Keywords: neural regeneration, peripheral nerve injury, iron overload, oxidative stress, diabetic peripheral neuropathy, reactive oxygen species, high glucose, PC12 cells, Nrf2/ARE, grants-supported paper, neuroregeneration
Abstract
Iron overload can lead to cytotoxicity, and it is a risk factor for diabetic peripheral neuropathy. However, the underlying mechanism remains unclear. We conjectured that iron overload-induced neurotoxicity might be associated with oxidative stress and the NF-E2-related factor 2 (Nrf2)/ARE signaling pathway. As an in vitro cellular model of diabetic peripheral neuropathy, PC12 cells exposed to high glucose concentration were used in this study. PC12 cells were cultured with ferric ammonium citrate at different concentrations to create iron overload. PC12 cells cultured in ferric ammonium citrate under high glucose concentration had significantly low cell viability, a high rate of apoptosis, and elevated reactive oxygen species and malondialdehyde levels. These changes were dependent on ferric ammonium citrate concentration. Nrf2 mRNA and protein expression in the ferric ammonium citrate groups were inhibited markedly in a dose-dependent manner. All changes could be inhibited by addition of deferoxamine. These results indicate that iron overload aggravates oxidative stress injury in neural cells under high glucose concentration and that the Nrf2/ARE signaling pathway might play an important role in this process.
INTRODUCTION
Diabetic peripheral neuropathy is one of most common chronic complications induced by diabetic hyperglycemia, and is associated with axonal atrophy, blunted regenerative potential, demyelination, and loss of peripheral nerve fibers[1]. Although numerous factors contribute to diabetic peripheral neuropathy, including insulin-induced resistance to neuronal trophic support[2], decreased (Na/K)-ATP-ase activity[3] and Schwann cell dysfunction[4], increased oxidative stress and mitochondrial dysfunction seem intimately associated with nerve dysfunction and diminished regenerative capacity. Oxidative stress and apoptosis have been found to play crucial roles in diabetic peripheral neuropathy[5,6]. Under hyperglycemia, large amounts of reactive oxygen species are produced by the mitochondrial respiratory chain, and neuronal apoptosis is increased[7]. Despite advances in understanding the etiology of diabetic peripheral neuropathy, few approved therapies exist for the pharmacological management of the disease. Therefore, identifying novel therapeutic strategies remains paramount.
Iron is ubiquitous in cells and is essential for biological functioning. Normal iron balance is maintained by meticulous regulation of its absorption from the intestine and release from macrophages. It is modulated in response to requirement from body iron stores and demand from erythropoiesis to prevent deleterious extremes of iron deficiency or excess[8]. However, without adequate management, excess amounts of free iron may cause progressive damage. In recent years, there has been increasing interest in brain iron metabolism during normal ageing, particularly as excessive iron deposition has been found in neurological disorders[9]. Iron overload is also a risk factor for diabetes. The link between iron and diabetes was first recognized in pathologic conditions (hereditary hemochromatosis and thalassemia), but high levels of dietary iron also confer diabetes risk. Iron plays a direct and causal role in diabetes pathogenesis, which involves both β cell failure and insulin resistance. Iron also regulates metabolism in most tissues involved in energy homeostasis, with the adipocyte in particular having an iron-sensing role. The molecular mechanisms underlying these processes are numerous and incompletely understood, but include oxidative stress and the modulation of adipokine and intracellular signal transduction pathways[10].
A large body of evidence shows that iron overload is closely related to diabetes mellitus as well as its chronic complications[11,12,13,14,15]; oxidative stress and inflammatory factors may play a pivotal role in this relationship[16]. However, there is no direct evidence on whether abnormal iron metabolism is related to diabetic neuropathy.
In this study, we made use of a cellular model of diabetic peripheral neuropathy using PC12 cells exposed to high glucose concentration, and examined cell viability and apoptosis under iron overload. We measured the levels of reactive oxygen species and malondialdehyde, and the expression of the transcriptional activator NF-E2-related factor 2 (Nrf2).
RESULTS
Iron overload aggravated high glucose concentration-induced neurotoxicity in PC12 cells
Hyperglycemia was recently shown to induce oxidative stress and generate reactive oxygen species in neurons, resulting in neuronal damage and dysfunction[17]. In addition, high glucose induced oxidative damage in PC12 cells[18]. Thus, we generated a cell culture model of diabetic peripheral neuropathy by culturing PC12 cells in high glucose (25 mmol/L). Iron overload was created by exposure to ferric ammonium citrate[19]. To determine the appropriate experimental concentration of ferric ammonium citrate, PC12 cells cultured under high glucose (25 mmol/L) were exposed to 12 different concentrations of the compound (0, 12.5, 25, 50, 100, 200, 300, 400, 500, 600, 700, 800 μmol/L) for 24 hours.
