Abstract
In vitro experiments have demonstrated that neuronal-like cells derived from bone marrow mesenchymal stem cells can survive, migrate, integrate and help to restore the function and behaviors of spinal cord injury models, and that they may serve as a suitable approach to treating spinal cord injury. However, it is very difficult to track transplanted cells in vivo. In this study, we injected superparamagnetic iron oxide-labeled neuronal-like cells into the subarachnoid space in a rabbit model of spinal cord injury. At 7 days after cell transplantation, a small number of dot-shaped low signal intensity shadows were observed in the spinal cord injury region, and at 14 days, the number of these shadows increased on T2-weighted imaging. Perl's Prussian blue staining detected dot-shaped low signal intensity shadows in the spinal cord injury region, indicative of superparamagnetic iron oxide nanoparticle-labeled cells. These findings suggest that transplanted neuronal-like cells derived from bone marrow mesenchymal stem cells can migrate to the spinal cord injury region and can be tracked by magnetic resonance in vivo. Magnetic resonance imaging represents an efficient noninvasive technique for visually tracking transplanted cells in vivo.
Keywords: neural regeneration, neuronal-like cells, bone marrow mesenchymal stem cells, stem cells, bone marrow, in vivo tracking, magnetic resonance, transplantation, grants-supported paper, neuroregeneration
Research Highlights
-
(1)
In vitro experiments have demonstrated that bone marrow mesenchymal stem cell-derived neuronal-like cells can survive, migrate, integrate and restore spinal cord function in spinal cord jury models; however, there is currently no reliable method for tracking transplanted cells in vivo.
-
(2)
In this study, we tracked superparamagnetic iron oxide nanoparticle-labeled neuronal-like cells derived from bone marrow mesenchymal stem cells, which were transplanted via the subarachnoid space of rabbit models of spinal cord injury, using magnetic resonance in vivo tracking techniques.
-
(3)
The results showed that transplanted neuronal-like cells derived from bone marrow mesenchymal stem cells can migrate to the spinal cord injury region and thereby improve the functional recovery of injured spinal cord. Moreover, these cells can be tracked by magnetic resonance in vivo.
INTRODUCTION
Spinal cord injury is a devastating traumatic injury leading to the loss of neurons, serious neurological deficit and permanent invalidity. Despite extensive research, current treatment methods for spinal cord injury exhibit poor and delayed efficiency[1,2]. One promising treatment approach tested in preclinical and clinical studies is transplantation of stem cells into the damaged spinal cord[3]. Bone marrow mesenchymal stem cells have been widely used in the treatment of stroke[4,5,6,7], amyotrophic lateral sclerosis[8], and spinal cord injury[9,10], in particular. Numerous studies have demonstrated their benefit in promoting anatomic and functional recovery after transplantation into animal models of spinal cord injury [11,12]. Bone marrow mesenchymal stem cells can be induced into neurons, help to reestablish neural networks and promote the functional recovery of injured spinal cord in animal models[13]. There is evidence that bone marrow mesenchymal stem cell-derived neuronal-like cells can survive, migrate, integrate and restore spinal cord function and behavior in models of spinal cord injury, and that they may serve as a suitable approach to treating spinal cord injury[14]. The current study focused on how to noninvasively and dynamically monitor the ability of transplanted cells to migrate from the transplanted location to the relevant lesions in vivo. To investigate the efficacy of cell transplantation, grafted cells can be labeled with superparamagnetic iron oxide nanoparticles and confirmed by magnetic resonance[15]. Recently, magnetic resonance tracking superparamagnetic iron oxide-labeled cells in vivo has become a novel technique[16,17]. The purpose of this study was to investigate the feasibility of using neuronal-like cells obtained from rabbit bone marrow mesenchymal stem cells in the treatment of spinal cord injury. Furthermore, superparamagnetic iron oxide-labeled neuronal-like cells were transplanted into rabbit models of spinal cord injury through the subarachnoid space to investigate the feasibility of magnetic resonance tracking of transplanted cells in vivo.
RESULTS
Quantitative analysis of experimental animals
A total of 22 New Zealand white rabbits were initially included in this study. All rabbits were treated as described in the Methods section to establish models of spinal cord injury and received catheterization in the subarachnoid space. During induction of spinal cord injury, one rabbit died of overdose injection of anesthetics, one died of postoperative infection. The remaining 20 rabbits were randomly and equally divided into two groups: a transplantation group, in which, bone marrow mesenchymal stem cell-derived neuronal-like cells were injected into the subarachnoid space through the use of microsyringe, and a control group, in which, an equal amount of PBS was injected into the subarachnoid space as a control.
Induced differentiation of bone marrow mesenchymal stem cells into neuronal-like cells in vitro
Bone marrow mesenchymal stem cells of rabbit iliac artery were obtained in preliminary experiments. At 6 hours after induced differentiation, some bone marrow mesenchymal stem cells changed from spindle-shaped to show a round and blunt appearance. A small number of cells exhibited a polygon appearance and became star-like and spread about (Figure 1A). At 24 hours after induction, some star-like cells began to stretch out the apophysis, with reticulation by mutual connection of the intercellular protuberance. Most cells showed typical neuronal-like cell morphology, with oval-shaped, triangular or irregular appearance and bipolar or multipolar protrusions (Figure 1B).
Figure 1.

