Abstract
Thirty pathologically diagnosed patients with grade III–IV primary or recurrent malignant glioma (tumor diameter 3–7 cm) were randomly divided into two groups. The control group underwent conventional radiotherapy and chemotherapy. In the hyperthermia group, primary cases received hyperthermia treatment, and patients with recurrent tumors were treated with hyperthermia in com-bination with radiotherapy and chemotherapy. Hyperthermia treatment was administered using a 13.56-MHz radio frequency hyperthermia device. Electrodes were inserted into the tumor with the aid of a CT-guided stereotactic apparatus and heat was applied for 1 hour. During 3 months after hyperthermia, patients were evaluated with head CT or MRI every month. Gliomas in the hyper-thermia group exhibited growth retardation or growth termination. Necrosis was evident in 80% of the heated tumor tissue and there was a decrease in tumor diameter. Our findings indicate that ra-dio frequency hyperthermia has a beneficial effect in the treatment of malignant glioma.
Keywords: neural regeneration, glioma, radio frequency hyperthermia, necrosis, malignant tumor, recurrence, CT, MRI, intracranial hypertension, clinical effects, grants-supported paper, neuroregeneration
Research Highlights
(1) With the aid of a CT-guided stereotactic apparatus, electrode needles were inserted into the center of lioma tumors, and the heat exposure of peripheral tissue was maintained at temperatures of ≤43°C.
(2) The blood-brain barrier within and surrounding the tumor was completely destroyed after hyper-thermia treatment, and chemotherapy drugs could then reach and target the tumor.
(3) It was found that hyperthermia and radiotherapy could contribute synergistically to the treatment of brain glioma.
INTRODUCTION
Malignant brain tumors, such as glioblastoma and anaplastic astrocytoma, are regarded as challenges for neurosurgeons[1]. Glioma accounts for 25–33% of all brain tumors. Malignant glioma usually relapses very quickly after surgery[2]. Patients with grade IV glioma generally relapse within 3 months after surgery and tumor size can return to pre-surgery size by 6 months after surgery[3]. Without treatment, malignant glioma patients only survive for an average of 17 weeks, and life span can be prolonged to 30 weeks after surgery and chemotherapy. Patients with recurrent malignant glioma repeatedly receive craniotomy at short time intervals, which is not only a severe physical burden but also a heavy economic cost for both family and society[4]. Therefore, clinicians urgently need a new treatment that has a low impact on patients and can be repeated multiple times at low cost.
Radio frequency ablation treatment is a sub-stantive tumor therapy. It is currently used to treat cancers of the liver, lung, and head and neck, and is now widely applied by clinicians. The treatment of malignant brain tumors is a challenge for neurosurgeons, especially recurrent malignant glioma that can grow rapidly after surgery. Surgical, radiotherapy and chemotherapy treatments for malignant brain tumors are unsatisfactory; thus it is an urgent clinical requirement to find a new therapeutic approach. Malignant glioma is very sensitive to heat. Therefore, malignant brain tumor heat treatment could potentially be used as a new modality. Perovskite-based tumor heat treatment has achieved good therapeutic efficacy in malignant glioma patients. Radiotherapy and chemotherapy have proved to be suboptimal modalities in the treatment of malignant brain tumors. Hyperthermia treatment has received increasing attention from clinicians as a new alternative treatment[5,6,7]. In this study, we used a brain tumor hyperthermia apparatus to treat malignant glioma patients, and subsequently performed medical imaging examinations to observe efficacy. The results of treatment are as follows.
RESULTS
Quantitative analysis of participants
Thirty malignant glioma patients (primary and recurrent) were included in the study and were randomly divided into control and hyperthermia groups, with 15 patients in each group. Patients in the hyperthermia group (patients with grade III–IV primary glioma did not receive concurrent chemotherapy and radiotherapy, while recurrent glioma patients underwent radiotherapy and chemotherapy) received hyperthermia. Patients in the control group received radiotherapy and chemotherapy but not hyperthermia. All 30 patients were involved in the final analysis.
Patient baseline data
The baseline information for the 30 patients enrolled in the study is detailed in Table 1. There were no significant differences regarding gender, age, tumor type and tumor diameter, between the control and hyperthermia groups (P > 0.05; Table 2).
Table 1.
