Abstract
Emerging studies of treating spinal cord injury (SCI) with adult stem cells led us to evaluate the effects of transplantation of hair follicle stem cells in rats with a compression-induced spinal cord lesion. Here, we proposed a hypothesis that rat hair follicle stem cell transplantation can promote the recovery of injured spinal cord. Compression-induced spinal cord injury was induced in Wistar rats in this study. The bulge area of the rat vibrissa follicles was isolated, cultivated and characterized with nestin as a stem cell marker. 5-Bromo-2′-deoxyuridine (BrdU) labeled bulge stem cells were transplanted into rats with spinal cord injury. Immunohistochemical staining results showed that some of the grafted cells could survive and differentiate into oligodendrocytes (receptor-interacting protein positive cells) and neuronal-like cells (βIII-tubulin positive cells) at 3 weeks after transplantation. In addition, recovery of hind limb locomotor function in spinal cord injury rats at 8 weeks following cell transplantation was assessed using the Basso, Beattie and Bresnahan (BBB) locomotor rating scale. The results demonstrate that the grafted hair follicle stem cells can survive for a long time period in vivo and differentiate into neuronal- and glial-like cells. These results suggest that hair follicle stem cells can promote the recovery of spinal cord injury.
Keywords: neural regeneration, spinal cord injury, cell transplantation, cell therapy, hair follicle stem cells, oligodendrocytes, nerve cells, glial cells, receptor-interacting protein, grants-supported paper, neuroregeneration
INTRODUCTION
About 2.5 million people all over the world suffer from spinal cord injury, with more than 130 000 cases added each year[1]. Contusive injury with subsequent compression is the most common type of spinal cord injury, resulting in neuronal and glial cell death and the demyelination of surviving axons[2]. Unfortunately, there has been no comprehensive approach for the treatment of spinal cord injury[3]. Studies are underway to develop strategies to restore structure and function of the damaged spinal cord[2,4]. Cell therapy plays a major role in the promotion of axonal growth and neuronal replacement in spinal cord injury[5], Alzheimer's disease[6], Parkinson's disease[7] and other central nervous system-related degenerative diseases[8,9]. Transplantation of peripheral nerve[10,11], fetal tissue[12], olfactory ensheathing glia[13],
oligodenrocyte precursor[14], Schwann cells[15] and growth factors[16] for the treatment of central nervous system disorder[5,17] has been assessed. Hair follicles undergo repeated cycles of growth (anagen), regression (catagen) and rest (telogen) throughout the adult life[18,19]. Hair follicle stem cells reside in the bulge area of the hair follicle and are morphologically undifferentiated and slow-cycling in vivo[20,21,22]. The bulge area is a niche highly enriched for stem cells with neuronal and glial differentiation potential[23,24]. Both hair follicle stem cells and central nervous system cells are applied because of their source from ectodermal plate[25] and their availability as well as without ethical concerns as other cells like embryonic stem cells or fetal stem cells[25]. There is strong evidence that hair follicle stem cells contribute to the regenerative process of skin[26], spinal cord[27,28,29], and sciatic nerve[24,30].
In the present study, we used a rat model of compression-induced spinal cord injury to simulate human spinal cord injuries[31] and studied the effects of rat hair follicle stem cells in vivo in terms of their survival and differentiation potential as reported previously[32], and the ability to reduce motor disability.
RESULTS
Syringomyelia formation in the spinal cord
Histological evidence confirmed that there was a significant damage to the spinal cord of rat spinal cord injury models. Lesion of spinal cord at the level of T10 segment was observed at 1 week after injury (Figure 1A). At 3 weeks after injury, post-traumatic syringomyelia developed (Figure 1B).
Figure 1.
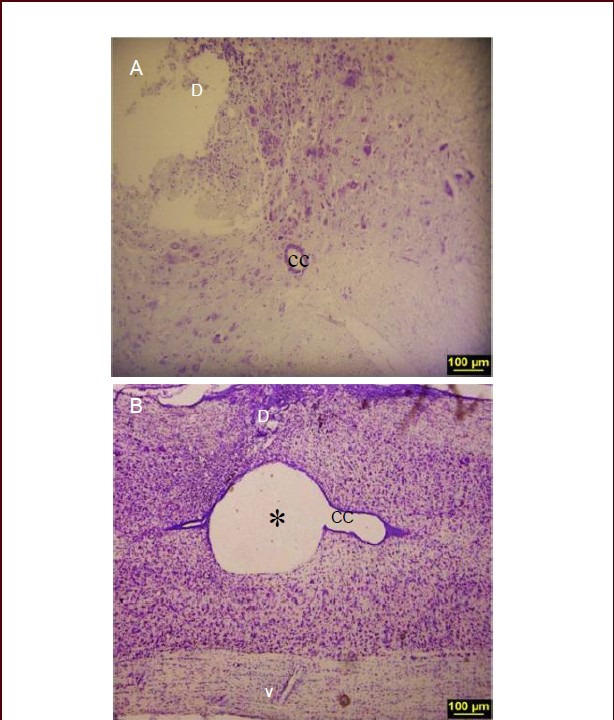
Histological evidence of spinal cord lesion in spinal cord injury (SCI) rat model.