Morphological characterization of bone marrow mesenchymal stem cell-derived neuronal-like cells after in vitro induced differentiation (inverted phase contrast microscope, × 100).
(A) At 6 hours after induced differentiation, bone marrow mesenchymal stem cells became round and blunt, and some cells exhibited a polygon appearance with protuberances (arrow). (B) At 24 hours after induced differentiation, bone marrow mesenchymal stem cells exhibited a neuronal-like cell appearance and intercellular protuberances connected to form a network (arrow).
In vitro identification of bone marrow mesenchymal stem cell-derived neuronal-like cells
At 24 hours after induced differentiation, bone marrow mesenchymal stem cells differentiated into neuronal-like cells. Some neuronal-like cells were separated for detection of the expression levels of the neuronal markers neuron specific-enolase and microtubule-associated protein 2. Immunocytochemical staining showed that neuronal-like cells were immunoreactive for neuron-specific enolase and brown particles were observed in the cytoplasm (Figure 2A, B). They were also immunoreactive for microtubule-associated protein 2 and brown particles in the cytoplasm were also observed; moreover, some axons were stained brown in microtubule-associated protein 2-positive cells (Figure 2C, D).
Figure 2.
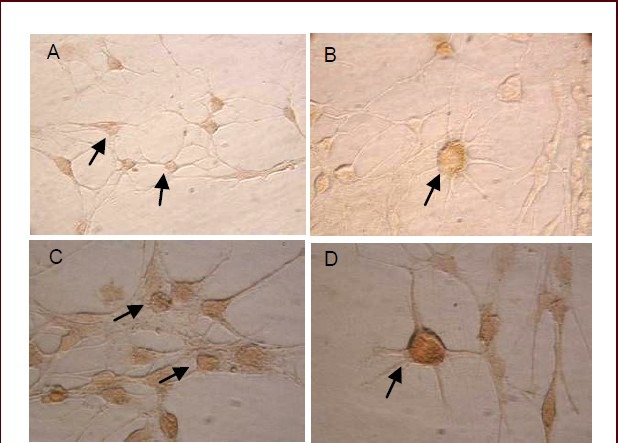
Morphological characterization of bone marrow mesenchymal stem cell-derived neuronal-like cells at 24 hours after induced differentiation (inverted phase contrast microscope, A: × 200; B–D: × 400).
Representative images of neuron-specific enolase staining (A, B). Representative images of microtubule-associated protein 2 staining (C, D) (arrows).
Morphology of bone marrow mesenchymal stem cells-derived neuronal-like cells after induced differentiation
Perl's Prussian blue staining revealed that neuronal-like cells contained blue-stained iron particles inside the cytoplasm (Figure 3). After superparamagnetic iron oxide nanoparticle labeling, the oriented differentiation capacity of bone marrow mesenchymal stem cells was not influenced, and nanoscale iron particles could be retained in the differentiated neuronal-like cells.
Figure 3.
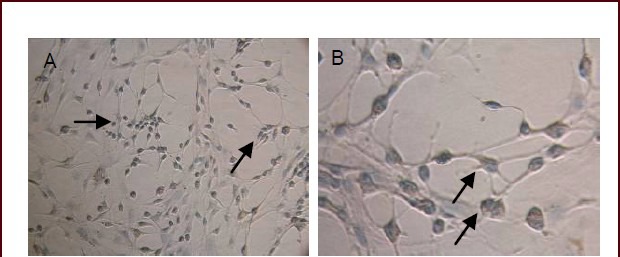
Bone marrow mesenchymal stem cell-derived neuronal-like cells at 24 hours after induced differentiation (inverted phase contrast microscope, A: × 200; B: × 400).
Blue-stained iron particles are visible in the cytoplasm of bone marrow mesenchymal stem cell-derived neuronal-like cells (arrows).
Survival of bone marrow mesenchymal stem cell-derived neuronal-like cells after in vitro induced differentiation
By laser scanning confocal microscopy, after calcein-AM/PI staining, viable cells exhibited green fluorescence while dead cells appeared red (Figure 4). After superparamagnetic iron oxide nanoparticle labeling, the survival rate of induced neuronal-like cells was 93.5%, indicating a high survival rate of induced neuronal-like cells after superparamagnetic iron oxide nanoparticle labeling.
Figure 4.
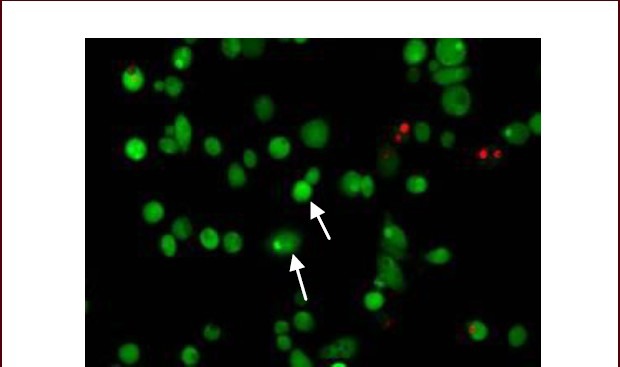
Survival of bone marrow mesenchymal stem cell-derived neuronal-like cells after in vitro induced differentiation (laser scanning confocal microscope, calcein-AM/PI staining, × 200).
Viable cell cytoplasm is green (arrows) and dead cells exhibit red nuclei.
