Keywords: neural regeneration, stem cells, peripheral nerve injury, human umbilical cord mesenchymal stem cells, radial nerve, amniotic membrane, nerve conduit, nerve regeneration chamber, electrophysiology, motor, sensory, neuroregeneration
Abstract
In this study, we loaded human umbilical cord mesenchymal stem cells onto human amniotic membrane with epithelial cells to prepare nerve conduits, i.e., a relatively closed nerve regeneration chamber. After neurolysis, the injured radial nerve was enwrapped with the prepared nerve conduit, which was fixed to the epineurium by sutures, with the cell on the inner surface of the conduit. Simultaneously, a 1.0 mL aliquot of human umbilical cord mesenchymal stem cell suspension was injected into the distal and proximal ends of the injured radial nerve with 1.0 cm intervals. A total of 1.75 × 107 cells were seeded on the amniotic membrane. In the control group, patients received only neurolysis. At 12 weeks after cell transplantation, more than 80% of patients exhibited obvious improvements in muscular strength, and touch and pain sensations. In contrast, these improvements were observed only in 55–65% of control patients. At 8 and 12 weeks, muscular electrophysiological function in the region dominated by the injured radial nerve was significantly better in the transplantation group than the control group. After cell transplantation, no immunological rejections were observed. These findings suggest that human umbilical cord mesenchymal stem cell-loaded amniotic membrane can be used for the repair of radial nerve injury.
INTRODUCTION
Radial nerve injury generally refers to structural and functional impairment, even successive discontinuance caused by drag, pressurization or transection, leading to a series of dysfunctions in the region dominated by the injured radial nerve. Functional recovery after peripheral nerve injury is limited by complex pathological processes, slow neural regeneration velocity, adhesion of regenerative nerve to peripheral tissue, neuromuscular atrophy and motor end plate degeneration, so clinical therapeutic effects are not satisfactory[1,2,3].
The current methods of treating radial nerve injury mainly include neurolysis, suturing and nerve transplantation. The fist key event after radial nerve injury is to recover the succession of the nerve stem in enough time to avoid neuronal death, actively promote axon regeneration and effectively prevent effector atrophy[4]. At present, for the treatment of peripheral nerve injury, the most common method with optimal therapeutic effect is end-to-end anastomosis or nerve autografting, but both treatments have limitations. In end-to-end anastomosis, scar formation during healing and uncertain anastomosis between motor nerve fibers and sensory nerve fibers hinder neurofunctional recovery, even leading to stretch injury of the proximal and distal normal nerve tissue. There are many limitations in the use of nerve autografts, including limited source of nerve grafts, unmatched sizes of donor nerve and injured nerve functions in the donor size. With the development of tissue engineering, peripheral nerve tissue engineering has been considered as one of the most effective methods to address these limitations.
Human umbilical cord mesenchymal stem cells, one of the common seed cells used in peripheral nerve tissue engineering, have strong self-renewal and multi-differentiation potentials and can be induced to differentiate into nerve cells in vitro[5]. Human umbilical cord mesenchymal stem cells have a rich source, can be easily harvested with less opportunity for contamination, high purity and low immunogenicity, and can tolerate HLA mismatch to a larger extent[5,6,7]. Umbilical cord mesenchymal stem cells can be induced to differentiate into dopaminergic neuron-like cells and choline acetyl transferase-positive cells, suggesting that these cells have a greater potential in the treatment of Parkinson's and Alzheimer's diseases[8,9]. Human umbilical cord mesenchymal stem cells can promote functional recovery after acute spinal cord injury[10], and can be induced to differentiate into functional Schwann cells and promote peripheral nerve regeneration[11,12]. Intravenous administration of umbilical cord mesenchymal stem cells into the injured axillary and radial nerves by oligotrophic nonunion has achieved obvious therapeutic effects in the clinic[13].
Amniotic membrane has been used as a biomaterial for the repair of peripheral nerve injury. It generally consists of an epithelial cell layer, basilar membrane layer and compact layer. The epithelial cell surface is rich in microvilli. The basilar membrane provides mechanical support and is composed of extracellular matrix, collagen IV, heparan sulfate, proteoglycan and other macromolecules[14], similar to Schwann cell basilar membrane components. All of these structural characteristics of the amniotic membrane play an important role in tissue engineering[15]. In this study, we investigated the therapeutic effect of transplantation of human amniotic membrane loaded with human umbilical cord mesenchymal stem cells for the repair of radial nerve injury.