(A) Nissl-stained section illustrating the lesion in dorsal horn 1 week after SCI. (B) Nissl-stained longitudinal section at 3 weeks after SCI showing cavity formation (× 10). * indicates the cavity; D: dorsal; V: ventral, CC: central canal.
Differentiation of hair follicle stem cells to neuronal and glial lineage
At 3 weeks after transplantation, cell aggregates were seen around the syrinx cavity forming in T10 segment (Figure 2C1, C2). The glial nature of transplanted cells was evaluated using receptor-interacting protein (RIP) (a marker of oligodenrocytes). Many of these transplanted cells were RIP-immunoreactive oligodendroglial cells with 5-bromo-2’-deoxyuridine (BrdU) positive nuclei (Figure 2D1, E1). In addition, to evaluate neuronal differentiation, βIII-tubulin antibody was used as a general marker for immature and mature neuronal cells. Our results showed that some of the grafted hair follicle stem cells were expressing βIII-tubulin 3 weeks after transplantation (Figure 2D2). The percentage of BrdU/βIII-tubulin (neuronal markers) and BrdU/RIP (glial markers) double labeled cells was 38.77 ± 4.07% and 23.07 ± 3.86%, respectively (Figure 2E1, E2).
Figure 2.
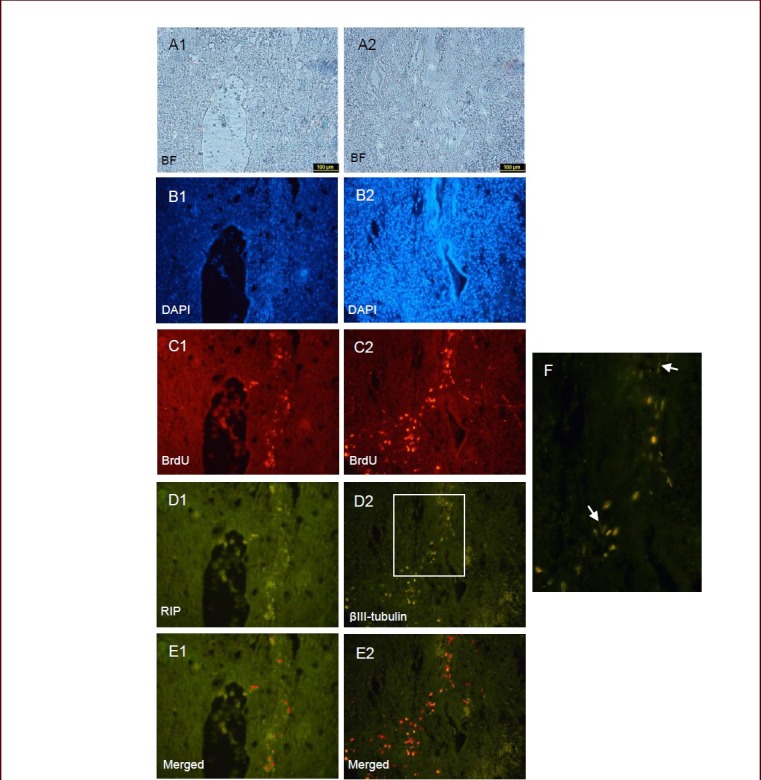
An immunohistochemical procedure was used to detect receptor-interacting protein (RIP) and βIII-tubulin expression in rat spinal cord sections (× 10).
Images (A1, A2 bright field & B1, B2 DAPI) showing the cystic lesions forming at 3 weeks after spinal cord injury. The sections show that BrdU-labeled hair follicle stem cells survived for 3 weeks after transplantation, and the red cell aggregates (C1, C2) are clearly visible. The cells were assessed by double immunostaining. Through double label immunohistochemistry, the same section shows that cells were positive for the oligodendrocyte marker RIP (D1) and some of them were βIII-tubulin positive (D2). Also, cells with neuronal morphology are shown by arrows in image F. Moreover, BrdU-labeled cells that express both RIP and βIII-tubulin are shown in merged images (E1 and E2). D: Dorsal; V: ventral; BF: bright field; DAPI: 4’,6-diamidino-2-phenylindole; BrdU: 5-bromo-2’-deoxyuridine.
Survival of transplanted cells around the central canal of the injured spinal cord
Nestin, as a well known neural stem cell marker, is also expressed in the bulge stem cells and precursor cells in spinal cord. Transplanted hair follicle stem cells were traced by double label immunostaining for nestin and BrdU in spinal cord injury (Figures 2C1, C2 and 3B1). BrdU labeled cells were observed around the cysts in the posterior horn, but not in the central canal of spinal cord (Figure 3B2). The transition from hair follicle stem cells to differentiated neural cells as evidenced by RIP and βIII tubulin staining (Figure 2D1, D2, F) likely requires the generation of more restricted cells that did not express nestin (Figure 3C1).