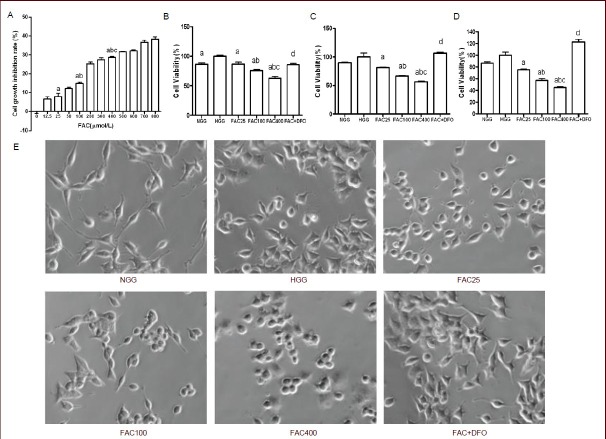
The 3-(4,5-dimethylthiazol-2-yl)2,5-diphenyl tetrazolium bromide (MTT) assay was used to assess PC12 cell growth inhibition. Ferric ammonium citrate inhibited the growth of PC12 cells in a concentration-dependent manner (Figure 1A). The degree of growth inhibition could be divided into three phases according to ferric ammonium citrate concentration—the initial phase (less than 50 μmol/L), the rapid rising phase (50–200 μmol/L), and the plateau phase (more than 200 μmol/L). The growth inhibitory effect was statistically significant when the 25, 100 and 400 μmol/L treatment doses were compared with each other (P < 0.05 or P < 0.01). Hence, we chose these three concentrations of ferric ammonium citrate for use in the subsequent experiments.
Figure 1.
PC12 cell growth and viability were inhibited by ferric ammonium citrate (FAC) and rescued by deferoxamine (DFO) (3-(4,5-dimethylthiazol-2-yl)2,5-diphenyl tetrazolium bromide assay).
(A) The growth inhibition ratio of PC12 cells after FAC treatment. Cells were exposed to 12 different concentrations of FAC (0, 12.5, 25, 50, 100, 200, 300, 400, 500, 600, 700 and 800 μmol/L) under high glucose (25 μmol/L) for 24 hours. The cell viability of PC12 cells after 24 hours (B), 48 hours (C) and 72 hours (D) of culture. Cell morphology of PC12 cells at 48 hours showing that cells subjected to high glucose and/or FAC treatment failed to extend long neurites compared with the normal glucose concentration group. Deferoxamine protected PC12 cells by promoting neurite growth and cell proliferation(E).
(A–D) All data are the percentage to control PC12 cells without supplementation with glucose, FAC or DFO. Data are shown as mean ± SD from triplicate experiments. One-way analysis of variance was adopted for multiple-group comparison; two-tailed Student's t-test was used for intergroup comparison. aP < 0.05, vs. HGG; bP < 0.01, vs. 25 μmol/L FAC group; cP < 0.01, vs. 100 μmol/L FAC group; dP < 0.01, vs. 400 μmol/L FAC group.
NGG: Normal glucose concentration group; HGG: high glucose concentration group; FAC25: 25 μmol/L FAC group; FAC100: 100 μmol/L FAC group; FAC400: 400 μmol/L FAC group; FAC + DFO: 400 μmol/L FAC + 200 μmol/L DFO group.
Deferoxamine is a chelating agent used to remove excess free iron from the body. Therefore, we examined the effect of deferoxamine on our cell culture model of diabetic peripheral neuropathy. After PC12 cells were exposed to high glucose (25 mmol/L) for 24 hours, ferric ammonium citrate and/or deferoxamine were added and the cells were cultured for an additional 24, 48 or 72 hours, and cell viability was assessed (Figure 1B-D).