RESULTS
Quantitative analysis of subjects
Twelve patients who received neurolysis followed by transplantation of human amniotic membrane loaded with human umbilical cord mesenchymal stem cells for the treatment of radial nerve injury (transplantation group) and twenty patients who received only neurolysis (control group) were included in the final analysis, without any loss.
Baseline information of patients from the two groups
There were no significant differences in gender, age distribution, injury cause and pre-treatment neurological function between the transplantation and control groups (P > 0.05; Table 1).
Table 1.
Baseline information of patients from two groups
Identification of human umbilical cord mesenchymal stem cells
Under an inverted microscope, passage 2 human umbilical cord mesenchymal stem cells grew well, exhibited a shuttle-shaped appearance similar to fibroblast-like cells. Through flow cytometry, CD34 and CD45 expression was not detected on the cell surface, but CD73, CD90 and CD105 expression was detected (Figure 1), indicative of umbilical cord mesenchymal stem cells[16] (Figure 2).
Figure 1.
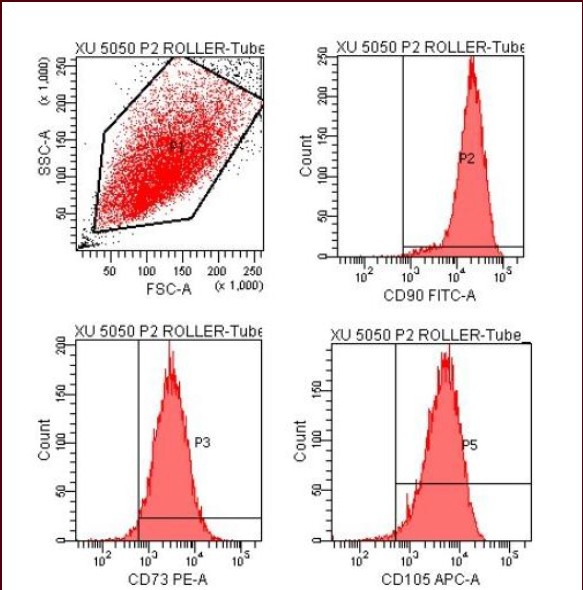
Identification of human umbilical cord mesenchymal stem cells by flow cytometry.
Passage 2 human umbilical cord mesenchymal stem cells express CD73, CD90 and CD105.
Figure 2.

Morphology of passage 2 human umbilical cord mesenchymal stem cells under an inverted microscope (× 40).
Cells grow evenly and exhibit a shuttle-shaped appearance similar to fibroblast-like cells.
Through electron microscopy, human umbilical cord mesenchymal stem cells loaded on amniotic membrane exhibited a shuttle-shaped appearance and evenly adhered onto human amniotic membrane (Figure 3). The amniotic membrane without loading human umbilical cord mesenchymal stem cells exhibited a basilar membrane-like structure (Figure 4).
Figure 3.
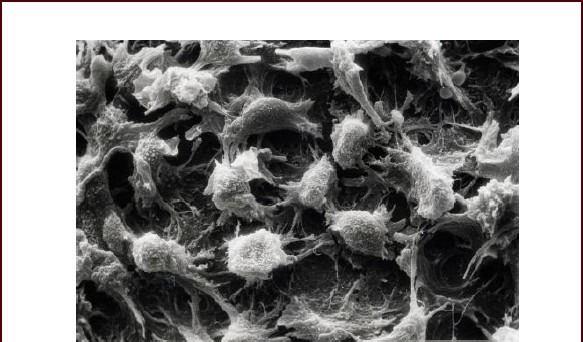
Morphology of passage 2 human umbilical cord mesenchymal stem cells loaded on human amniotic membrane under a scanning microscope (× 2 000).
Cells exhibit a shuttle-shaped appearance and adhere to the human amniotic membrane.
Figure 4.
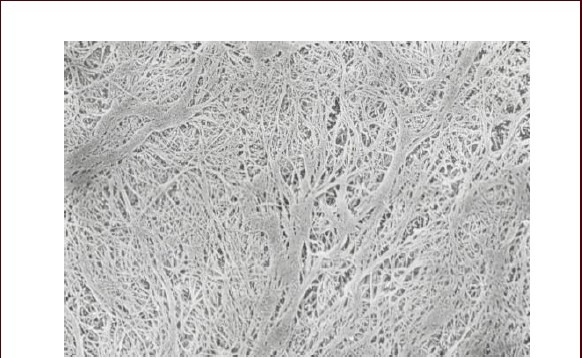
Human amniotic membrane without umbilical cord mesenchymal stem cells exhibits a basilar membrane-like structure under an electron microscope (× 5 000).