Magnetic resonance imaging of spinal cord injury region after transplantation of neuronal-like cells
At 3 days after cell transplantation, high signal intensity shadows were present on T1- and T2-weighted images taken from the transplantation and control groups. They represent acute hematoma shadows. In the transplantation group, a small number of dot-shaped low intensity shadows were present in the spinal cord injury region on T2-weighted images at 7 days after cell transplantation (Figure 5A); more dot-shaped low signal intensity shadows were observed at 14 days (Figure 5B), and these shadows were reduced in number at 21 days (Figure 5C). However, no dot-shaped low signal intensity shadows were observed at the identical time points in the control group. These findings suggest that the transplanted neuronal-like cells labeled by superparamagnetic iron oxide nanoparticle can be dynamically tracked in vivo by magnetic resonance imaging.
Figure 5.
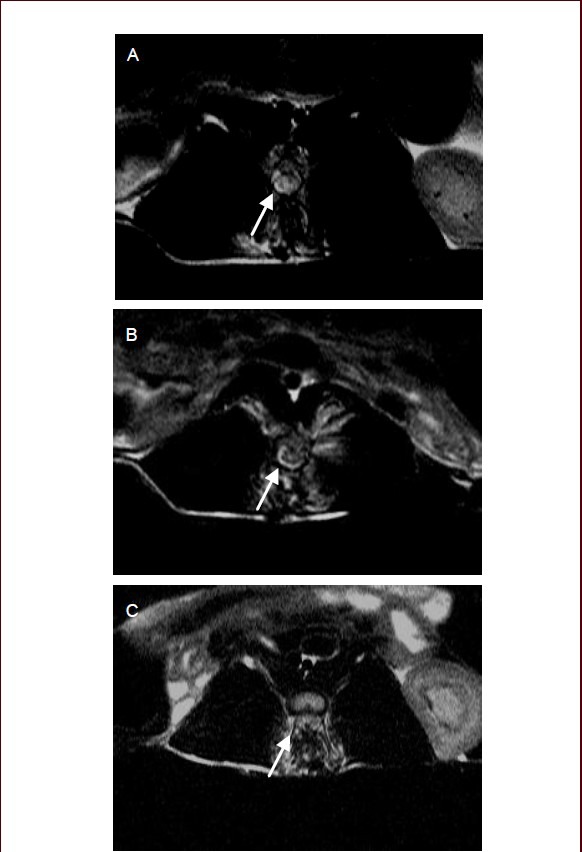
T2-weighted images of the spinal cord injury region after cell transplantation.
(A) At 7 days after cell transplantation, a small number of dot-shaped low signal intensity shadows were present in the spinal cord injury region; (B) at 14 days after cell transplantation, the low signal intensity shadows increased in number compared with that at 7 days after cell transplantation; (C) at 21 days after cell transplantation, low signal intensity shadows were reduced in number. Arrows point to dot-shaped low signal intensity shadows.
Morphology of injured spinal cord tissue
Perl's Prussian blue staining revealed that, in the transplantation group, a large number of red blood cells were observed in the spinal cord injury region, but no cells with blue-stained iron particles were observed at 3 days after cell transplantation. A small number of cells with blue-stained iron particles were present in the spinal cord injury region at 7 days after cell transplantation (Figure 6A). At 14 days after cell transplantation, more cells with blue-stained iron particles appeared in the spinal cord injury region compared with the number at 7 days after cell transplantation, and more of them accumulated in the most injured region (Figure 6B).
Figure 6.
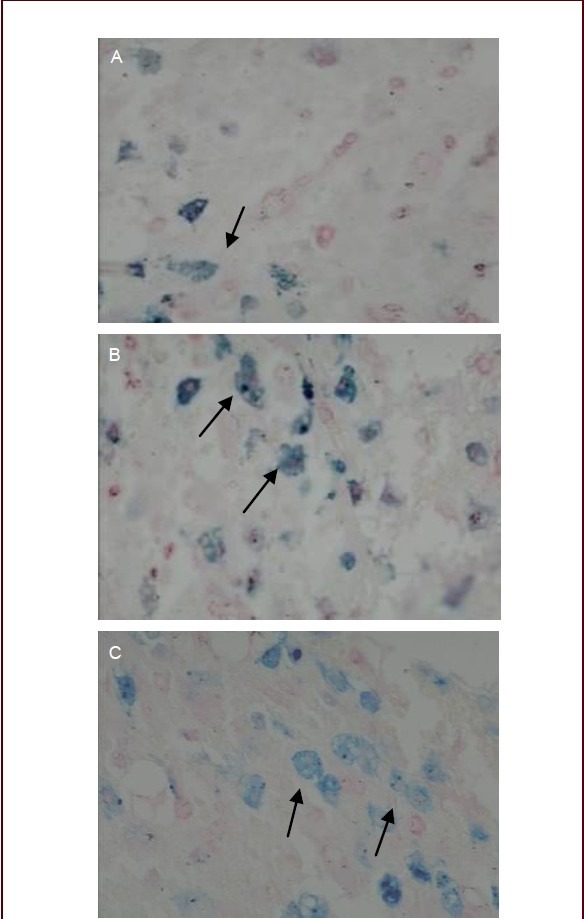
Perl's Prussian blue staining of rabbit spinal cord injury tissue after transplantation of bone marrow mesenchymal stem cell-derived neuronal-like cells (phrase contrast microscope, × 200).