Figure 3.
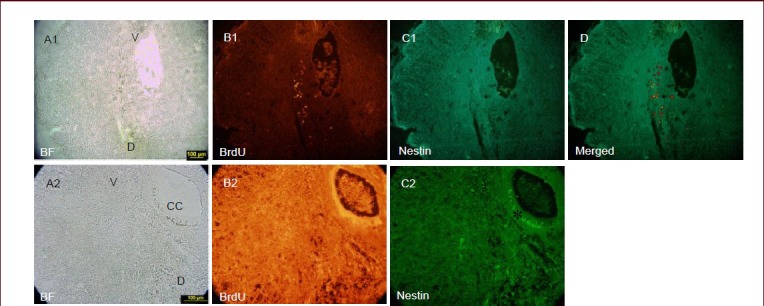
Nestin expression around the central canal.
In the cystic spinal cord (A1), 5-bromo-2’-deoxyuridine (BrdU)-labeled cells are seen in the lesion site (B1). The images (C1, D) did not show immunoreactivity for nestin 3 weeks after transplantation. Nestin expression was restricted to ependymal cell layer (star) (C2). In the same region, labeled cells were not observed around the central canal (B2). Magnification at 10 × for A1, B1, C1, D and at 40 × for A2, B2, C2. V: Ventral; D: dorsal; CC: central canal; BF: bright field.
Motor functional recovery
Hind limb motor function was assessed by the open field test using the Basso, Beattie and Bresnahan (BBB) locomotor rating scale. Spinal cord compression injury was induced. Ten days later, hair follicle stem cells were transplanted and the animals were tested at weekly intervals. BBB scores were gradually increased in the transplantation group over the following 8 weeks. Eight weeks later, the locomotor activity of these animals was significantly improved (Figure 4). The rats that received hair follicle stem cell transplantation exhibited consistent weight-supported plantar stepping, with forelimb-hindlimb coordination. In contrast, the spinal cord injury rats showed frequent weight-supported plantar steps and occasional forelimb-hindlimb coordination (Figure 4).
Figure 4.
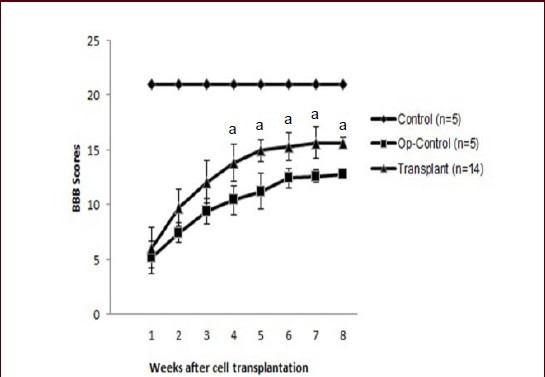
Locomotor function assessed using the Basso, Beattie and Bresnahan (BBB) locomotor rating scale.
Within 3 weeks after the initial compression of the spinal cord, BBB scores were in the range 5.2-12.07, and the same range found in the control animals. Following transplantation of hair follicle stem cells, the use of the hindlimbs increased gradually, starting 4 weeks after transplantation. By 8 weeks after transplantation, BBB scores were significantly greater in transplant rats than in Op-Control rats. The mean BBB score for rats receiving hair follicle stem cell transplantation was significantly higher than that for spinal cord injury control (op-control) rats (aP < 0.05, Student's t-test).
DISCUSSION
It has been well known that the bulge area of hair follicles comprise the cells with stem cell properties[33,34]. Hair follicle stem cells are characterized by high proliferation potential, slow cycling and label retaining[34,35], which fulfilling the required criteria for a stem cell population. Nestin, a protein marker for neural stem cells, is also expressed in human and mouse hair follicle stem cells and dermal papilla[36,37,38,39,40]. Rat hair follicle stem cells have the ability to express nestin and differentiate into neuronal and glial lineages[6,28,32]. Nestin is present in human and mouse hair follicle stem cells, confirming our original observation in rats[37,41].
Spinal cord is prone to injuries caused by spinal vertebral fracture and disc displacement[42]. Among several models developed in rodents, compression models are the most used paradigms to mimic human spinal cord injury condition[43]. In compression injury, neuronal loss is more protracted than in contusion injury[44]. This technique can cause the changes in motor disturbances by applying a 20 g weight for 20 minutes at the injured site[45].
In the present study, a well developed rat spinal cord compression injury model was used[43]. In animals with spinal cord injury, cavities and cysts developed in the lesion site of the spinal cord may cause interruption of the descending and ascending axonal tracts, and hence cause paralysis of the lower extremities similar to partial spinal cord injury in human[46]. This is evidenced by significantly low BBB scores in spinal cord injury animals as compared to the control group both in our study and previously published literatures[31,47].