Recovery of radial nerve functions after transplantation of human amniotic membrane loaded with umbilical cord mesenchymal stem cells
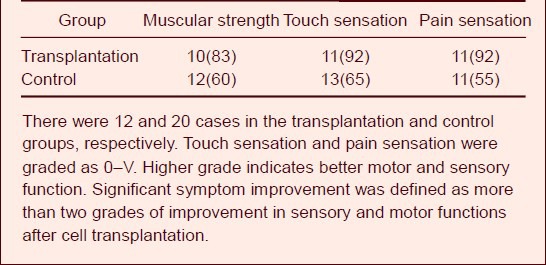
Motor and sensory functional recovery after peripheral nerve injury was assessed according to the criteria formulated by Neurotrauma Society, British Medical Academy[17]. At 12 weeks after transplantation of human amniotic membrane loaded with umbilical cord mesenchymal stem cells, the muscular power, touch sensation and pain sensation were significantly improved in most patients. However, only 55–65% of patients from the control group exhibited obvious improvements in motor and sensory functions (Table 2).
Table 2.
Symptom improvements [n (%)] in patients with radial nerve injury at 12 weeks after transplantation of human amniotic membrane loaded with human umbilical cord mesenchymal stem cells
Muscular electrophysiological function in the region dominated by injured radial nerve after transplantation of human amniotic membrane loaded with umbilical cord mesenchymal stem cells
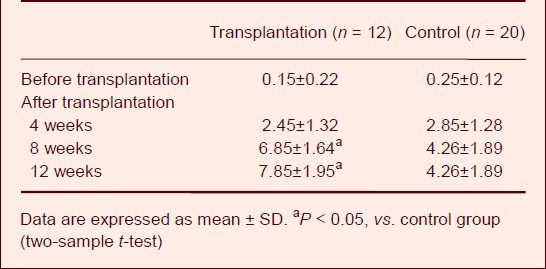
At 4 weeks after transplantation of human amniotic membrane loaded with umbilical cord mesenchymal stem cells, the muscular electrophysiological function in the region dominated by the injured radial nerve was not significantly changed (P > 0.05). However, at 8 and 12 weeks after cell transplantation, muscular electrophysiological function in the transplantation group was significantly improved compared to the control group (P < 0.05; Table 3).
Table 3.
Amplitude (mV) of muscular electromyogram of the region dominated by the injured radial nerve at 4, 8 and 12 weeks after transplantation of human amniotic membrane loaded with human umbilical cord mesenchymal stem cells
Adverse events after transplantation of human amniotic membrane loaded with umbilical cord mesenchymal stem cells
During 12 weeks after transplantation of human amniotic membrane loaded with umbilical cord mesenchymal stem cells, no severe adverse events, complications or fever were found. After cell transplantation, swelling, hemorrhage, exudation, infection, and allergic reaction did not occur. There was no need to remove the transplant in any of the patients.
DISCUSSION
The mechanism underlying repair and regeneration after peripheral nerve injury is very complex and influenced by many factors. One single method provides limited effects on the regeneration of injured peripheral nerve. A combined method can overcome the axon regeneration-inhibiting factors to the largest extent and effectively promote peripheral nerve regeneration and functional recovery. There is evidence that the most effective method to strengthen nerve regeneration is the combination of the following four methods: (1) remove axonal growth-inhibiting factors[18,19,20]; (2) provide axonal growth-promoting factors, such as neurotrophic factors[21,22]; (3) transplantation of seed cells[23]; (4) provide a pathway suitable for axonal growth[24].
The structural characteristics of the amniotic membrane provide substance basis for its use in nerve tissue engineering: the complex maze-shaped conduit system promotes molecular exchange; enough collagen fibers to strengthen its elasticity and tenacity; a special basilar membrane structure that facilitates the migration of epithelial cells, increases epithelial cell adhesion, acts a good cell carrier, which benefits cell adhesion and survival; an avascular matrix that prevents the excessive formation of fibrous scar tissue and inhibits inflammation[25].
Strong evidence exists that human amniotic membrane exhibits a distinct advantage and feasibility in the repair of peripheral nerve injury[26,27]. Yoshii et al[28,29] found that abundant collagen fibers in the amniotic membrane can promote axonal regeneration in transected spinal cord and sciatic nerve. Toba et al[30] reported that the collagen component in the basilar membrane of the amniotic membrane plays an important role in the repair of peripheral nerve injury. In this study, we prepared a nerve conduit, a relatively closed nerve regeneration chamber, using human amniotic membrane with epithelial cells maintained and loaded with human umbilical cord mesenchymal stem cells. The prepared nerve conduit is an ideal biological nerve conduit because it can be twisted, expanded, easily sutured, and sterilized during preparation without influences on its physiological characteristics.