At 7 (A), 14 (B) and 21 days after cell transplantation (C). Arrows indicate cells containing blue-stained iron particles.
Compared with the number at 14 days, there were more cells with blue-stained iron particles at 21 days, but the staining intensity was decreased, which occurred possibly because of the decreased level of iron particles caused by cell division and proliferation (Figure 6C). The results obtained mirrored those seen by MRI. This suggests that bone marrow mesenchymal stem cells transplanted via the subarachnoid space can survive and migrate towards the spinal cord injury region.
Functional recovery of rabbit spinal cord injury after transplantation of neuronal-like cells
At 14, 21, 28 and 35 days after spinal cord injury, Basso-Beattie-Bresnehan (BBB) locomotor rating scores in the transplantation group were significantly higher than those in the control group (P < 0.05). This suggests that bone marrow mesenchymal stem cell-derived neuronal-like cells can promote the functional recovery of rabbit spinal cord injury.
Figure 7.

Rabbit spinal cord functional recovery after transplantation of bone marrow mesenchymal stem cellderived neuronal-like cells.
aP < 0.05, vs. 7 days after cell transplantation (in the same group); bP < 0.05, vs. PBS control group (at the same time point). Mean ± SD, n = 10 rabbits per group. Repeated measures analysis of variance was used.
DISCUSSION
Stem cells are often considered as the best candidates for cell therapy owing to their self-renewal capacity and strong differentiation potential[18]. Repair after spinal cord injury remains a major problem in neuroscience. Neural stem cell transplantation in the treatment of spinal cord injury provides a novel way to address this problem[19,20]. However, application of neural stem cell transplantation is greatly hampered because neural stem cells are difficult to obtain. Bone marrow mesenchymal stem cells are easily isolated and expand and exhibit immunodepressive characteristics, which make them less sensitive to rejection by the host immune system[21,22,23].
Bone marrow mesenchymal stem cells are capable of differentiating into diverse cell types unrelated to their phenotypical embryonic origin, including nerve cells[24]. Bone marrow mesenchymal stem cells can be induced to differentiate into neuronal-like cells[25,26] and enteric neuronal-like cells, and express neuronal markers and neurotransmitters[27]. Therefore, if bone marrow mesenchymal stem cell-derived neuronal-like cells can promote functional recovery of spinal cord injury as seed cells, these cells will hold some promise for clinical application. The results from this study revealed that bone marrow mesenchymal stem cells can be induced to differentiate into neuronal-like cells and that they exhibit neuronal-like cell changes. Immunocytochemical staining detected the expression of the neuronal markers neuron-specific enolase and microtubule-associated protein 2. Neuronal-like cells may serve as a useful alternative for potential clinical applications in spinal cord injury. In other experiments, cell patch clamp recordings revealed that bone marrow mesenchymal stem cell-derived neuronal-like cells exhibit the electrophysiological properties of neurons, including action potentials. Neuronal-like cells can be efficiently derived from bone marrow mesenchymal stem cells and they may serve as a useful alternative to neural stem cells for potential clinical applications such as autologous cell replacement therapies[28]. These studies demonstrated that neuronal-like cells derived from bone marrow mesenchymal stem cell may integrate as well as restore spinal function and behavior in models of spinal cord injury.
However, it is very important to use noninvasive methods for cell tracking in vivo. Cell tracking can be used to ensure the appropriate route of transplantation, provide feedback into the preferred sites of engraftment and aid in determining the optimal dosing schedule and numbers of cells to be used to achieve the desired therapeutic outcome[29]. MRI is the most commonly used imaging modality for in vivo tracking of labeled stem cells, because it is noninvasive, generates high-resolution images, and does not rely on radioactive isotopes[30]. Cellular MRI combines the ability of MRI with contrast agents for labeling cells providing dynamic assessment of cell migration into target tissues[31]. Labeling cells with superparamagnetic iron oxide nanoparticles allows for the possibility of detecting single or clusters of labeled cells within target tissues[32].
Magnetic resonance molecular imaging, a recent microscopic imaging technology, provides high resolution and can be used to distinguish structures similar in size to cells. In magnetic resonance molecular imaging, superparamagnetic iron oxide nanoparticles enter cells through endocytosis to produce markers, and these are then injected into living bodies in which signal contrast can be used to display images by magnetic resonance scanning. Superparamagnetic iron oxide is a new type of magnetic resonance intracellular contrast agent made of nanoscale particles based on single or multi-crystalline iron oxide, which appears as low signal intensity on T2-weighted images. Heyn et al[33] detected superparamagnetic iron oxide nanoparticle-labeled cells in the brains of mice using rapid equilibrium steady state imaging sequences on 1.5-T conventional MR devices. Peldschusl[34] detected single superparamagnetic iron oxide nanoparticle-labeled bone marrow mesenchymal stem cells using 3-T MR equipment, and proposed that quantitative analysis of labeled stem cells using magnetic resonance was feasible. Magnetic resonance is an ideal means of detection and tracing of stem cells[35]. It can provide detailed non-invasive anatomical information and show the outcomes of procedures using migrating labeled cells. Magnetic resonance tracking techniques show good prospects, and this approach has give rise to increasingly widespread interest in recent years[36].