Baseline information for malignant glioma patients
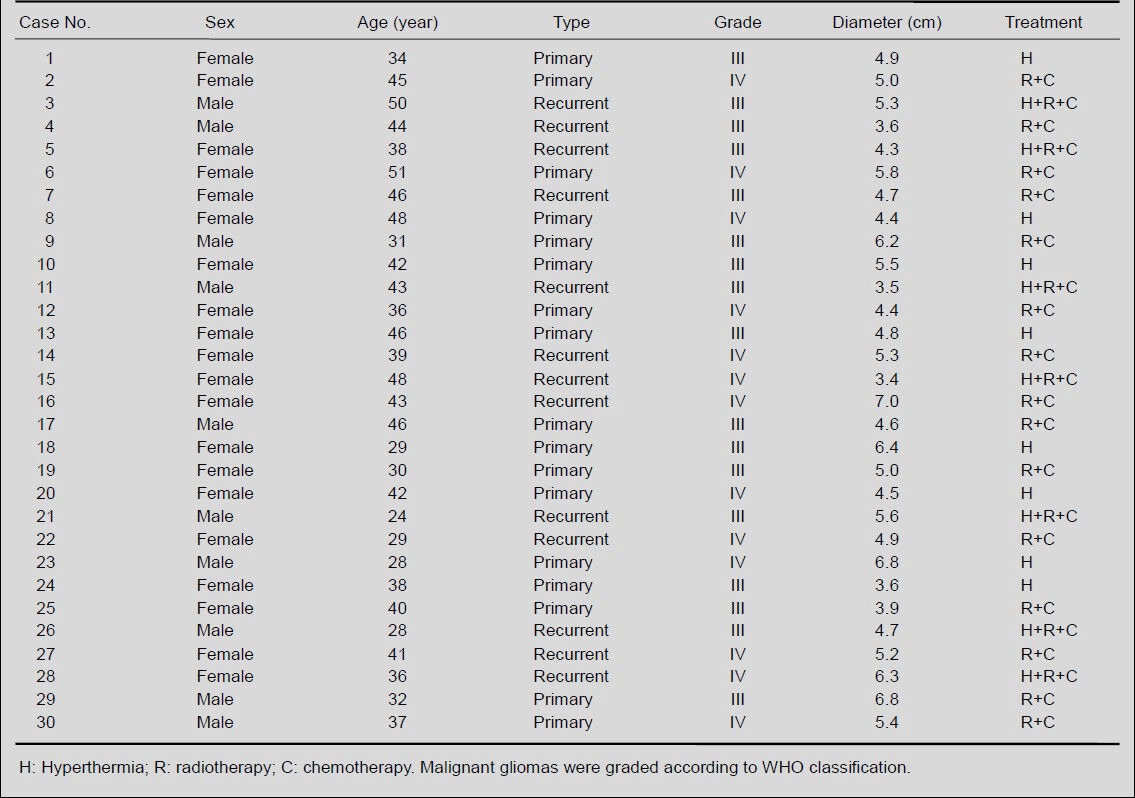
Table 2.
Comparison of baseline information for malignant glioma patients between the control and hyperthermia groups
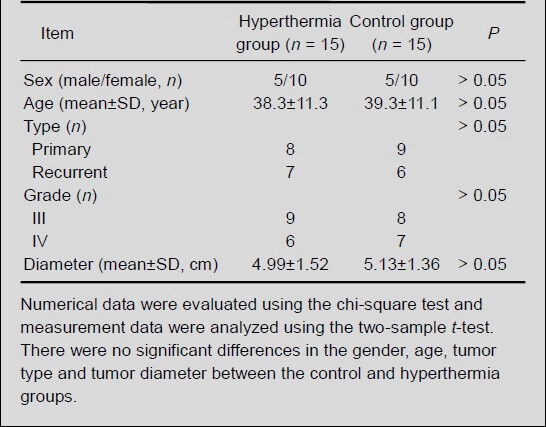
Effects of hyperthermia
Patients in the two groups underwent head CT or MRI examination at 3 months after treatment to monitor changes in tumor size. In patients in the control group, tumor growth was very rapid, there was no significant necrosis in the center of the tumor, and symptoms of intracranial hypertension were apparent. In patients in the hyperthermia group, tumor growth slowed or stopped and the tumor had low density. The heated centers of 80% of the tumors presented with necrosis, intracranial hypertension symptoms were improved and life expectancy was prolonged. Thus, the results indicated that hyperthermia was effective in the treatment of malignant glioma.
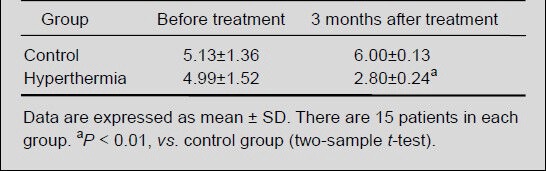
Patients in the two groups also underwent head CT or MRI examination before and after hyperthermia treatment to measure tumor diameter. There was no significant difference in tumor diameter between the control and hyperthermia groups before treatment; at 3 months after treatment, tumor diameter in the hyperthermia group was significantly smaller than that in the control group (P < 0.01; Table 3).