There is no consensus regarding whether the epithelial cells should be removed from the amniotic membrane to completely expose the basilar membrane for nerve regeneration. Mohammad et al[31] prepared a nerve conduit using amniotic membrane without epithelial cells to bridge 10 mm rat sciatic nerve defects. Results showed that in the amniotic membrane group, an even nerve tissue grew and penetrated the amniotic membrane conduit, showing similar repair effects to nerve grafting. Researchers also used amniotic membrane with epithelial cells to prepare a nerve regeneration chamber, in which rat autologous nerve tissue, containing active Schwann cells and their secreted neurotrophic factors, were seeded within to strengthen the effects of the nerve scaffold in guiding nerve regeneration[32,33,34]. Thus, the bridged graft can provide a pathophysiological microenvironment closer to the nerve autograft, providing an in vivo environment suitable for axon regeneration. These findings suggest that amniotic membrane with or without epithelial cells can be used as a bridge scaffold to guide nerve growth to a certain extent. In this study, we used amniotic membrane with epithelial cells because human amniotic membrane epithelial cells have characteristics similar to neural stem cells. Uchida et al[35] found that under certain culture conditions, human amniotic membrane epithelial cells can synthesize and release various neurotrophic factors, including neurotrophic factor 3, nerve growth factor, basic fibroblast growth factor and brain-derived neurotrophic factor, and these factors can regulate the proliferation and differentiation of neural stem cells and promote axon regeneration.
Results from this study showed that at 12 weeks after transplantation, sensory and motor functions in the region dominated by the injured radial nerve were significantly recovered; in particular the muscular strength was obviously recovered compared to before transplantation. The possible mechanism may be as follows:
(1) The special structure of human amniotic basilar membrane facilitates the migration of epithelial cells, and the adhesion and survival of human umbilical cord mesenchymal stem cells[35].
(2) The injured radial nerve was enwrapped moderately with the artificial nerve conduit prepared using amniotic membrane (containing epithelial cells) loaded with human umbilical cord mesenchymal stem cells. Thus, a relatively closed nerve regeneration chamber was established to isolate the peripheral tissue and reduce the invasion of fibrous tissue and inflammatory cells, thereby effectively preventing the adhesion and pressurization caused by the scar formed in the anastomosis site.
(3) The human umbilical cord mesenchymal stem cell suspension injected into the nerve regeneration chamber exerted a synergistic effect together with the human umbilical cord mesenchymal stem cells adhered to the amniotic membrane, i.e., under the effect of various neurotrophic factors, increasing Schwann cells in number and promoting cell functional output, accelerating axon regeneration in the proximal injured nerve and elongating the axonal growth cone[35,36].
Taken together, the nerve regeneration chamber formed by the nerve conduit prepared using human amniotic membrane (epithelial cells were not removed) loaded with human umbilical cord mesenchymal stem cells provides not only a bridging role but also a microenvironment suitable for regeneration and repair of the injured radial nerve. In addition, human amniotic membrane and human umbilical cord mesenchymal stem cells can be easily obtained and there is no need to perform the second surgery after transplantation. Therefore, amniotic membrane loaded with human umbilical cord mesenchymal stem cells for the repair of radial nerve injury exerts promising potential for clinical application.
SUBJECTS AND METHODS
Design
A non-randomized, concurrent controlled study.
Time and setting
This study was performed in Affiliated Central Hospital of Shenyang Medical College, China, between December 2011 and June 2012.
Subjects
Thirty-two patients with radial nerve injury who received treatment in the Department of Bone Surgery, Fengtian Hospital, Shenyang Medical College, China, were included in this study. According to disease condition and patient selection, 12 patients received treatment by transplantation with human amniotic membrane loaded with human umbilical cord mesenchymal stem cells, and the remainder received only neurolysis.
All cases suffered from radial shaft fracture complicated by radial nerve injury. The time period between nerve injury and surgery lasted for 1–9 months.
Inclusion criteria: (1) Patients with clinically diagnosed radial nerve injury. (2) Patients that did not satisfy the therapeutic effects of intravenous injection or oral administration of neurotrophic medicine after first surgery. (3) Patients and their family agreed to accept human umbilical cord mesenchymal stem cell transplantation and signed the informed consent.