In this study, before transplantation into the injury site, hair follicle stem cells were labeled with 3 μg/mL BrdU in vitro to characterize their proliferation status. Our results demonstrated that the BrdU-labeled cells could survive in the injured spinal cord for 3 weeks after transplantation and differentiate into neuronal and glial lineages. Surprisingly, 23% of the transplanted cells were RIP (oligodendrocyte marker) positive and 39% of them were βIII-tubulin (neural cell marker) positive with some neuronal fibers. Consistent with our previous report, these results confirm that hair follicle stem cells can differentiate to not only neuronal and glial lineages in vitro[32], but also neural cell lineage upon transplantation. A previous study showed that spinal cord recovery is related to Schwann cell derived myelination[48]. Our results indicate that transplanted hair follicle stem cells differentiating into oligodendroglial cells may be part of spinal cord regeneration.
To the best of our knowledge, this is the first report stating that rat hair follicle stem cells can differentiate into neural like cells in the compression model of spinal cord injury. Hair follicle is readily accessible, therefore, this can overcome the risks of harvesting stem cells from the brain[25,26].
Sieber-Blum et al[49] transplanted a novel type of multipotent adult stem cells known as epidermal neural crest stem cells into the lesioned spinal cord and found that transplanted epidermal neural crest stem cells survive and differentiate into βIII-tubulin, glutamic acid decarboxylase (GAD67) and RIP positive cells, and even integrated and intermingled with host neurites in the lesioned spinal cord[28]. In the present experiment, the cell types produced by hair follicle stem cells are oligodendrocytes and neuronal-like cells. It is proved that oligodendrocytes produce myelin sheaths around the spared and demyelinated host axons[14], but the extent to which demyelinated axons survive or become remyelinated by oligodendrocytes needs to be addressed in future using electron microscopy. Although remyelination of the injured axons is a possible mechanism behind functional recovery, transplanted hair follicle stem cells may participate in recovery through other ways rather than replacing the damaged oligodendroglial cells[17].
Locomotor function analysis in freely moving animals using the BBB locomotor rating scale following spinal cord compression and subsequent hair follicle stem cell transplantation showed that hair follicle stem cell could enable limb coordination and plantar stepping based on walking scale. These biological and behavioral results show that hair follicle stem cells can promote the recovery of spinal cord injury.
In consistent with our study, Hu et al[29] also showed that transplantation of bulge-derived neural crest stem cells in spinal cord contusive injury models caused a 24% recovery in sensory connectivity and touch perception. Likewise, Liu et al[40] also found that nestin expressing stem cells from bulge area and dermal papilla could differentiate into neuronal and glial cells after transplantation into the hemisection spinal cord injury and enhance locomotor recovery.
Importantly, according to our results, hair follicle stem cells gradually lose nestin expression within 3 weeks after transplantation and finally most of the transplanted cells were nestin negative. This confirms that hair follicle stem cells are safe source of cells with limited proliferation potential after transplantation and thus may not develop into tumor like pathologies after transplanation.
In summary, the transplanted rat hair follicle stem cells can differentiate into neuronal and glial lineages and promote recovery following spinal cord injury. This suggests the applicability of the same cell source for the treatment of human spinal cord injury.
MATERIALS AND METHODS
Materials
All rats used in this study were purchased from Razi Research Institute, Tehran. Fourteen female Wistar rats, aged 3–5 weeks, were sacrificed for the isolation of hair follicle stem cells. Experimental protocols were permitted by the Animal Ethics Committee of the Tehran University of Medical Sciences, Iran. Twenty-nine female 8-week-old Wistar rats were used for later experiments: 24 rats for establishing spinal cord compression injury model and the remainder five rats serving as normal controls.
The rat models of spinal cord compression injury were randomly divided into three groups as follows:
a) Hair follicle stem cell transplantation group (transplant group, n = 14): Rats received hair follicle stem cell transplantation on day 10 after injury, and behavioral changes in the hind limbs were evaluated by the BBB scale during a 8-week post transplantation period.
b) Control group (n = 5): rats received hair follicle stem cell transplantation and were sacrificed at 3 weeks after injury and then immunohistochemical staining was performed.
c) The Op-control group (n = 5): rats only received 10 μL Hanks balanced salt solution (HBSS). Animals were maintained on a dark-light cycle at 20°C. The rats were given free access to water and food at all time.
Methods
Isolation and culture of rat vibrissa follicles
Rat vibrissae follicles were isolated by using modified methods described by Sieber-Blum and Kobayashi[33]. Briefly, the whisker pads were removed and placed in Dulbecco's modified Eagle's medium and Ham's F12 medium (DMEM/F12; 3:1) supplemented with 100 U penicillin and 100 mg streptomycin per mL. Each selected vibrissa was then dissected under an Olympus Stereo Microscope (SZX12, Japan). Most of the connective tissue and dermis around the follicles were removed. Vibrissa follicles were lifted out, rinsed and the bulge area was dissected from hair follicle by making two transverse cuts above and below the bulge area using a fine blade and the follicle capsule was incised longitudinally[35].