Interestingly, our data showed that cell viability in the high glucose concentration group was significantly higher than in the normal glucose concentration group 24 hours after adding the drug (P < 0.05), but there was no significant difference at 48 or 72 hours (P > 0.05). Irrespective of how long PC12 cells were cultured (24, 48 or 72 hours), cell viability after ferric ammonium citrate treatment was significantly lower than in the high glucose concentration group (P < 0.05). At higher ferric ammonium citrate concentrations, there was a statistically higher toxicity compared with the lower doses (P < 0.05). Furthermore, compared with the 400 μmol/L ferric ammonium citrate group, the ferric ammonium citrate + deferoxamine group had significantly higher cell viability (P < 0.01). These results indicate that deferoxamine rescues PC12 cells under iron overload and high glucose. PC12 cells subjected to high glucose and/or ferric ammonium citrate treatment failed to extend long neurites compared with the normal glucose concentration group. Deferoxamine protected PC12 cells by promoting neurite growth and cell proliferation (Figure 1E).
To further evaluate the neurotoxicity of ferric ammonium citrate, annexin V-FITC/PI staining and flow cytometry analysis were performed to assess apoptosis in PC12 cells (Figure 2). Similar to the cell viability results, the apoptosis rate in the normal glucose concentration group was significantly higher than that in the high glucose concentration group 48 hours after adding the iron compound (P < 0.05). The apoptosis rates in PC12 cells cultured for 48 hours in the three different ferric ammonium citrate concentrations were significantly higher than the apoptosis rate in the high glucose concentration group (P < 0.05). In addition, the apoptosis rate in PC12 cells rose in tandem with increasing ferric ammonium citrate concentration. Compared with the 400 μmol/L ferric ammonium citrate group, ferric ammonium citrate combined with deferoxamine significantly reduced the apoptosis rate (P < 0.01), indicating that deferoxamine inhibits neurotoxicity by chelating excess iron extracellularly.
Figure 2.
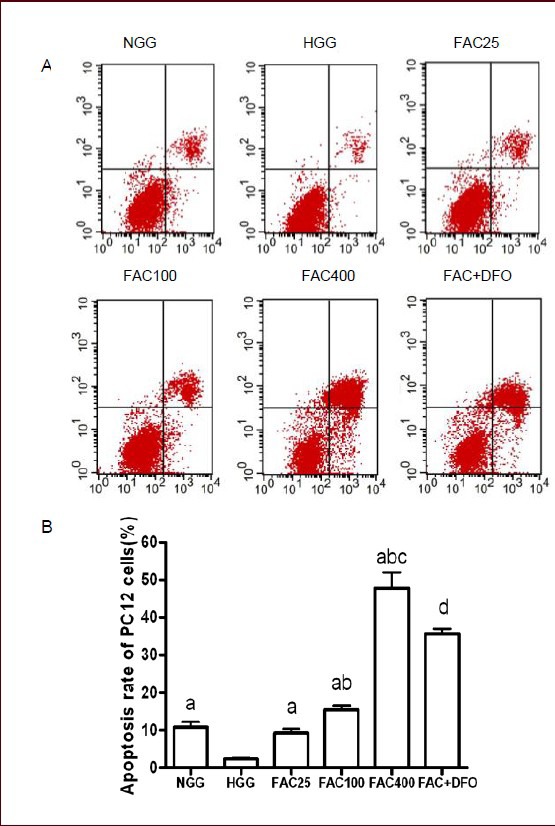
Apoptosis rate was increased by ferric ammonium citrate (FAC) and decreased by deferoxamine (DFO) in PC12 cells after 48 hours of culture (annexin V-FITC/PI staining and flow cytometry).
(A) Apoptosis rate of PC12 cells in the six groups was measured by annexin V-FITC/PI staining and flow cytometry. X-axis: The number of cells positive for annexin V-FITC staining; Y-axis: the number of propidium iodide-stained cells. First quadrant: Annexin V-FITC and propidium iodide double-stained cells, representing late apoptotic cells. Second quadrant: Annexin V-FITC-negative and propidium iodide-positive cells, representing necrotic cells. Third quadrant: Annexin V-FITC-negative and propidium iodide-negative cells, representing normal cells. Fourth quadrant: Annexin V-FITC-positive and propidium iodide-negative cells, representing early apoptotic cells. (B) A histogram of the apoptosis rate of PC12 cells in the different groups. FAC increased apoptosis rate in a concentration-dependant manner. DFO rescued the neurotoxicity caused by FAC.
Data are shown as mean ± SD from triplicate experiments. One-way analysis of variance was adopted for multiple-group comparison. Two-tailed Student's t-test was used for intergroup comparison. aP < 0.05, vs. HGG; bP < 0.01, vs. 25 μmol/L FAC group; cP < 0.01, vs. 100 μmol/L FAC group; dP < 0.01, vs. 400 μmol/L FAC group.