Exclusion criteria: (1) Patients with space-occupying lesion. (2) Pregnancy. (3) Those unable to communicate because of severe associated injury or severe nerve injuries, or those with severe mental disorders. (4) Patients with alcoholism, diabetes mellitus, gout, collagenosis or those who use immunosuppressive drugs. (5) Patients with acute infection, severe contamination of wounds, neurological defects or the presence of unstable vital signs. Umbilical cord and amniotic membrane source: Healthy full-term parturient women from Department of Gynaecology and Obstetrics, Fengtian Hospital, Shenyang Medical College, China agreed to the use of umbilical cord and amniotic membrane in the experiments and signed informed consent.
Methods
Preparation of human umbilical cord mesenchymal stem cells
According to a previously described method[37], human umbilical cord mesenchymal stem cells were cultured, purified, and phenotyped. Then, cells were identified by flow cytometry (BD, San Jose, CA, USA). A 10 mL aliquot of passage 2 cell suspension containing 1.75 × 107 cells was used.
Preparation of human amniotic membrane
The dissected human amniotic membrane was washed repeatedly with physiological saline, sterilized in alcohol for 1 minute and then washed with physiological saline. Thereafter, the amniotic membrane was cut into small pieces of required size using a sterile shears and placed in a 30 mL Petri dish. According to the amniotic membrane size, human umbilical cord mesenchymal stem cells were seeded on the amniotic membrane at a density of 1 × 105/cm2. The Petri dish was incubated at 37°C in an incubator containing 5% CO2 saturated humidity (Kogyo). Cell growth state was periodically observed under an inverted microscope (Olympus, Tokyo, Japan) and a scanning electron microscope.
Transplantation of human amniotic membrane loaded with human umbilical cord mesenchymal stem cells
In the transplantation group, after neurolysis of radial nerve, three or four incisions, each of 1.0–1.5 cm, were longitudinally cut on the epineurium along the injured radial nerve segment. Then, the injured radial nerve segment was enwrapped with the human amniotic membrane loaded with umbilical cord mesenchymal stem cells, with the cells on the inner surface, and the enwrapping should not be so tight that it causes pressurization. The two ends of the amniotic membrane were sutured to the epineurium. Thus, a biomembrane nerve conduit was prepared (Figure 5). A 7.0 mL aliquot of human umbilical cord mesenchymal stem cell suspension was injected into the injured radial nerve segments enwrapped with the biomembrane nerve conduit via seven points from the center towards the distal (three points) and proximal ends (three points) with 1.0 cm intervals on the epineurium, 1.0 mL per point. Then, another 3.0 mL aliquot of cell suspension was equally injected into the injured segment via two points, i.e., 0.5 cm distal and proximal away from the center. A total of 10 mL cell suspension containing 1.75 × 107 human umbilical cord mesenchymal stem cells was injected. In the control group, only neurolysis and layer-by-layer skin suturing of the injured radial nerve were performed. After surgery, 10 g first generation cephalosporin was orally administered, twice a day, for total 3 days in both groups to prevent infection.
Figure 5.
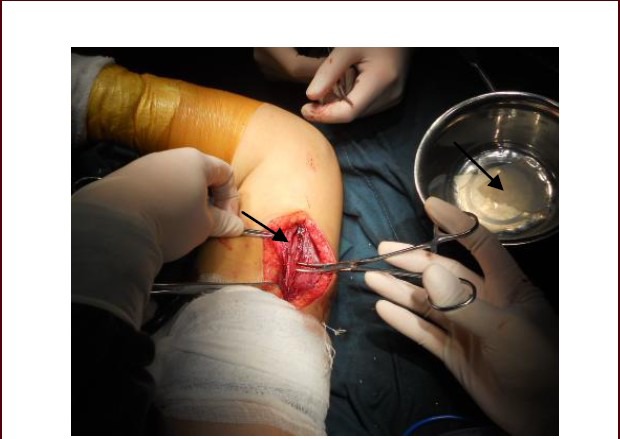
Neurolysis of the radial nerve followed by transplantation of amniotic membrane loaded with human umbilical cord mesenchymal stem cells.
The right arrow indicates the amniotic membrane and the left arrow indicates the injured radial nerve that needs to be enwrapped.
Assessment of functional recovery of injured peripheral nerve
According to the criteria formulated by the Neurotrauma Society, British Medical Academy, motor and sensory functional recovery after peripheral nerve injury was assessed[17]. Sensory function: 0, no recovery; I, recovery of skin deep sensation in the injured radial nerve-dominated region; II, partial recovery of superficial sensation and touch sensation in the dominated region; III, recovery of skin pain and touch sensation, and absence of hyperesthesia; IV, partial recovery of two point discrimination; V, complete recovery. Motor function: 0, no muscle contraction; I, proximal muscle contraction; II, proximal and distal muscle contraction; III, all important muscles can contract against resistance; IV, can perform all activities, including independent or synergetic; V; completely normal. After surgery, significant symptom improvement was designated for improvement in all sensory and motor functions by more than two grades.