The culture procedure was used as previously described by Yang and colleagues with slight modifications[36]. Briefly, 20 isolated bulges were cut into small pieces, plated in T-25 flask and immersed in a 3:1 supplemented mixture DMEM/F12 containing 5% fetal bovine serum, antibiotics (100 U/mL penicillin, 100 μg/mL streptomycin, 0.5 μg/mL fungizone), 10 ng/mL epidermal growth factor (EGF; E4127, Sigma-Aldrich, St. Louis, MO, USA), 1 × 10-9 mol/L cholera toxin (Sigma-Aldrich; C8052), 0.5 mg/mL hydrocortisone, 3.4 mmol/L L-glutamine, 5 μg/mL insulin and 0.135 mmol/L adenine. Primary culture was done for 14 days; after three passages, the cells were labeled with BrdU and transplanted into spinal cord compression injury model.
Establishment of spinal cord compression injury model
Spinal cord compression injury was induced in accordance with the modified weight compression method[39]. Briefly, following anesthesia by intraperitoneal injection of 80 mg/kg ketamine and 20 mg/kg xylazine (both from Alfasan, Woerden, Holland), rats were immobilized to minimize movement of the thoracic spine on the stereotaxic instrument, skin overlaying the spine was opened with a razor blade and the muscles were detached from the spinal processes. Partial laminectomy was done at T12 level (T10 segment) to expose dura mater and compression lesion was performed by placing a 20 g weight on the dura matter for 20 minutes. The weight used had a cylindrical appearance with a round concave lower surface of 6 mm diameter which exactly fitted the lesion site. The weight was placed in a cylindrical tube (8 mm in diameter) and fixed to a stereotaxic frame. Then, hair follicle stem cells were transplanted in the transplant group and control group. In the Op-control group, HBSS was injected to lesion site the same as cell transplantation. The body temperature was kept at 37°C during the operation.
Cell transplantation
Cell transplantation was performed 10 days after spinal cord compression injury. The cells were labeled with 3 μg/mL BrdU (Sigma-Aldrich) in cell culture medium for 30–45 minutes. Then, 48 hours after labeling, animals were placed in a stereotaxic frame and the injury site was exposed. BrdU-labeled hair follicle stem cells were suspended using a Hamilton syringe with 30 gauge needle and 5 μL of cell suspension (2 × 105 hair follicle stem cells/5 μL HBSS) was injected to the injury center and 5 μL cell suspension (2 × 105 hair follicle stem cells/5 μL HBSS) was injected to both 2 mm rostral and caudal of the injury site. After cell transplantation, rats were intramuscularly administered 10 mg/kg cefazolin, once a day for 7 days postoperatively, and the bladders were manually compressed twice a day until the animals were killed. In this study, the composition of the hair follicle stem cell culture, in respect to the absence of differentiated cells, was verified before transplantation by staining with βIII-tubulin for neurons and RIP for oligodendrocytes. To verify the purity of hair follicle stem cells, they were immunostained with nestin as a marker of hair follicle stem cells[24,40].
Tissue preparation
At 3 weeks after cell transplantation, following anesthesia, rats were transcardially perfused with 200 mL of 0.1 mol/L PBS (pH 7.4) followed by 500 mL of 4% paraformaldehyde. The T10 segment of spinal cord was then removed and postfixed. For histological and double-label immonohistochemistry analysis, tissues were embedded in paraffin and then cut into 10 μm-thick sections.
Histological and double-label immunohistochemistry procedure
Briefly, 10-μm thick sections were placed on slides and deparaffinized and dehydrated. Histological analysis was performed based on Nissl staining (cresyl violet acetate dye; C5042, Sigma-Aldrich) using light microscopy to confirm spinal cord compression injury model[23]. For double-label immunohistochemistry, sections were incubated in blocking buffer containing 10% normal goat serum (Invitrogen, Carlsbad, CA, USA), 0.3% Triton X-100 (Sigma-Aldrich) and 1% bovine serum albumin (BSA) at room temperature for 60 minutes. The following primary antibodies were used during BrdU/βIII-tubulin and BrdU/RIP double-label staining: sheep anti-BrdU polyclonal antibody (1:100; AB1893, Abcam, Cambridge, MA, USA); mouse anti-nestin monoclonal antibody (1:100, MAB353; Millipore, Billerica, MA, USA); mouse anti-βIII-tubulin antibody (1:200; Sigma-Aldrich); mouse anti-RIP monoclonal antibody (1:50 000; MAB1580; Millipore). Sheep anti-mouse FITC conjugates IgG (1:200; Sigma-Aldrich) and Alexa Fluor 546 donkey anti-sheep IgG (1:400 Molecular Probes, Eugene, OR, USA) were used as the secondary antibodies. All sections were counterstained with 4’,6-diamidino-2-phenylindole (DAPI) and the glass coverslips were mounted using final mounting media containing glycerol (1:3) 2.5% 1,0-4-diazabicyclo-(2.2.2) octane for visualization on an Olympus photomicroscope (PROVIS AX70, Japan). Negative controls were obtained by omitting of the primary antibodies.