Table 3.
Effects of hyperthermia on the tumor diameter (cm) in malignant glioma patients
Analysis of representative cases
Case 1 was a 24-year-old man (No. 21 in Table 1). He received hyperthermia treatment on December 26, 2007, and remained in good health during a long-term follow-up period. He died suddenly of grand mal epilepsy at 20 months after treatment. Figure 1 shows the changes in the CT and MRI images before and after treatment.
Figure 1.
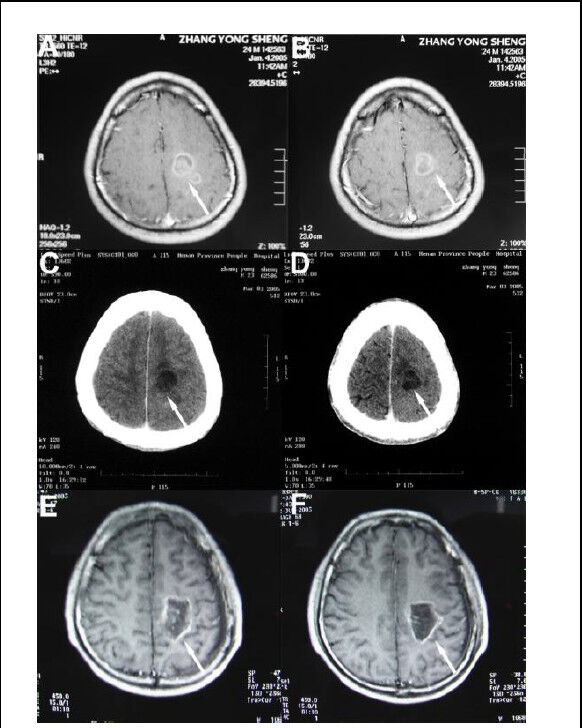
A 24-year-old man with grade III recurrent glioma.
(A, B) Brain tumor showing an irregular enhancing signal shadow (arrows) on CT before treatment.
(C, D) CT scan showing internal liquefaction and tumor necrosis (arrows) in low-density images at 7 days after hyperthermia (hyperthermia + radiotherapy + chemotherapy).
(E, F) MRI scan showing increased tumor necrosis (arrows) and surrounding tissue with no obvious enhancement at 12 months after hyperthermia.
Case 2 was a 28-year-old man (No. 26 in Table 1) who experienced the recurrence of astrocytoma after surgical removal and radiotherapy in 2002. He experienced headache and nausea in 2009, and underwent hyperthermia treatment in the same year. The patient remained healthy during an 18-month follow-up period. Figure 2 shows changes in the CT and MRI images before and after treatment.
Figure 2.
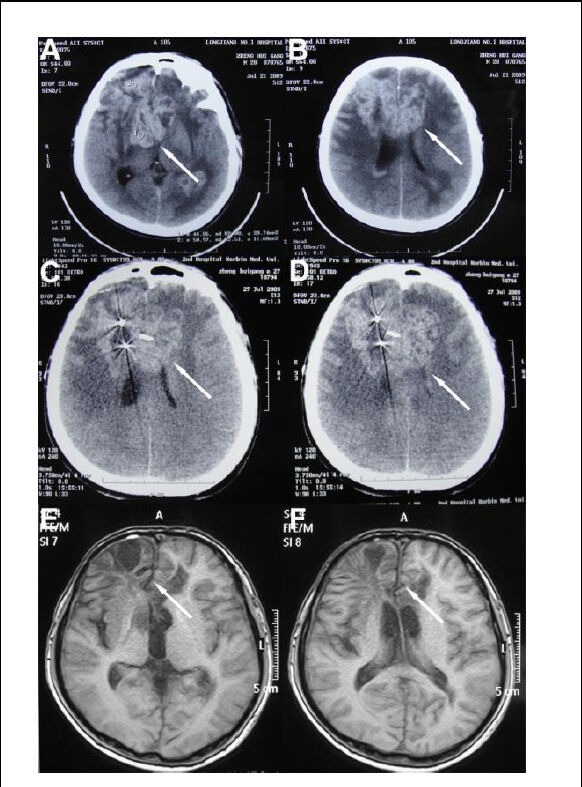
A 28-year-old man with grade III recurrent astrocytoma.