Muscular electrophysiological examination
The amplitude (mV) of muscular electromyogram of the region dominated by the injured radial nerve was detected using a needle electrode to assess the functional recovery of injured radial nerve.
Safety observation
After transplantation of human amniotic membrane loaded with human umbilical cord mesenchymal stem cells, no foreign body reaction, inflammation, swelling, infection, hemorrhage, wound healing, hyperesthesia or allergic reaction was observed.
Statistical analysis
All data were statistically processed using SPSS13.0 software (SPSS, Chicago, IL, USA). Measurement data were expressed as mean ± SD. Two-sample t-test was used for comparison of the means between two groups. A level of P < 0.05 was considered statistically significant.
Research background: After peripheral nerve injury, a good microenvironment for neural regeneration protects injured neurons and promotes effective axon regeneration.
Research frontiers: A conduit fabricated from amniotic membrane, which exhibits good histocompatibility and cellular compatibility, and human umbilical cord mesenchymal stem cells is an ideal biological nerve conduit because it can be twisted, expanded and easily sutured, and provides a good microenvironment for injured peripheral nerve.
Clinical significance: This study was the first to validate the efficacy and safety of human umbilical cord mesenchymal stem cells combined with human amniotic membrane containing epithelial cells for the treatment of peripheral nerve injury, providing a novel strategy for the treatment of radial nerve injury.
Academic terminology: Nerve regeneration chambers refer to the nerve conduit, an empty conduit that guides peripheral nerve regeneration. The proximal and distal ends of injured peripheral nerve were firmly anastomosed to the two ends of the conduit. Neural regeneration is achieved within the conduit cavity.
Peer review: This study used a novel method, human umbilical cord mesenchymal stem cell-loaded amniotic membrane, to repair injured radial nerve in patients, which is an increasing research area. However, this was a non-randomized clinical trial involving a small sample size. More meaningful outcomes need to be further validated by completely randomized or multi-center clinical trials involving more cases.
Footnotes
Funding: the Science and Technology Foundation of Shenyang in China, No. F10-217-1-00.
Conflicts of interest: None declared.
Ethical approval: The therapeutic protocol received full approval by Central Hospital, Shenyang Medical College, China.
(Reviewed by Wallace M, Raye W, Zhang L, Li R)
(Edited by Li CH, Song LP, Liu WJ, Zhao M)
REFERENCES
- [1].Niver GE, Ilyas AM. Management of radial nerve palsy following fractures of the humerus. Orthop Clin North Am. 2013;44:419–424. doi: 10.1016/j.ocl.2013.03.012. [DOI] [PubMed] [Google Scholar]
- [2].Caldwell JM, Kim HM, Levine WN. Radial nerve injury associated with application of a hinged elbow external fixator: a report of 2 cases. J Shoulder Elbow Surg. 2013;22:e12–16. doi: 10.1016/j.jse.2012.11.012. [DOI] [PubMed] [Google Scholar]
- [3].Korompilias AV, Lykissas MG, Kostas-Agnantis IP, et al. Approach to radial nerve palsy caused by humerus shaft fracture: Is primary exploration necessary? Injury. doi: 10.1016/j.injury.2013.01.004. in press. [DOI] [PubMed] [Google Scholar]
- [4].Tom VJ, Sandrow-Feinberg HR, Miller K, et al. Combining peripheral nerve grafts and chondroitinase promotes functional axonal regeneration in the chronically injured spinal cord. J Neurosci. 2009;29:14881–14890. doi: 10.1523/JNEUROSCI.3641-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ning X, Li D, Wang DK, et al. Changes of biological characteristics and gene expression profile of umbilical cord mesenchymal stem cells during senescence in culture. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2012;20:458–465. [PubMed] [Google Scholar]