Cell quantification
BrdU-positive hair follicle stem cells and BrdU/RIP, BrdU/βIIIubulin positive cells were counted in three sections, in the middle, 200 μm above and below the lesion site. The ImageJ software (a free software) was used to analyze these images and the data were presented as mean ± SD.
Neurological evaluation
Rats were placed in a circular open field and observed for 4 minutes once a week. The hind limb movements were examined by two different persons[50] and the locomotor behavior was evaluated by the open field test and scored by the BBB scale from 0 (no movements) to 21 (normal movement)[50,51].
Statistical analysis
Student's t-test was used for comparison of immunostained cells between experimental groups. SPSS 13.0 software (SPSS, Chicago, IL, USA) was used for statistical analysis. A level of P < 0.05 was considered statistically significant.
Acknowledgments
We would like to thank Mahmoudian M, Sharifi M and Rahbar Roushandel N (Department of Pharmacology, Iran University of Medical Sciences, Tehran, Iran) for providing useful equipments.
Footnotes
Funding: This study was financially supported by a grant from Iran University of Medical Sciences (Tehran–Iran), No. 531.
(Reviewed by Barreiro-Iglesias A, Toulouse A, Timothy Himes B, Zhang N, Wang LS)
(Edited by Li CH, Song LP, Liu WJ, Zhao M)
REFERENCES
- [1].Kundi S, Bicknell R, Ahmed Z. Spinal cord injury: current mammalian models. Am J Neurosci. 2013;4:1–12. [Google Scholar]
- [2].Ruff CA, Wilcox JT, Fehlings MG. Cell-based transplantation strategies to promote plasticity following spinal cord injury. Exp Neurol. 2012;235:78–90. doi: 10.1016/j.expneurol.2011.02.010. [DOI] [PubMed] [Google Scholar]
- [3].Thuret S, Moon LD, Gage FH. Therapeutic interventions after spinal cord injury. Nat Rev Neurosci. 2006;7:628–643. doi: 10.1038/nrn1955. [DOI] [PubMed] [Google Scholar]
- [4].Boulenguez P, Vinay L. Strategies to restore motor functions after spinal cord injury. Curr Opin Neurobiol. 2009;19:587–600. doi: 10.1016/j.conb.2009.10.005. [DOI] [PubMed] [Google Scholar]
- [5].Reier PJ. Cellular transplantation strategies for spinal cord injury and translational neurobiology. NeuroRx. 2004;1:424–451. doi: 10.1602/neurorx.1.4.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Esmaeilzade B, Nobakht M, Joghataei MT, et al. Delivery of epidermal neural crest stem cells (EPI-NCSC) to hippocamp in Alzheimer's disease rat model. Iran Biomed J. 2012;16(1):1–9. doi: 10.6091/IBJ.1029.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Sundberg M, Bogetofte H, Lawson T, et al. Improved cell therapy protocols for Parkinson's disease based on differentiation efficiency and safety of hESC-, hiPSC-, and non-human primate iPSC-derived dopaminergic neurons. Stem cells. 2013;31:1548–1562. doi: 10.1002/stem.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Feng SQ, Kong XH, Liu Y, et al. Regeneration of spinal cord with cell and gene therapy. Orthop Surg. 2009;1:153–163. doi: 10.1111/j.1757-7861.2009.00018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Onose G, Anghelescu A, Muresanu D, et al. A review of published reports on neuroprotection in spinal cord injury. Spinal Cord. 2009;47:716–726. doi: 10.1038/sc.2009.52. [DOI] [PubMed] [Google Scholar]
- [10].Richardson P, McGuinness U, Aguayo A. Peripheral nerve autografts to the rat spinal cord: studies with axonal tracing methods. Brain Res. 1982;237:147–162. doi: 10.1016/0006-8993(82)90563-7. [DOI] [PubMed] [Google Scholar]
- [11].Myckatyn TM, Mackinnon SE, McDonald JW. Stem cell transplantation and other novel techniques for promoting recovery from spinal cord injury. Transpl Immunol. 2004;12:343–358. doi: 10.1016/j.trim.2003.12.017. [DOI] [PubMed] [Google Scholar]
- [12].Houlé JD, Reier PJ. Transplantation of fetal spinal cord tissue into the chronically injured adult rat spinal cord. J Comp Neurol. 1988;269:535–547. doi: 10.1002/cne.902690406. [DOI] [PubMed] [Google Scholar]
- [13].Ramón-Cueto A, Cordero MI, Santos-Benito FF, et al. Functional recovery of paraplegic rats and motor axon regeneration in their spinal cords by olfactory ensheathing glia. Neuron. 2000;25:425–435. doi: 10.1016/s0896-6273(00)80905-8. [DOI] [PubMed] [Google Scholar]