(A, B) A tumor located in the contralateral frontal with an irregular mass shadow and unclear boundaries (arrows) on CT before treatment.
(C, D) A CT scan at 3 months after hyperthermia (hyperthermia + radiotherapy + chemotherapy), showing that stereotactic radiosurgery had targeted the tumor (arrows).
(E, F) An MRI scan at 18 months after hyperthermia showing that the tumor signals and pressure on the brain ventricles had disappeared (arrows).
Side effects
Adverse reactions including early induced hemorrhage and infection may aggravate cerebral edema a short time after treatment. There were no significant differences between the two groups in terms of side effects.
DISCUSSION
The survival rate of patients with malignant glioma remains disappointing. In two decades, conventional methods of treatment for this disease have failed to control tumor progression[8,9]. In basic and clinical research studies a new treatment modality, hyperthermia, has shown remarkable effectiveness in killing neoplastic cells. Primary glioma patients account for about one third of all brain tumors, and there are also many recurrent patients. Selecting patients for clinical trial is easy.
The effects of hyperthermia on malignant glioma can be determined in a few days or weeks after treatment. Recently, great clinical progress has been made in the treatment of liver and lung cancer using hyperthermia, but evidence regarding its efficacy in the treatment of malignant glioma is lacking[10,11,12,13]. Hyperthermia and radio frequency ablation are two different treatments. Hyperthermia treatment mainly involves application with radiotherapy and chemotherapy as an auxiliary modality. Radio frequency ablation treatment can be used alone and is targeted at certain substantive tumors, such as those occurring in the liver and lung; it is estimated that tens of thousands of liver cancer patients receive radio frequency therapy every year. However, radio frequency ablation therapy for brain tumors has seldom been reported.
The superiority of hyperthermia
(1) Both animal and cellular studies have proved that tumor cells are more sensitive to heat than normal cells. The center of the tumor is a hypoxic low nutritional environment. Because of active glycolysis in tumor cells, the center of the lesion has an acidic pH and is easily influenced by temperature. As compared with normal vascular structures, tumor blood vessels have a rough structure and no nerves, which make them more sensitive to temperature.
(2) Hyperthermia is a safe treatment involving a low power, slow heating process and minimal damage to surrounding normal tissue. It also results in little discomfort to the patient. The treatment efficacy of hyperthermia is superior to that of laser, freezing and tumor immunotherapy[14].
(3) The center of tumors can be heated to a temperature ≥ 43°C. However, there are normal neurons located around the periphery of the tumor, so the temperature in this region should be maintained at ≤ 43°C to avoid side effects[15].
(4) The blood-brain barrier around the tumor can be destroyed by heat, thus facilitating tumor targeting by chemotherapy reagents. Hyperthermia can increase treatment efficacy, and reduce the concentration and side effects of chemotherapy reagents[16].
(5) Heat can prevent DNA and RNA synthesis in tumor cells and cause protein denaturation, thereby preventing tumor cell division[17].
(6) Heat can block tumor cell respiration, decrease oxygen consumption and reduce the pH in the extracellular environment, thus inhibiting cell proliferation[18].
(7) Heat can enhance the activity of cellular lysosomes and promote lysozyme function which damages mitochondria, the Golgi apparatus and other organelles, leading to tumor cell destruction[19].
Hyperthermia treatment for malignant brain tumors utilizes a CT scan assisted stereotactic apparatus to insert the electrode needle into the center of tumors. Two electrode needles can be placed together. In the present study, thermometry catheters, which are fine soft tubes, were inserted around the tumor periphery. The positions of the electrode needles were adjusted using a pre-operation simulated temperature distribution model. Temperature was monitored constantly with the temperature around tumor periphery kept around 42–43°C. Stereotactic surgery is simple, convenient and minimally invasive, with short surgery time and can be performed under local anesthesia. Hyperthermia was carried out in the ward. Patients experience no heat, pain or headaches. The treatment rarely has complications and can be completed within 1 hour. One advantage of hyperthermia is its minimal invasiveness; it can be carried out with no complications on inoperable patients with systemic failure, on elderly patients with poor cardiopulmonary function, and on patients with tumors in functional areas of the cerebral cortex in which surgery can lead to disability. In the current study, the survival rate of glioma patients was not the main outcome measure. We aimed to observe changes in tumor size before and after treatment. After treatment, the glioma signal shadow increased, but the tumor became necrotic and showed low signal intensity; this indicated that radio frequency heat treatment was effective.