- [6].Can A, Karahuseyinoglu S. Concise review: human umbilical cord stroma with regard to the source of fetus-derived stem cells. Stem Cells. 2007;25:2886–2895. doi: 10.1634/stemcells.2007-0417. [DOI] [PubMed] [Google Scholar]
- [7].Koh SH, Kim KS, Choi MR, et al. Implantation of human umbilical cord-derived mesenchymal stem cells as a neuroprotective therapy for ischemic stroke in rats. Brain Res. 2008;1229:233–248. doi: 10.1016/j.brainres.2008.06.087. [DOI] [PubMed] [Google Scholar]
- [8].Yang S, Sun HM, Yan JH, et al. Conditioned medium from human amniotic epithelial cells may induce the differentiation of human umbilical cord blood mesenchymal stem cells into dopaminergic neuron-like cells. J Neurosci Res. 2013;91:978–986. doi: 10.1002/jnr.23225. [DOI] [PubMed] [Google Scholar]
- [9].Zhang L, Tan X, Dong C, et al. In vitro differentiation of human umbilical cord mesenchymal stem cells (hUCMSCs), derived from Wharton's jelly, into choline acetyltransferase (ChAT)-positive cells. Int J Dev Neurosci. 2012;30:471–477. doi: 10.1016/j.ijdevneu.2012.05.006. [DOI] [PubMed] [Google Scholar]
- [10].Hu SL, Luo HS, Li JT, et al. Functional recovery in acute traumatic spinal cord injury after transplantation of human umbilical cord mesenchymal stem cells. Crit Care Med. 2010;38:2181–2189. doi: 10.1097/CCM.0b013e3181f17c0e. [DOI] [PubMed] [Google Scholar]
- [11].Peng J, Wang Y, Zhang L, et al. Human umbilical cord Wharton's jelly-derived mesenchymal stem cells differentiate into a Schwann-cell phenotype and promote neurite outgrowth in vitro. Brain Res Bull. 2011;84:235–243. doi: 10.1016/j.brainresbull.2010.12.013. [DOI] [PubMed] [Google Scholar]
- [12].Matsuse D, Kitada M, Kohama M, et al. Human umbilical cord-derived mesenchymal stromal cells differentiate into functional Schwann cells that sustain peripheral nerve regeneration. J Neuropathol Exp Neurol. 2010;69:973–985. doi: 10.1097/NEN.0b013e3181eff6dc. [DOI] [PubMed] [Google Scholar]
- [13].Xue G, He M, Zhao J, et al. Intravenous umbilical cord mesenchymal stem cell infusion for the treatment of combined malnutrition nonunion of the humerus and radial nerve injury. Regen Med. 2011;6:733–741. doi: 10.2217/rme.11.83. [DOI] [PubMed] [Google Scholar]
- [14].Enosawa S, Sakuragawa N, Suzuki S. Possible use of amniotic cells for regenerative medicine. Nihon Rinsho. 2003;61:396–400. [PubMed] [Google Scholar]
- [15].Solomon A, Wajngarten M, Alviano F, et al. Suppression of inflammatory and fibrotic responses in allergic inflammation by the amniotic membrane stromal matrix. Clin Exp Allergy. 2005;35:941–948. doi: 10.1111/j.1365-2222.2005.02285.x. [DOI] [PubMed] [Google Scholar]
- [16].Qian YX, Shu Q, Cai HX, et al. Surface marker changes in human umbilical cord-derived mesenchymal stem cells after cryopreservation and resuscitation. Zhongguo Zuzhi Gongcheng Yanjiu yu Linchuang Kangfu. 2011;15:187–190. [Google Scholar]
- [17].Zhu SX. Changsha: Science and Technology Publishing House of Hunan; 1994. Modern Microsurgery. [Google Scholar]
- [18].Brosamle C, Huber AB, Fiedler M, et al. Regeneration of lesioned corticospinal tract fibers in the adult rat induced by a recombinant, humanized IN-1 antibody fragment. J Neurosci. 2000;20:8061–8068. doi: 10.1523/JNEUROSCI.20-21-08061.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5(2):146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- [20].Li S, Strittmatter SM. Delayed systemic Nogo-66 receptor antagonist promotes recovery from spinal cord injury. J Neurosci. 2003;23:4219–4227. doi: 10.1523/JNEUROSCI.23-10-04219.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Pizzorusso T, Medini P, Landi S, et al. Structural and functional recovery from early monocular deprivation in adult rats. Proc Natl Acad Sci U S A. 2006;103:8517–8522. doi: 10.1073/pnas.0602657103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Blesch A, Tuszynski MH. Cellular GDNF delivery promotes growth of motor and dorsal column sensory axons after partial and complete spinal cord transections and induces remyelination. J Comp Neurol. 2003;467:403–417. doi: 10.1002/cne.10934. [DOI] [PubMed] [Google Scholar]