- [14].Wilkins A, Majed H, Layfield R, et al. Oligodendrocytes promote neuronal survival and axonal length by distinct intracellular mechanisms: a novel role for oligodendrocyte-derived glial cell line-derived neurotrophic factor. J Neurosci. 2003;23:4967–4974. doi: 10.1523/JNEUROSCI.23-12-04967.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Fouad K, Schnell L, Bunge MB, et al. Combining Schwann cell bridges and olfactory-ensheathing glia grafts with chondroitinase promotes locomotor recovery after complete transection of the spinal cord. J Neurosci. 2005;25:1169–1178. doi: 10.1523/JNEUROSCI.3562-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wang J, Sun J, Tang Y, et al. Basic fibroblast growth factor attenuates the degeneration of injured spinal cord motor endplates. Neural Regen Res. 2013;8:2213–2224. doi: 10.3969/j.issn.1673-5374.2013.24.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Coutts M, Keirstead HS. Stem cells for the treatment of spinal cord injury. Exp Neurol. 2008;209:368–377. doi: 10.1016/j.expneurol.2007.09.002. [DOI] [PubMed] [Google Scholar]
- [18].Stenn KS, Paus R. Controls of hair follicle cycling. Physiol Rev. 2001;81:449–494. doi: 10.1152/physrev.2001.81.1.449. [DOI] [PubMed] [Google Scholar]
- [19].Paus R, Cotsarelis G. The biology of hair follicles. N Engl J Med. 1999;341:491–497. doi: 10.1056/NEJM199908123410706. [DOI] [PubMed] [Google Scholar]
- [20].Cotsarelis G. Epithelial stem cells: a folliculocentric view. J Invest Dermatol. 2006;126:1459–1468. doi: 10.1038/sj.jid.5700376. [DOI] [PubMed] [Google Scholar]
- [21].Zhang Y, Xiang M, Wang Y, et al. Bulge cells of human hair follicles: segregation, cultivation and properties. Colloids Surf B Biointerfaces. 2006;47:50–56. doi: 10.1016/j.colsurfb.2005.11.017. [DOI] [PubMed] [Google Scholar]
- [22].Hsu Y-C, Pasolli HA, Fuchs E. Dynamics between stem cells, niche, and progeny in the hair follicle. Cell. 2011;144:92–105. doi: 10.1016/j.cell.2010.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Yu H, Kumar SM, Kossenkov AV, et al. Stem cells with neural crest characteristics derived from the bulge region of cultured human hair follicles. J Invest Dermatol. 2010;130:1227–1236. doi: 10.1038/jid.2009.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Amoh Y, Aki R, Hamada Y, et al. Nestin-positive hair follicle pluripotent stem cells can promote regeneration of impinged peripheral nerve injury. J Dermatol. 2012;39:33–38. doi: 10.1111/j.1346-8138.2011.01413.x. [DOI] [PubMed] [Google Scholar]
- [25].Amoh Y, Katsuoka K, Hoffman RM. The advantages of hair follicle pluripotent stem cells over embryonic stem cells and induced pluripotent stem cells for regenerative medicine. J Dermatol Sci. 2010;60:131–137. doi: 10.1016/j.jdermsci.2010.09.007. [DOI] [PubMed] [Google Scholar]
- [26].Jaks V, Kasper M, Toftgård R. The hair follicle-a stem cell zoo. Exp Cell Res. 2010;316:1422–1428. doi: 10.1016/j.yexcr.2010.03.014. [DOI] [PubMed] [Google Scholar]
- [27].Liu F, Uchugonova A, Kimura H, et al. The bulge area is the major hair follicle source of nestin-expressing pluripotent stem cells which can repair the spinal cord compared to the dermal papilla. Cell Cycle. 2011;10:830–839. doi: 10.4161/cc.10.5.14969. [DOI] [PubMed] [Google Scholar]
- [28].Sieber-Blum M. Epidermal neural crest stem cells and their use in mouse models of spinal cord injury. Brain Res Bull. 2010;83:189–193. doi: 10.1016/j.brainresbull.2010.07.002. [DOI] [PubMed] [Google Scholar]
- [29].Hu YF, Gourab K, Wells C, et al. Epidermal neural crest stem cell (EPI-NCSC)--mediated recovery of sensory function in a mouse model of spinal cord injury. Stem Cell Rev. 2010;6:186–198. doi: 10.1007/s12015-010-9152-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hejazian L, Hejazian M, Moradi F, et al. Isolation and culture of hair follicle stem cells (HFSCs) and their use for regeneration of the sciatic nerve in rat. J Neurol Sci. 2013;333:e671–672. [Google Scholar]
- [31].Tiede S, Kloepper JE, Bodo E, et al. Hair follicle stem cells: walking the maze. Eur J Cell Biol. 2007;86:355–376. doi: 10.1016/j.ejcb.2007.03.006. [DOI] [PubMed] [Google Scholar]
- [32].Nobakht M, Najafzadeh N, Safari M, et al. Bulge cells of rat hair follicles: Isolation, cultivation, morphological and biological features. Yakhteh Med J. 2010;12:51–58. [Google Scholar]