Cases suitable for hyperthermia
Cases suitable for hyperthermia treatment include the following. (1) Malignant astrocytoma (astroblastoma), glioblastoma, other malignant brain tumors and brain metastases. (2) Recurrent malignant brain tumors. (3) Elderly patients with poor cardiopulmonary function who are unsuitable for multiple surgeries[20]. (4) Patients that have already received radiotherapy and chemotherapy[21]. (5) Patients with tumor located in deep brain areas who are unsuitable for surgery[22]. (6) Tumors located in brain functional areas. (7) Fast-recurrent tumors that are unsuitable for surgery, and tumors with a diameter ≥ 3 cm that are unsuitable for γ-knife treatment. (8) Patients with poor economic circumstances.
Cases unsuitable for hyperthermia
Benign brain tumor is not sensitive to heat; thus hyperthermia usually has poor treatment efficacy.
Characteristics of the brain tumor hyperthermia apparatus
The hyperthermia apparatus is a new advanced instrument with a frequency of 13.56 MHz, which has been designed specifically for the treatment of malignant brain tumors. The device is simple to operate and has both an artificial intelligent control and a manual control. It also has a security control circuit and an electromagnetic shield, which makes the instrument more stable[23]. Using an amplified sine wave to heat local brain tissue, the hyperthermia apparatus can destroy diseased tissue or change the architecture of the local tissue to accomplish treatment[24].
Malignant brain tumors, such as Glioblastoma, Anaplatic astrocytoma, are challenges for neurosurgeons in the 21st century. Glioma accounts for 1/3–1/4 of all brain tumors. Malignant glioma usually relapses very quickly after surgery[25]. Patients with grade IV gliomas generally relapse within 3 months after surgery and tumor size can return to pre-surgery size 6 months after surgery. Recurrent patients with malignant glioma repeatedly receive craniotomy in short intervals, which can only be physically unbearable for patients, but also brings heavy economic burden to both family and society.
Combination of hyperthermia with other methods
It is difficult for chemotherapy drugs to cross the blood-brain barrier and effectively target brain tumors; hence, brain tumor radio frequency ablation treatment is carried out. Changing from water-soluble drugs to lipophilic drugs has not satisfactory improved tumor targeting. Using CT-assisted stereotactic surgery, electrode needles that generate hyperthermia are inserted into the center of the tumor, and the temperature around the periphery of tumor is controlled in the range 42–43°C[26]. Heat can completely disrupt the blood-brain barrier in the tumor, thus enhancing targeting by chemotherapy drugs[27]. High concentrations of drugs help to boost the treatment efficacy of chemotherapy. In addition, hyperthermia and radiation treatment have synergistic effects[28]. For example, radiation cannot kill quiescent tumor cells (G0 phase), while hyperthermia can kill tumor cells in all phases of the cell cycle. It has been confirmed in a large number of clinical studies that hyperthermia can be used together with radiation therapy and chemotherapy to treat cancer[29,30].
Prospects for application
Treatment of glioblastoma is a challenge for a neurosurgeon. In particular, malignant glioma, which accounts for 25–33% of brain tumors, usually relapses very quickly after surgery. For example, a grade IV glioma tumor which relapses by 3 months after surgery returns to its pre-operation size by 6 months after surgery. Recurrent patients will receive repeated craniotomy in short intervals, which places unbearable stress on the body. It also results in a heavy economic burden for the family and society. Therefore, clinicians urgently need a new treatment that has a low impact on patients and can be repeated multiple times at low cost. Hyperthermia is considered by clinicians as a promising new method to treat malignant brain tumors because radiotherapy, chemotherapy and other treatments give unsatisfactory results. With recent advances in the development of medical equipment, malignant glioma hyperthermia treatment has made remarkable progress in clinical practice, whereas immunotherapy and gene therapy remain unsatisfactory.
The Brain Research Institute of Neurosurgery and the Faculty of Engineering at Niigata University co-developed the brain tumor hyperthermia instrument. A considerable amount of basic and clinical research regarding brain glioma hyperthermia treatment has been carried out at the Brain Research Institute of Neurosurgery at Niigata University. After hyperthermia, tumors disappeared completely. The treatment delivered very satisfactory results with significantly prolonged patient survival with minimal invasiveness and less side effects. All of the studies to date have demonstrated that hyperthermia is minimally invasive, has minimal side effects, and can achieve satisfactory therapeutic results[31,32].
We firmly believe that as the hyperthermia instrument is improved and experience using the treatment grows, there will be a positive new chapter in the treatment of malignant glioma.
SUBJECTS AND METHODS
Design
A clinical case-controlled study.