In this study, we labeled bone marrow mesenchymal stem cells using 25 μg Fe/mL superparamagnetic iron oxide nanoparticles and found that the labeled cells could be induced to differentiate into neuronal-like cells. The cell viability of these neuronal-like cells was good, laying the foundation for tracking the distribution and migration of transplanted cells by magnetic resonance in vivo. We transplanted superparamagnetic iron oxide nanoparticle-labeled neuronal-like cells into spinal cord injury models, which had been scanned by magnetic resonance. At 14 days after cell transplantation, cells with blue-stained iron particles were increased in number in the spinal cord injury region and mainly accumulated in the severely injured region. Compared with 14 days after cell transplantation, cells with blue-stained iron particles were increased in number at 21 days, but the staining intensity was slightly decreased, which may have been caused by cell division and proliferation. These findings suggest that bone marrow mesenchymal stem cell-derived neuronal-like cells transplanted via the subarachnoid space can migrate towards the spinal cord injury region and that they will gradually increase in number with time after transplantation. Cells can be tracked in vivo when the number of migrated cells reaches the threshold for detection by magnetic resonance. With the increase in cell numbers, the number of dot-shaped low signal intensity shadows increased on T2-weighted images. However, if labeled cells survive in vivo for a long time, intracellular iron content will decrease because of cell division and proliferation. Low signal intensity will continue to decline so that, ultimately, the cells cannot be tracked, which may be a defect of tracking in vitro by magnetic resonance.
Our results indicate that bone marrow mesenchymal stem cell-derived neuronal-like cells can migrate towards the spinal cord injury region and improve functional recovery, which overcomes the need to obtain neural stem cells. Furthermore, magnetic resonance can monitor these superparamagnetic iron oxide nanoparticle-labeled cells in vivo, which has important significance for evaluating the effects of cell transplantation and optimizing cell transplantation treatment of spinal cord injury.
MATERIALS AND METHODS
Design
A randomized, controlled animal experiment.
Time and setting
This experiment was performed at the Department of Molecular Biology of Shanxi Medical University, China from September 2008 to November 2011.
Materials
Twenty healthy New Zealand white rabbits, male or female, weighing 600–700 g, were provided by the Experimental Animal Center of Shanxi Medical University, China (experimental animal license No. key SCXK (Jin) 2009-0001).
The method of processing the animals was in accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals[37]. Superparamagnetic iron oxide nanoparticles (Resovist) were provided by Schering Company, Kenilworth, NJ, USA.
Methods
Superparamagnetic iron oxide nanoparticles labeling bone marrow mesenchymal stem cells
Rabbit bone marrow mesenchymal stem cells were obtained according to a previously published method[38]. Passage 3 bone marrow mesenchymal stem cells were cultured with Resovist containing 25 μg/mL superparamagnetic iron oxide nanoparticle (Schering Co., Ltd., Berlin, Germany) at 37°C, in 5% CO2 and saturated humidity for 24 hours.
In vitro induced differentiation of bone marrow mesenchymal stem cells into neuronal-like cells
Passage 3 bone marrow mesenchymal stem cells were pre-induced with 5 mL of 1 mmol/L β-mercaptoethanol (Sigma, St. Louis, MO, USA) for 24 hours, washed three times with PBS, cultured with serum-free medium (Sigma) containing 40 ng/mL basic fibroblast growth factor (Sigma) for 24 hours and finally observed under an inverted phase contrast microscope (Olympus, Tokyo, Japan).
Identification of neuronal-like cells by immunocytochemical staining
At 24 hours after induced differentiation of bone marrow mesenchymal stem cells, the neuronal markers neuron-specific enolase and microtubule-associated protein 2 were detected by immunocytochemical staining. Bone marrow mesenchymal stem cells were fixed with 4% paraformaldehyde for 30 minutes, treated with 3% H2O2 for 30 minutes to inactivate endogenous peroxidase, then with normal goat serum for 20 minutes, and incubated with rabbit anti-mouse neuron-specific enolase or microtubule-associated protein 2 monoclonal antibodies (1:200; Biosynthesis Biotechnology Co., Ltd., Beijing, China) at 4°C for 12 hours. On the next day, the cells were incubated with biotinylated goat anti-rabbit secondary antibody (1:200; Wuhan Bio-Tech Co., Ltd., China) at room temperature for 20 minutes and developed with 3,3’-diaminobenzidine at room temperature for 5–30 minutes, and finally observed under an inverted phase contrast microscope (OLYMPUS-IX70; Olympus).
Perl's Prussian blue staining of neuronal-like cells
At 24 hours after induction, bone marrow mesenchymal stem cells differentiated into neuronal-like cells. After incubation with superparamagnetic iron oxide nanoparticles, Perl's Prussian blue staining was used for detection of iron particles within the cell cultures. Superparamagnetic iron oxide nanoparticle-labeled neuronal-like cells were washed twice with D-Hank's solution (Wuhan Bio-Tech Co., Ltd., China) and fixed in 4% paraformaldehyde for 10 minutes.
After washes with distilled water, the labeling efficiency of superparamagnetic iron oxide nanoparticle was determined by manual counting of Prussian blue-stained cells.
Determination of the survival rates of differentiated neuronal-like cells by calcein-AM/PI staining
At 24 hours after induction, bone marrow mesenchymal stem cells differentiated into neuronal-like cells. Calcein-AM/PI (Dojindo Institute, Japan) staining was used to evaluate the viability of labeled neuronal-like cells and the cells were observed by confocal microscopy (Olympus; excitation 490 nm, emission 617 nm). The dead cells were marked red by propidium iodide and viable cells were marked green by calcein-AM. The unlabeled and labeled neuronal-like cells were cultured in neural induction medium, and then the cells were stained with calcein-AM/PI for 20 minutes at 1 and 6 days of induction. Three fields of view were selected randomly for counting the number of viable cells and the percentage of viable cells was calculated.