- [33].Kobayashi K, Rochat A, Barrandon Y. Segregation of keratinocyte colony-forming cells in the bulge of the rat vibrissa. Proc Natl Acad Sci U S A. 1993;90:7391–7395. doi: 10.1073/pnas.90.15.7391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Cotsarelis G, Cheng SZ, Dong G, et al. Existence of slow-cycling limbal epithelial basal cells that can be preferentially stimulated to proliferate: implications on epithelial stem cells. Cell. 1989;57:201–209. doi: 10.1016/0092-8674(89)90958-6. [DOI] [PubMed] [Google Scholar]
- [35].Lavker RM, Miller S, Wilson C, et al. Hair follicle stem cells: their location, role in hair cycle, and involvement in skin tumor formation. J Invest Dermatol. 1993;101:16S–26S. doi: 10.1111/1523-1747.ep12362556. [DOI] [PubMed] [Google Scholar]
- [36].Hoffman RM. The potential of nestin-expressing hair follicle stem cells in regenerative medicine. Expert Opin Biol Ther. 2007;7(3):289–291. doi: 10.1517/14712598.7.3.289. [DOI] [PubMed] [Google Scholar]
- [37].Amoh Y, Li L, Katsuoka K, Penman S, et al. Multipotent nestin-positive, keratin-negative hair-follicle bulge stem cells can form neurons. Proc Natl Acad Sci U S A. 2005;102(15):5530–5534. doi: 10.1073/pnas.0501263102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Li L, Mignone J, Yang M, et al. Nestin expression in hair follicle sheath progenitor cells. Proc Natl Acad Sci U S A. 2003;100:9958–9961. doi: 10.1073/pnas.1733025100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Uchugonova A, Duong J, Zhang N, et al. The bulge area is the origin of nestin-expressing pluripotent stem cells of the hair follicle. J Cell Biochem. 2011;112:2046–2050. doi: 10.1002/jcb.23122. [DOI] [PubMed] [Google Scholar]
- [40].Liu F, Uchugonova A, Kimura H, et al. The bulge area is the major hair follicle source of nestin-expressing pluripotent stem cells which can repair the spinal cord compared to the dermal papilla. Cell Cycle. 2011;10:830–839. doi: 10.4161/cc.10.5.14969. [DOI] [PubMed] [Google Scholar]
- [41].Yu H, Fang D, Kumar SM, et al. Isolation of a novel population of multipotent adult stem cells from human hair follicles. Am J Pathol. 2006;168:1879–1888. doi: 10.2353/ajpath.2006.051170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Norenberg MD, Smith J, Marcillo A. The pathology of human spinal cord injury: defining the problems. J Neurotrauma. 2004;21:429–440. doi: 10.1089/089771504323004575. [DOI] [PubMed] [Google Scholar]
- [43].Taoka Y, Okajima K, Murakami K, et al. Role of neutrophil elastase in compression-induced spinal cord injury in rats. Brain Res. 1998;799:264–269. doi: 10.1016/s0006-8993(98)00459-4. [DOI] [PubMed] [Google Scholar]
- [44].Huang W, George K, Ibba V, et al. The characteristics of neuronal injury in a static compression model of spinal cord injury in adult rats. Eur J Neurosci. 2007;25:362–372. doi: 10.1111/j.1460-9568.2006.05284.x. [DOI] [PubMed] [Google Scholar]
- [45].Taoka Y, Okajima K. Spinal cord injury in the rat. Prog Neurobiol. 1998;56:341–358. doi: 10.1016/s0301-0082(98)00049-5. [DOI] [PubMed] [Google Scholar]
- [46].Hill CE, Beattie MS, Bresnahan JC. Degeneration and sprouting of identified descending supraspinal axons after contusive spinal cord injury in the rat. Exp Neurol. 2001;171:153–169. doi: 10.1006/exnr.2001.7734. [DOI] [PubMed] [Google Scholar]
- [47].Profyris C, Cheema SS, Zang D, et al. Degenerative and regenerative mechanisms governing spinal cord injury. Neurobiol Dis. 2004;15:415–436. doi: 10.1016/j.nbd.2003.11.015. [DOI] [PubMed] [Google Scholar]
- [48].Amoh Y, Li L, Campillo R, et al. Implanted hair follicle stem cells form Schwann cells that support repair of severed peripheral nerves. Proc Natl Acad Sci U S A. 2005;102:17734–17738. doi: 10.1073/pnas.0508440102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Sieber-Blum M, Grim M, Hu YF, et al. Pluripotent neural crest stem cells in the adult hair follicle. Dev Dyn. 2004;231:258–269. doi: 10.1002/dvdy.20129. [DOI] [PubMed] [Google Scholar]
- [50].Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- [51].Basso DM, Fisher LC, Anderson AJ, et al. Basso Mouse Scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J Neurotrauma. 2006;23:635–659. doi: 10.1089/neu.2006.23.635. [DOI] [PubMed] [Google Scholar]