Time and setting
The study was performed between 2005 and 2008 in the Second Hospital of Harbin Medical University in China.
Subjects
Thirty patients were recruited from the Second Hospital of Harbin Medical University in China between 2005 and 2008, who had grade III–IV primary or recurrent malignant gliomas (tumor diameter 3–7 cm) according to the WHO classification[1].
Inclusion criteria: Primary malignant glioma patients (tumor diameter 3–7 cm) received no surgery, radiotherapy or chemotherapy, and their health condition deteriorated rapidly. (2) Recurrent patients (tumor diameter 3–7 cm) had tumor recurrence and rapid disease progression after surgery alone, after surgery and radiotherapy, or after surgery, radiotherapy and chemotherapy. (3) Each patient underwent CT or MRI examination once a month to monitor tumor changes.
Thirty malignant glioma patients were enrolled after being given a full explanation of the possible side effects and benefits of hyperthermia. They were selected over the same time period and randomly divided into control and hyperthermia groups. In accordance with the ethical requirements of the Declaration of Helsinki, patients gave informed consent prior to enrollment in the study.
Methods
Hyperthermia treatment
The brain tumor hyperthermia apparatus was provided by Toshiba Corporation (Tokyo, Japan). It consisted of a 13.56 MHz radio frequency generator and needle-shaped electrodes (radio frequency antennas). The radio frequency antennas were made from medical stainless steel, and were 12 cm in length and 1.0 mm in diameter. The electrode needle was inserted into the center of the tumor by means of stereotactic surgery. Our hyperthermia treatment utilized a CT scan assisted stereotactic apparatus to insert the electrode needle into the center of tumors. The two electrode needles could be placed together. The thermometry catheters were inserted around the tumor periphery. The positions of the electrode needles were adjusted using the pre-operation simulated temperature distribution model. Temperature was monitored constantly with the tumor periphery temperature maintained at 42–43°C for approximately 60 minutes. All of the procedures were carried out by the same physician.
Radiotherapy and chemotherapy
Seven recurrent patients in the hyperthermia group underwent radiotherapy and chemotherapy at 2 days after hyperthermia treatment. The control group received a conventional radiotherapy and chemotherapy regimen. In brief, CT was applied to simulate the position of the tumor prior to radiotherapy and the target area was plotted based on the preoperative and postoperative brain enhanced MRI. Gross tumor volume refers to the postoperative residual tumor, and clinical target volume refers to preoperative brain enhancement. External radiotherapy was delivered with a margin of up to 1 cm from the edema area on MRI T2 phase images. The planning target volume was 1–2 cm from the clinical target volume. Using an electron linear accelerator, the planning target volume was irradiated with 6 MV X-rays at total doses of 44–46 Gy; the clinical target volume received 50–54 Gy and the gross tumor volume 60 Gy. Radiation was delivered in 2 Gy fractions once per day, 5 days per week. When external radiation began, patients were orally administered with Temozolomide (Schering-Plough Brands, Merck & Co., Whitehouse Station, NJ, USA) at a dose of 75 mg/m2 for 42 days; 2 weeks after the completion of radiotherapy, patients underwent sequential chemotherapy and oral administration of Temozolomide 150–200 mg/m2, once per day over a 1–5 day period. A single treatment period was 28 days and treatment was administered over a total of two periods.
Clinical evaluation
To verify the effectiveness of hyperthermia in treating malignant glioma, patients received either head CT or MRI (Siemens, Munich, Germany) examinations at 3 months after treatment at monthly intervals to monitor changes in tumor size, tumor growth rate and tumor necrosis. If tumor necrosis, tumor shrinkage or slowed tumor growth was evident, this confirmed that the hyperthermia apparatus was effective in the treatment of malignant glioma; otherwise, it was considered ineffective. The standards used to evaluate the effectiveness of the hyperthermia apparatus in treating malignant glioma were as follows: (1) After hyperthermia treatment, head CT or MRI examination showed tumors with partial or full necrosis. (2) After hyperthermia treatment, head CT or MRI examination revealed that tumor growth had slowed or stopped for a few weeks to months.
Statistical analysis
Data were analyzed using SPSS 13.0 software (SPSS, Chicago, IL, USA) and data were represented as mean ± SD. Statistically significant differences in mean values between the groups were compared using the two-sample t-test, and P < 0.05 was considered to be statistically significant.
Footnotes
Funding: This study was financially sponsored by the Overseas Returnees of Heilongjiang Province in China, No. IC03C18 2003-2005.
Conflicts of interest: None declared.