NGG: Normal glucose concentration group; HGG: high glucose concentration group; FAC25: 25 μmol/L FAC group; FAC100: 100 μmol/L FAC group; FAC400: 400 μmol/L FAC group; FAC + DFO: 400 μmol/L FAC + 200 μmol/L DFO group.
Iron overload increased intracellular reactive oxygen species and malondialdehyde levels in PC12 cells
To determine whether iron overload induced apoptosis via oxidative stress, reactive oxygen species and malondialdehyde levels in PC12 cells were measured after treatment with ferric ammonium citrate and/or deferoxamine for 48 hours (Figure 3). Compared with the normal glucose concentration group, the reactive oxygen species and malondialdehyde levels were significantly higher in the high glucose concentration group (P < 0.01 or P < 0.05).
Figure 3.
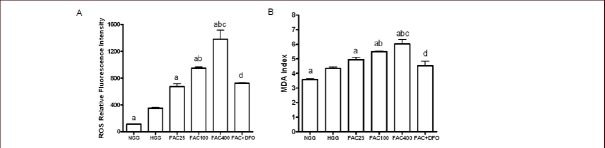
Reactive oxygen species (ROS) and malondialdehyde (MDA) levels were increased by ferric ammonium citrate (FAC) and decreased by deferoxamine (DFO) in PC12 cells after 48 hours of culture (flow cytometry).
(A) ROS level was significantly higher in FAC groups under high glucose concentration compared to HGG. ROS level was significantly decreased after adding DFO.
(B) MDA index was significantly increased in FAC groups under high glucose concentration compared to HGG. MDA index was significantly decreased after adding DFO.
Data are shown as mean ± SD from triplicate experiments. One-way analysis of variance was adopted for multiple-group comparison. Two-tailed Student's t-test was used for intergroup comparison. aP < 0.05, vs. HGG; bP < 0.05, vs. 25 μmol/L FAC group; cP < 0.05, vs. 100 μmol/L FAC group; dP < 0.05, vs. 400 μmol/L FAC group.
NGG: Normal glucose concentration group; HGG: high glucose concentration group; FAC25: 25 μmol/L FAC group; FAC100: 100 μmol/L FAC group; FAC400: 400 μmol/L FAC group; FAC + DFO: 400 μmol/L FAC + 200 μmol/L DFO group.
Reactive oxygen species and malondialdehyde levels were significantly higher in the ferric ammonium citrate groups compared with the high glucose concentration group (P < 0.01 or P < 0.05). These levels rose in parallel with increasing ferric ammonium citrate concentration. Malondialdehyde and reactive oxygen species levels in the ferric ammonium citrate + deferoxamine group were significantly lower than those in the 400 μmol/L ferric ammonium citrate group (P < 0.05).
Iron overload inhibited Nrf2 expression in PC12 cells
To explore whether the Nrf2/ARE signaling pathway participates in oxidative stress induced by iron overload in PC12 cells, we analyzed Nrf2 mRNA expression using real-time PCR and Nrf2 protein expression using western blot assay.
The amplification curves for the quantitative real-time PCR were smooth, and each curve had a significant exponential amplification period (data not shown), indicating that the assay could be used for the experiment. The amplification efficiency of the target and reference genes were consistent. Thus, the 2-ΔΔCt method could be used for the relative quantification of mRNA. Compared with the normal glucose concentration group, Nrf2 mRNA expression in the high glucose concentration group was significantly lower (P < 0.05; Figure 4A). Nrf2 mRNA levels in the ferric ammonium citrate groups (100 and 400 μmol/L, but not 25 μmol/L) were significantly lower than in the high glucose concentration group, and the reductions in expression were dose-dependent (P < 0.05; Figure 4A). In PC12 cells treated with ferric ammonium citrate and deferoxamine, expression of Nrf2 mRNA was relatively higher. The difference was statistically significant when compared with the 400 μmol/L ferric ammonium citrate group (P < 0.05; Figure 4A).
Figure 4.
