually dimorphic nucleus in the preoptic area of the rat brain is inhibited by postnatal treatment with an estrogen antagonist. Neuroendocrinology. 1984;38:297–301. doi: 10.1159/000123907. [DOI] [PubMed] [Google Scholar]
- [61].Dohler KD, Coquelin A, Davis F, et al. Pre- and postnatal influence of an estrogen antagonist and an androgen antagonist on differentiation of the sexually dimorphic nucleus of the preoptic area in male and female rats. Neuroendocrinology. 1986;42:443–448. doi: 10.1159/000124484. [DOI] [PubMed] [Google Scholar]
- [62].Vancutsem PM, Roessler ML. Neonatal treatment with tamoxifen causes immediate alterations of the sexually dimorphic nucleus of the preoptic area and medial preoptic area in male rats. Teratology. 1997;56:220–228. doi: 10.1002/(SICI)1096-9926(199709)56:3<220::AID-TERA5>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- [63].Scallet AC, Divine RL, Newbold RR, et al. Increased volume of the calbindin D28k-labeled sexually dimorphic hypothalamus in genistein and nonylphenol-treated male rats. Toxicol Sci. 2004;82:570–576. doi: 10.1093/toxsci/kfh297. [DOI] [PubMed] [Google Scholar]
- [64].Murray JF, Dakin CL, Siddiqui A, et al. Neonatal 5HT activity antagonizes the masculinizing effect of testosterone on the luteinizing hormone release response to gonadal steroids and on brain structures in rats. Eur J Neurosci. 2004;19:387–395. doi: 10.1111/j.0953-816x.2003.03158.x. [DOI] [PubMed] [Google Scholar]
- [65].Jeong JK, Ryu BJ, Choi J, et al. NELL2 participates in formation of the sexually dimorphic nucleus of the pre-optic area in rats. J Neurochem. 2008;106:1604–1613. doi: 10.1111/j.1471-4159.2008.05505.x. [DOI] [PubMed] [Google Scholar]
- [66].Lauber ME, Sarasin A, Lichtensteiger W. Transient sex differences of aromatase (CYP19) mRNA expression in the developing rat brain. Neuroendocrinology. 1997;66:173–180. doi: 10.1159/000127235. [DOI] [PubMed] [Google Scholar]
- [67].Lauber ME, Sarasin A, Lichtensteiger W. Sex differences and androgen-dependent regulation of aromatase (CYP19) mRNA expression in the developing and adult rat brain. J Steroid Biochem Mol Biol. 1997;61:359–364. [PubMed] [Google Scholar]
- [68].Brager DH, Sickel MJ, McCarthy MM. Developmental sex differences in calbindin-D(28K) and calretinin immunoreactivity in the neonatal rat hypothalamus. J Neurobiol. 2000;42:315–322. [PubMed] [Google Scholar]
- [69].Lephart ED. Dimorphic expression of calbindin-D28K in the medial basal hypothalamus from perinatal male and female rats. Brain Res Dev Brain Res. 1996;96:281–284. doi: 10.1016/0165-3806(96)00100-9. [DOI] [PubMed] [Google Scholar]
- [70].Quadros PS, Lopez V, De Vries GJ, et al. Progesterone receptors and the sexual differentiation of the medial preoptic nucleus. J Neurobiol. 2002;51:24–32. doi: 10.1002/neu.10040. [DOI] [PubMed] [Google Scholar]
- [71].Kim JY, Han EH, Kim HG, et al. Bisphenol A-induced aromatase activation is mediated by cyclooxygenase-2 up-regulation in rat testicular Leydig cells. Toxicol Lett. 2010;193:200–208. doi: 10.1016/j.toxlet.2010.01.011. [DOI] [PubMed] [Google Scholar]
- [72].Xu XH, Wang YM, Zhang J, et al. Perinatal exposure to bisphenol-A changes N-methyl-D-aspartate receptor expression in the hippocampus of male rat offspring. Environ Toxicol Chem. 2010;29:176–181. doi: 10.1002/etc.18. [DOI] [PubMed] [Google Scholar]