Ethical approval: The study was approved by the Ethics Committee of the Second Affiliated Hospital of Harbin Medical University in China.
(Reviewed by Morris G, Dong L, You YP, Sun H)
(Edited by Wang LM, Yang Y, Li CH, Song LP, Liu WJ, Zhao M)
REFERENCES
- [1].Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Qaddoumi I, Sultan I, Gajjar A. Outcome and prognostic features in pediatric gliomas: a review of 6212 cases from the Surveillance, Epidemiology, and End Results database. Cancer. 2009;115(24):5761–5770. doi: 10.1002/cncr.24663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Paugh BS, Qu C, Jones C, et al. Integrated molecular genetic profiling of pediatric high-grade gliomas reveals key differences with the adult disease. J Clin Oncol. 2010;28(18):3061–3068. doi: 10.1200/JCO.2009.26.7252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Liang ML, Ma J, Ho M, et al. Tyrosine kinase expression in pediatric high grade astrocytoma. J Neurooncol. 2008;87(3):247–253. doi: 10.1007/s11060-007-9513-1. [DOI] [PubMed] [Google Scholar]
- [5].Guthkelch AN, Carter LP, Cassady JR, et al. Treatment of malignant brain tumors with focused ultrasound hyperthermia and radiation: results of a phase I trial. J Neurooncol. 1991;10(3):271–284. doi: 10.1007/BF00177540. [DOI] [PubMed] [Google Scholar]
- [6].Kakinuma K, Tanaka R, Takahashi H, et al. Targeting chemotherapy for malignant brain tumor using thermosensitive liposome and localized hyperthermia. J Neurosurg. 1996;84(2):180–184. doi: 10.3171/jns.1996.84.2.0180. [DOI] [PubMed] [Google Scholar]
- [7].Kakinuma K, Tanaka R, Takahashi H, et al. Drug delivery to the brain using thermosensitive liposome and local hyperthermia. Int J Hyperthermia. 1996;12(1):157–165. doi: 10.3109/02656739609023698. [DOI] [PubMed] [Google Scholar]
- [8].Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360(8):765–773. doi: 10.1056/NEJMoa0808710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Christensen BC, Smith AA, Zheng S, et al. DNA methylation, isocitrate dehydrogenase mutation, and survival in glioma. J Natl Cancer Inst. 2011;103(2):143–153. doi: 10.1093/jnci/djq497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Marchosky JA, Moran CJ, Fearnot NE, et al. Hyperthermia catheter implantation and therapy in the brain. Technical note. J Neurosurg. 1990;72(6):975–979. doi: 10.3171/jns.1990.72.6.0975. [DOI] [PubMed] [Google Scholar]
- [11].Moriyama E, Salcman M, Broadwell RD. Blood-brain barrier alteration after microwave-induced hyperthermia is purely a thermal effect: I. Temperature and power measurements. Surg Neurol. 1991;35(3):177–182. doi: 10.1016/0090-3019(91)90068-k. [DOI] [PubMed] [Google Scholar]
- [12].Roberts DW, Coughlin CT, Wong TZ, et al. Interstitial hyperthermia and iridium brachytherapy in treatment of malignant glioma. A phase I clinical trial. J Neurosurg. 1986;64(4):581–587. doi: 10.3171/jns.1986.64.4.0581. [DOI] [PubMed] [Google Scholar]
- [13].Ryan TP, Hoopes PJ, Taylor JH, et al. Experimental brain hyperthermia: techniques for heat delivery and thermometry. Int J Radiat Oncol Biol Phys. 1991;20(4):739–750. doi: 10.1016/0360-3016(91)90017-x. [DOI] [PubMed] [Google Scholar]
- [14].Dayanc BE, Beachy SH, Ostberg JR, et al. Dissecting the role of hyperthermia in natural killer cell mediated anti-tumor responses. Int J Hyperthermia. 2008;24(1):41–56. doi: 10.1080/02656730701858297. [DOI] [PubMed] [Google Scholar]
- [15].Peake J, Peiffer JJ, Abbiss CR, et al. Body temperature and its effect on leukocyte mobilization, cytokines and markers of neutrophil activation during and after exercise. Eur J Appl Physiol. 2008;102(4):391–401. doi: 10.1007/s00421-007-0598-1. [DOI] [PubMed] [Google Scholar]
- [16].Roberts GT, Ghebeh H, Chishti MA, et al. Microvascular injury, thrombosis, inflammation, and apoptosis in the pathogenesis of heatstroke: a study in baboon model. Arterioscler Thromb Vasc Biol. 2008;28(6):1130–1136. doi: 10.1161/ATVBAHA.107.158709. [DOI] [PubMed] [Google Scholar]