Spinal cord injury model establishment and magnetic resonance scanning
Rabbits were fixed in the prone position on an experimental table, with L1–3 spinous process and vertebral laminae exposed. Laminectomy was performed at level L2 of the spinal cord. The bone was trimmed into a circular bone window and until the spinal dura mater was expressed. A plastic sheet consistent with the diameter of the dural sac was placed on the dura mater surface. A spinal cord injury model was established through the use of a self-designed experimental device[39] (designed in Taiyuan, China and made in Beijing Zhixiang Industrial Design Co., Ltd., China) with the injury power of 60 g·cm. Spinal cord injury was considered successful when the posterior limbs of rabbits were instantly retracted and twitched once they were attacked and bilateral posterior limbs showed flaccid paralysis after recovery of consciousness. At 1 week after spinal cord injury, 50 μL of a cell suspension containing 1 × 106 neuronal-like cells was injected into the subarachnoid space of rabbits in the transplantation group using a microsyringe, and 50 μL of PBS was injected into the subarachnoid space of rabbits in the control group. At 3, 7, 14 and 21 days after cell transplantation, after anesthesia and immobilization in the position of supination, spinal cord injury rabbits were scanned by magnetic resonance.
MRI was performed using a clinical 1.5T MR (SIMENS Medical Systems, SONATA, Germany). The imaging parameters acquired were as follows: field of view = 150 mm × 150 mm, slice thickness = 3.0; and matrix size = 512 × 224. The imaging protocol consisted of (1) spin echo T1-weighted imaging (SE T1WI): repetition time = 492 ms, echo time = 12 ms; (2) spin echo T2-weighted imaging (SE T2WI): repetition time = 3 430 ms, echo time = 105 ms.
Pathological observation of spinal cord tissues at the injury site
Rabbits in each group were sacrificed at different time points and were transcardially perfused with 200 mL of 0.1 mol/L PBS (pH 7.4), followed by 400 mL PBS (pH 7.4) containing 4% paraformaldehyde. Upon dissection, the dura mater was marked at the injury epicenter and the entire spinal cord was dissected and rostrally/caudally labeled. A 3-cm piece of spinal cord tissue, centered on the injury epicenter, was excised from the spinal cord. The specimens were embedded in paraffin and sliced into 5-μm-thick sections. Then, these sections were subjected to hematoxylin-eosin staining and Perl's Prussian blue staining. Staining experiments were performed in triplicate.
Assessment of the functional recovery of spinal cord injury rabbits
The functional status of spinal cord injury rabbits was assessed at 7, 14, 21, 28 and 35 days after spinal cord injury according to the Basso-Beattie-Bresnehan locomotor rating score[40]. Ranking standard was detailed as follows: the first portion (0–7 points) evaluates the activity of hindlimb joints; the second (8–13 points) evaluates the pace and coordination of hindlimbs; the third part (14–21 points) evaluates the delicate activities of paws during locomotion. Two individuals without knowledge of the treatment independently graded neurological function.
Statistical analysis
Measurement data are expressed as mean ± SD and were analyzed using SPSS 11.5 software (SPSS, Chicago, IL, USA). Repeated measures analyses of variance were used. A value of P < 0.05 was considered statistically significant.
Acknowledgments
We thank the staff from Department of Molecular Biology, Shanxi Medical University, China, for their assistance in the induced differentiation of bone marrow mesenchymal stem cells.
Footnotes
Funding: This study was supported by a grant from Science and Technology Research Projects of Shanxi Province, No. 20120321028-02; a grant from the Scientific and Technical Foundation of Shanxi Provincial Health Department, No. 201201067; and a grant from University Research and Development Projects of Shanxi Province, No. 20131101; and grant from the National Natural Science Foundation of China, No. 81371628.
Conflicts of interest: None declared.
Ethical approval: All surgical and animal care procedures were approved by the Shanxi Medical University Animal Care and Use Committee, China.