- [23].Takami T, Oudega M, Bates ML, et al. Schwann cell but not olfactory ensheathing glia transplants improve hindlimb locomotor performance in the moderately contused adult rat thoracic spinal cord. J Neurosci. 2002;22:6670–6681. doi: 10.1523/JNEUROSCI.22-15-06670.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Zhang LX, Tong XJ, Sun XH, et al. Experimental study of low dose ultrashortwave promoting nerve regeneration after acellular nerve allografts repairing the sciatic nerve gap of rats. Cell Mol Neurobiol. 2008;28:501–509. doi: 10.1007/s10571-007-9226-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Solomon A, Wajngarten M, Alviano F, et al. Suppression of inflammatory and fibrotic responses in allergic inflammation by the amniotic membrane stromal matrix. Clin Exp Allergy. 2005;35:941–948. doi: 10.1111/j.1365-2222.2005.02285.x. [DOI] [PubMed] [Google Scholar]
- [26].Henry FP, Goyal NA, David WS, et al. Improving electrophysiologic and histologic outcomes by photochemically sealing amnion to the peripheral nerve repair site. Surgery. 2009;145:313–321. doi: 10.1016/j.surg.2008.11.005. [DOI] [PubMed] [Google Scholar]
- [27].Ozgenel GY, Fílíz G. Combined application of human amniotic membrane wrapping and hyaluronic acid injection in epineurectomized rat sciatic nerve. J Reconstr Microsurg. 2004;20:153–157. doi: 10.1055/s-2004-820772. [DOI] [PubMed] [Google Scholar]
- [28].Yoshii S, Oka M, Shima M, et al. Bridging a spinal cord defect using collagen filament. Spine (Phila Pa 1976) 2003;28:2346–2351. doi: 10.1097/01.BRS.0000085302.95413.16. [DOI] [PubMed] [Google Scholar]
- [29].Yoshii S, Oka M, Shima M, et al. Bridging a 30-mm nerve defect using collagen filaments. J Biomed Mater Res A. 2003;67:467–474. doi: 10.1002/jbm.a.10103. [DOI] [PubMed] [Google Scholar]
- [30].Toba T, Nakamura T, Lynn AK, et al. Evaluation of peripheral nerve regeneration across an 80-mm gap using a polyglycolic acid (PGA)--collagen nerve conduit filled with laminin-soaked collagen sponge in dogs. Int J Artif Organs. 2002;25:230–237. doi: 10.1177/039139880202500310. [DOI] [PubMed] [Google Scholar]
- [31].Mohammad J, Shenaq J, Rabinovsky E, et al. Modulation of peripheral nerve regeneration: a tissue-engineering approach. The role of amnion tube nerve conduit across a 1-centimeter nerve gap. Plast Reconstr Surg. 2000;105:660–666. doi: 10.1097/00006534-200002000-00027. [DOI] [PubMed] [Google Scholar]
- [32].Henry FP, Goyal NA, David WS, et al. Improving electrophysiologic and histologic outcomes by photochemically sealing amnion to the peripheral nerve repair site. Surgery. 2009;145:313–321. doi: 10.1016/j.surg.2008.11.005. [DOI] [PubMed] [Google Scholar]
- [33].O’Neill AC, Randolph MA, Bujold KE, et al. Photochemical sealing improves outcome following peripheral neurorrhaphy. J Surg Res. 2009;151:33–39. doi: 10.1016/j.jss.2008.01.025. [DOI] [PubMed] [Google Scholar]
- [34].Sankar V, Muthusamy R. Role of human amniotic epithelial cell transplantation in spinal cord injury repair research. Neuroscience. 2003;118:11–17. doi: 10.1016/s0306-4522(02)00929-6. [DOI] [PubMed] [Google Scholar]
- [35].Uchida S, Suzuki Y, Araie M, et al. Factors secreted by human amniotic epithelial cells promote the survival of rat retinal ganglion cells. Neurosci Lett. 2003;341:1–4. doi: 10.1016/s0304-3940(02)01454-4. [DOI] [PubMed] [Google Scholar]
- [36].Dalous J, Larghero J, Baud O. Transplantation of umbilical cord-derived mesenchymal stem cells as a novel strategy to protect the central nervous system: technical aspects, preclinical studies, and clinical perspectives. Pediatr Res. 2012;71:482–490. doi: 10.1038/pr.2011.67. [DOI] [PubMed] [Google Scholar]
- [37].Sun L, Wang D, Liang J, et al. Umbilical cord mesenchymal stem cell transplantation in severe and refractory systemic lupus erythematosus. Arthritis Rheum. 2010;62:2467–2475. doi: 10.1002/art.27548. [DOI] [PubMed] [Google Scholar]