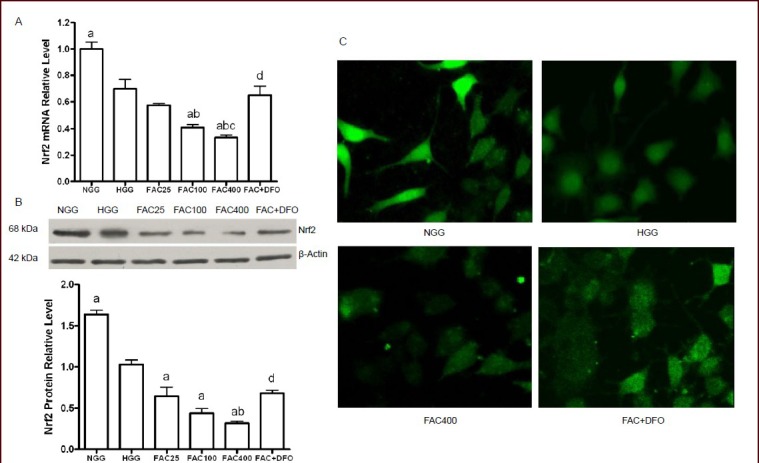
Iron overload inhibits NF-E2-related factor 2 (Nrf2) expression in PC12 cells after 48 hours of culture.
(A) Quantification of Nrf2 mRNA in PC12 cells in the different groups as shown by quantitative PCR. â-Actin mRNA served as an internal standard. The relative levels of Nrf2 mRNA were analyzed using the comparative threshold cycle method (2 -ÄÄCt).
(B) Western blot assay results showing Nrf2 protein expression in PC12 cells. Nrf2 protein level is expressed as the absorbance ratio to â-actin.
(C) Immunofluorescence microscopy on PC12 cells showing that Nrf2 localizes both in the cytoplasm and nucleus, and that it is primarily localized in the nucleus in the NGG and HGG. In the 400 μmol/L FAC group, the fluorescence intensity was reduced and labeling was dispersed. Deferoxamine promoted the expression of Nrf2 in the FAC + DFO group.
(A, B) Data are shown as mean ± SD from triplicate experiments. One-way analysis of variance was adopted for multiple-group comparison. Two-tailed Student's t-test was used for intergroup comparison. aP < 0.05, vs. HGG; bP < 0.05, vs. 25 μmol/L FAC group; cP < 0.05, vs. 100 μmol/L FAC group; dP < 0.05, vs. 400 μmol/L FAC group.
NGG: Normal glucose concentration group; HGG: high glucose concentration group; FAC25: 25 μmol/L FAC group; FAC100: 100 μmol/L FAC group; FAC400: 400 μmol/L FAC group; FAC + DFO: 400 μmol/L FAC + 200 μmol/L DFO group.
Western blot analysis was used to determine the levels of Nrf2 protein (Figure 4B). PC12 cells in the high glucose concentration group had reduced Nrf2 protein expression compared with the normal glucose concentration group (P < 0.05; Figure 4C). Furthermore, we found that the relative protein levels of Nrf2 in PC12 cells were significantly decreased by ferric ammonium citrate treatment (25, 100, and 400 μmol/L) compared with the high glucose concentration group (P < 0.05; Figure 4C). The relative protein level of Nrf2 was significantly decreased by 400 μmol/L ferric ammonium citrate treatment compared with the 25 μmol/L ferric ammonium citrate group (P < 0.05; Figure 4C). Similar to its effect on Nrf2 mRNA expression, deferoxamine treatment resulted in higher Nrf2 protein expression (in the ferric ammonium citrate + deferoxamine group compared with the 400 μmol/L ferric ammonium citrate group; P < 0.05; Figure 4C).
DISCUSSION
The pathogenesis of diabetic peripheral neuropathy is complex and involves an intricate web of mechanisms. The majority of studies have focused on glucose and lipid metabolism disorders, oxidative stress, apoptosis and autoimmune dysfunction.
Numerous studies[20,21] have shown that oxidative stress plays a major role in the disease, and have implicated the polyol pathway, microangiopathy, advanced glycation end product formation, protein kinase C pathway activation, and the hexosamine biosynthetic pathway.
Oxidative stress induced by iron overload is a major contributor to neural cell injury[22]. In vitro experiments have shown that neural cells cultured with high concentrations of iron (≥ 10 μmol/L) die in large quantities after 7 days because of oxidative stress[23]. In diabetic rats, both motor and sensory nerve conduction velocities in the sciatic nerve can be restored by adding deferoxamine in the diet, and the drug was found to increase endoneurial blood flow[24,25].