(Reviewed by McGowan D, Raye Z, Kong XY, He SM, Shuang WB)
(Edited by Wang J, Li CH, Song LP, Liu WJ, Zhao M)
REFERENCES
- [1].Kubinová S, Syková E. Biomaterials combined with cell therapy for treatment of spinal cord injury. Regen Med. 2012;7(2):207–224. doi: 10.2217/rme.11.121. [DOI] [PubMed] [Google Scholar]
- [2].Talac R, Friedman JA, Moore MJ, et al. Animal models of spinal cord injury for evaluation of tissue engineering treatment strategies. Biomaterials. 2004;25(9):1505–1510. doi: 10.1016/s0142-9612(03)00497-6. [DOI] [PubMed] [Google Scholar]
- [3].Arboleda D, Forostyak S, Jendelova P, et al. Transplantation of predifferentiated adipose-derived stromal cells for the treatment of spinal cord injury. Cell Mol Neurobiol. 2011;31(7):1113–1122. doi: 10.1007/s10571-011-9712-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kim J, Kim IS, Cho TH, et al. Bone regeneration using hyaluronic acid-based hydrogel with bone morphogenic protein-2 and human mesenchymal stem cells. Biomaterials. 2007;28(10):1830–1837. doi: 10.1016/j.biomaterials.2006.11.050. [DOI] [PubMed] [Google Scholar]
- [5].Fan H, Hu Y, Zhang C, et al. Cartilage regeneration using mesenchymal stem cells and a PLGA-gelatin/chondroitin/hyaluronate hybrid scaffold. Biomaterials. 2006;27(26):4573–4580. doi: 10.1016/j.biomaterials.2006.04.013. [DOI] [PubMed] [Google Scholar]
- [6].Gaebel R, Ma N, Liu J, et al. Patterning human stem cells and endothelial cells with laser printing for cardiac regeneration. Biomaterials. 2011;32(35):9218–9230. doi: 10.1016/j.biomaterials.2011.08.071. [DOI] [PubMed] [Google Scholar]
- [7].Borlongan CV, Glover LE, Tajiri N, et al. The great migration of bone marrow-derived stem cells toward the ischemic brain: therapeutic implications for stroke and other neurological disorders. Prog Neurobiol. 2011;95(2):213–228. doi: 10.1016/j.pneurobio.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Forostyak S, Jendelova P, Kapcalova M, et al. Mesenchymal stromal cells prolong the lifespan in a rat model of amyotrophic lateral sclerosis. Cytotherapy. 2011;13(9):1036–1046. doi: 10.3109/14653249.2011.592521. [DOI] [PubMed] [Google Scholar]
- [9].Kubinová S, Syková E. Nanotechnology for treatment of stroke and spinal cord injury. Nanomedicine (Lond) 2010;5(1):99–108. doi: 10.2217/nnm.09.93. [DOI] [PubMed] [Google Scholar]
- [10].Urdzikova L, Jendelova P, Glogarova K, et al. Transplantation of bone marrow stem cells as well as mobilization by granulocyte-colony stimulating factor promotes recovery after spinal cord injury in rats. J Neurotrauma. 2006;23(9):1379–1391. doi: 10.1089/neu.2006.23.1379. [DOI] [PubMed] [Google Scholar]
- [11].Osaka M, Honmou O, Murakami T, et al. Intravenous administration of mesenchymal stem cells derived from bone marrow after contusive spinal cord injury improves functional outcome. Brain Res. 2010;1343:226–235. doi: 10.1016/j.brainres.2010.05.011. [DOI] [PubMed] [Google Scholar]
- [12].Kamada T, Koda M, Dezawa M, et al. Transplantation of bone marrow stromal cell-derived Schwann cells promotes axonal regeneration and functional recovery after complete transaction of adult rat spinal cord. J Neuropathol Exp Neurol. 2005;64(1):37–45. doi: 10.1093/jnen/64.1.37. [DOI] [PubMed] [Google Scholar]
- [13].Park SS, Lee YJ, Lee SH, et al. Functional recovery after spinal cord injury in dogs treated with a combination of Matrigel and neural-induced adipose-derived mesenchymal Stem cells. Cytotherapy. 2012;14(5):584–597. doi: 10.3109/14653249.2012.658913. [DOI] [PubMed] [Google Scholar]
- [14].Zhang ZS, Wen YM. Bone marrow mesenchymal stem cells derived neuron-like cells for spinal cord injury of adult rats. Xiandai Shengwu Yixue Jinzhan. 2010;10(22):4227–4230. [Google Scholar]
- [15].Willenbrock S, Knippenberg S, Meier M, et al. In vivo MRI of intraspinally injected SPIO-labelled human CD34+ cells in a transgenic mouse model of ALS. In Vivo. 2012;26(1):31–38. [PubMed] [Google Scholar]
- [16].Ren Z, Wang J, Zou C, et al. Labeling of cynomolgus monkey bone marrow-derived mesenchymal stem cells for cell tracking by multimodality imaging. Sci China Life Sci. 2011;54(11):981–987. doi: 10.1007/s11427-011-4239-x. [DOI] [PubMed] [Google Scholar]
- [17].Delcroix GJ, Jacquart M, Lemaire L, et al. Mesenchymal and neural stem cells labeled with HEDP-coated SPIO nanoparticles: In vitro characterization and migration potential in rat brain. Brain Res. 2009;19(1255):18–31. doi: 10.1016/j.brainres.2008.12.013. [DOI] [PubMed] [Google Scholar]
- [18].Chickera S, Willert C, Mallet C, et al. Cellular MRI as a suitable, sensitive non-invasive modality for correlating in vivo migratory efficiencies of different dendritic cell populations with subsequent immunological outcomes. Int Immunol. 2012;24(1):29–41. doi: 10.1093/intimm/dxr095. [DOI] [PubMed] [Google Scholar]