- [17].Nagarsekar A, Greenberg RS, Shah NG, et al. Febrile- range hyperthermia accelerates caspase-dependent apoptosis in human neutrophils. J Immunol. 2008;181(4):2636–2643. doi: 10.4049/jimmunol.181.4.2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Huang TQ, Lee MS, Oh E, et al. Molecular responses of Jurkat T-cells to 1763 MHz radiofrequency radiation. Int J Radiat Biol. 2008;84(9):734–741. doi: 10.1080/09553000802317760. [DOI] [PubMed] [Google Scholar]
- [19].Zerbini A, Pilli M, Laccabue D, et al. Radiofrequency thermal ablation for hepatocellular carcinoma stimulates autologous NK-cell response. Gastroenterology. 2010;138(5):1931–1942. doi: 10.1053/j.gastro.2009.12.051. [DOI] [PubMed] [Google Scholar]
- [20].Tanaka R, Kim CH, Yamada N, et al. Radiofrequency hyperthermia for malignant brain tumors: preliminary results of clinical trials. Neurosurgery. 1987;21(4):478–483. doi: 10.1227/00006123-198710000-00007. [DOI] [PubMed] [Google Scholar]
- [21].Maier-Hauff K, Rothe R, Scholz R, et al. Intracranial thermotherapy using magnetic nanoparticles combined with external beam radiotherapy: results of a feasibility study on patients with glioblastoma multiforme. J Neurooncol. 2007;81(1):53–60. doi: 10.1007/s11060-006-9195-0. [DOI] [PubMed] [Google Scholar]
- [22].van Landeghem FK, Maier-Hauff K, Jordan A, et al. Post-mortem studies in glioblastoma patients treated with thermotherapy using magnetic nanoparticles. Biomaterials. 2009;30(1):52–57. doi: 10.1016/j.biomaterials.2008.09.044. [DOI] [PubMed] [Google Scholar]
- [23].Bouchama A, Knochel JP. Heat stroke. N Engl J Med. 2002;346(25):1978–1988. doi: 10.1056/NEJMra011089. [DOI] [PubMed] [Google Scholar]
- [24].Marchosky JA, Welsh DM, Moran CJ. Hyperthermia treatment of brain tumors. Mo Med. 1990;87(1):29–33. [PubMed] [Google Scholar]
- [25].Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359(5):492–507. doi: 10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- [26].Atanackovic D, Pollok K, Faltz C, et al. Patients with solid tumors treated with high-temperature whole body hyperthermia show a redistribution of naive/memory T-cell subtypes. Am J Physiol Regul Integr Comp Physiol. 2006;290(3):R585–594. doi: 10.1152/ajpregu.00014.2005. [DOI] [PubMed] [Google Scholar]
- [27].Dayanc BE, Beachy SH, Ostberg JR, et al. Dissecting the role of hyperthermia in natural killer cell mediated anti-tumor responses. Int J Hyperthermia. 2008;24(1):41–56. doi: 10.1080/02656730701858297. [DOI] [PubMed] [Google Scholar]
- [28].Meinander A, Söderström TS, Kaunisto A, et al. Fever-like hyperthermia controls T Lymphocyte persistence by inducing degradation of cellular FLIPshort. J Immunol. 2007;178(6):3944–3953. doi: 10.4049/jimmunol.178.6.3944. [DOI] [PubMed] [Google Scholar]
- [29].Sharma HS, Hoopes PJ. Hyperthermia induced pathophysiology of the central nervous system. Int J Hyperthermia. 2003;19(3):325–354. doi: 10.1080/0265673021000054621. [DOI] [PubMed] [Google Scholar]
- [30].Sharma HS. Hyperthermia induced brain oedema: current status and future perspectives. Indian J Med Res. 2006;123(5):629–652. [PubMed] [Google Scholar]
- [31].Hurwitz MD, Hansen JL, Prokopios-Davos S, et al. Hyperthermia combined with radiation for the treatment of locally advanced prostate cancer: long-term results from Dana-Farber Cancer Institute study 94-153. Cancer. 2011;117(3):510–516. doi: 10.1002/cncr.25619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Balivada S, Rachakatla RS, Wang H, et al. A/C magnetic hyperthermia of melanoma mediated by iron(0)/iron oxide core/shell magnetic nanoparticles: a mouse study. BMC Cancer. 2010;10:119. doi: 10.1186/1471-2407-10-119. [DOI] [PMC free article] [PubMed] [Google Scholar]