- [19].Lu P, Wang Y, Graham L, et al. Long-distance growth and connectivity of neural stem cells after severe spinal cord injury. Cell. 2012;150(6):1264–1273. doi: 10.1016/j.cell.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Xu W, Wang X, Li P, et al. miR-124 regulates neural stem cells in the treatment of spinal cord injury. Neurosci Lett. 2012;19(12):1243–1248. doi: 10.1016/j.neulet.2012.09.025. [DOI] [PubMed] [Google Scholar]
- [21].Le Blanc K, Tammik C, Rosendahl K, et al. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol. 2003;31(10):890–896. doi: 10.1016/s0301-472x(03)00110-3. [DOI] [PubMed] [Google Scholar]
- [22].Maitra B, Szekely E, Gjini K, et al. Human mesenchymal stem cells support unrelated donor hematopoietic stem cells and suppress T-cell activation. Bone Marrow Transplant. 2004;33(6):597–604. doi: 10.1038/sj.bmt.1704400. [DOI] [PubMed] [Google Scholar]
- [23].Nasef A, Mathieu N, Chapel A, et al. Immunosuppressive effects of mesenchymal stem cells: involvement of HLA-G. Transplantation. 2007;84(2):231–237. doi: 10.1097/01.tp.0000267918.07906.08. [DOI] [PubMed] [Google Scholar]
- [24].Nakamura S, Yamada Y, Baba S. Culture medium study of human mesenchymal stem cells for practical use of tissue engineering and regenerative medicine. Biomed Mater Eng. 2008;18(3):129–136. [PubMed] [Google Scholar]
- [25].Bae KS, Park JB, Kim HS, et al. Neuron-like differentiation of bone marrow-derived mesenchymal stem cells. Yonsei Med J. 2011;52(3):401–412. doi: 10.3349/ymj.2011.52.3.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Alexanian AR. An efficient method for generation of neural-like cells from adult human bone marrow-derived mesenchymal stem cells. Regen Med. 2010;5(6):891–900. doi: 10.2217/rme.10.67. [DOI] [PubMed] [Google Scholar]
- [27].Gao H, Wei M, Wang Y, et al. Differentiation of GDNF and NT-3 dual gene-modified rat bone marrow mesenchymal stem cells into enteric neuron-like cells. J Huazhong Univ Sci Technolog Med Sci. 2012;32(1):87–91. doi: 10.1007/s11596-012-0015-9. [DOI] [PubMed] [Google Scholar]
- [28].Ma K, Fox L, Shi G, et al. Generation of neural stem cell-like cells from bone marrow-derived human mesenchymal stem cells. Neurol Res. 2011;33(10):1083–1093. doi: 10.1179/1743132811Y.0000000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Lijkwan MA, Bos EJ, Wu JC, et al. Role of molecular imaging in stem cell therapy for myocardial restoration. Trends Cardiovasc Med. 2010;20(6):183–188. doi: 10.1016/j.tcm.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Barnett BP, Ruiz-Cabello J, Hota P, et al. Use of perfluorocarbon nanoparticles for non-invasive multimodal cell tracking of human pancreatic islets. Contrast Media Mol Imaging. 2011;6(4):251–259. doi: 10.1002/cmmi.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Mamani JB, Malheiros JM, Cardoso EF, et al. In vivo magnetic resonance imaging tracking of C6 glioma cells labeled with superparamagnetic iron oxide nanoparticles. Einstein (Sao Paulo) 2012;10(2):164–170. doi: 10.1590/s1679-45082012000200009. [DOI] [PubMed] [Google Scholar]
- [32].Saito K, Yoshimura N, Saguchi T, et al. MR characterization of focal nodular hyperplasia: gadoxetic acid versus superparamagnetic iron oxide imaging. Magn Reson Med Sci. 2012;11(3):163–169. doi: 10.2463/mrms.11.163. [DOI] [PubMed] [Google Scholar]
- [33].Heyn C, Ronald JA, Mackenzie LT, et al. In vivo magnetic resonance imaging of single cells in mouse brain with optical validation. Magn Reson Med. 2006;55(1):23–29. doi: 10.1002/mrm.20747. [DOI] [PubMed] [Google Scholar]
- [34].Peldschus K, Kaul M, Lange C, et al. Magnetic resonance imaging of single SPIO labeled mesenchymal stem cells at 3 Tesla. Rofo. 2007;179(5):473–479. doi: 10.1055/s-2006-927370. [DOI] [PubMed] [Google Scholar]
- [35].Reddy AM, Kwak BK, Shim HJ, et al. In vivo tracking of mesenchymal stem cells labeled with a novel chitosan-coated superparamagnetic iron oxide nanoparticles using 3.0T MRI. J Korean Med Sci. 2010;25(2):211–219. doi: 10.3346/jkms.2010.25.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Novotna B, Jendelova P, Kapcalova M, et al. Oxidative damage to biological macromolecules in human bone marrow mesenchymal stromal cells labeled with various types of iron oxide nanoparticles. Toxicol Lett. 2012;210(1):53–63. doi: 10.1016/j.toxlet.2012.01.008. [DOI] [PubMed] [Google Scholar]
- [37].The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals 2006-09-30 [Google Scholar]
- [38].Zhang RP, Liu Qiang, Li JD, et al. Biological characteristics and MR imaging of superparamagnetic iron oxide labeled BMSCs. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2009;23(7):851–855. [PubMed] [Google Scholar]
- [39].Shuang WB, Liu Q. The structure and use of self-designed experimental device on preparation animal model of spinal cord injury. Shiyong Yiji Zazhi. 2010;17(8):703–705. [Google Scholar]
- [40].Basso DM, Beattie MS, Bresnahan JC, et al. MASCIS evaluation of open field locomotor scores: effects of experience and teamwork on reliability. Multicenter Animal Spinal Cord Injury Study. J Neurotrauma. 1996;13(7):343–359. doi: 10.1089/neu.1996.13.343. [DOI] [PubMed] [Google Scholar]