The PC12 cell is often used as a neuron model because of its characteristic neuronal features. PC12 cells exposed to high glucose are often employed as in vitro model of diabetic peripheral neuropathy[26]. Therefore, in this study, we investigated the effect of iron overload on oxidative stress in diabetic peripheral neuropathy using this PC12 cell model. Here, we found that iron overload (induced with ferric ammonium citrate) inhibited the growth of PC12 cells, induced apoptosis, increased the levels of reactive oxygen species and malondialdehyde, and reduced the expression of Nrf2. It is interesting that the viability of PC12 cells in the high glucose concentration group was higher than in the normal glucose concentration group after 24 hours in culture. Concomitantly, the apoptotic rate was decreased in the high glucose concentration group compared with the normal glucose concentration group at 24 hours of culture. We speculate that stress caused by 25 mmol/L glucose protects cells acutely, but is toxic in the long term. This is supported by another study which showed that short pretreatment with high glucose protects H9c2 cells against hypoxia[27]. In this study, cell viability and apoptotic rate were not significantly different between the high glucose concentration and normal glucose concentration groups at 48 or 72 hours. Although the findings support our notion, further study is required to clarify how glucose protects cells in the short term.
Global clinical data and epidemiological investigations show that there is an intimate relationship between in vitro iron overload and diabetes mellitus (type 1, type 2 or gestational diabetes mellitus) and its chronic complications[28,29,30,31,32]. Hereditary hemochromatosis is a typical example of iron overload leading to diabetes. Iron overload results in apoptosis of pancreatic beta cells, which results in reduced insulin secretion. Furthermore, liver iron deposition can cause insulin resistance, impairing glucose tolerance. Bloodletting therapy can reverse pancreatic islet injury in patients with diabetes mellitus. Another study found that therapeutic bloodletting alleviates glucose metabolism disorder in 35–45% of hereditary hemochromatosis patients[33].
Accumulating evidence indicates that iron overload plays a pathological role in diabetic complications[34]. It has been recognized that in diabetic patients, compensatory mechanisms are impaired by hyperglycemia or hyperlipidemia, which makes tissues and organs more susceptible to oxidative stress injury[35,36,37]. Therefore, complications, including macrovascular and microvascular disorders (cardiomyopathy, neuropathy, retinopathy, nephropathy) and vascular dysfunction (arteriosclerosis and hypertension), may be enhanced in diabetic patients with increased serum and organ iron. Indeed, studies indicate that reduction of serum ferritin levels is beneficial for delaying or preventing cardiovascular complications in diabetic patients[38,39].
It is known that iron overload causes oxidative stress. The toxicity of iron is mediated by the Fenton reaction, Fe2+ + H2O2→Fe3+ +OH·+OH-, in which the reaction between iron and hydrogen peroxide generates the highly toxic hydroxyl radical (OH·)[40]. Free radicals can damage biological macromolecules (such as lipids, DNA and protein), produce extensive cellular oxidative stress injury, and induce tissue destruction. Both in vitro and in vivo studies show that an increase in iron content can aggravate lipid peroxidation, leading to neuronal apoptosis. An increase in reactive oxygen species is a direct index of oxidative stress, and malondialdehyde is a major product of lipid peroxidation within cells, and can reflect the degree of lipid peroxidation. Therefore, reactive oxygen species and malondialdehyde are representative indexes of oxidative stress damage. This study showed that after iron is overloaded in PC12 cells with ferric ammonium citrate, the levels of reactive oxygen species and malondialdehyde in the ferric ammonium citrate groups increased prominently in a dose-dependent manner compared with the high glucose concentration group. Their levels decreased substantially when iron was removed with deferoxamine. This demonstrates that iron overload is responsible for the increased levels of reactive oxygen species and malondialdehyde. Some studies have shown that neuronal apoptosis underlies diabetic neurological disorder and damage[17]. Along with the increase in iron concentration, oxidative stress within cells increases rapidly, leading to a reduction in cell viability and a gradual enhancement of apoptotic rate, which is the result of iron overload-induced injury.
Nrf2, a member of the basic leucine zipper protein family, is strongly associated with oxidative stress[41]. Under normal physiological conditions, Nrf2 is bound to and negatively regulated by Keap1. Nrf2 dissociates from Keap1 during oxidative stress and activates the transcription of genes containing an ARE, which participate in multiple antioxidative and detoxification reactions. These genes include glutathione S transferases, catalase and superoxide dismutase. This increases resistance to oxidative stress and protects cells against injury[42]. However, if the expression of Nrf2 is obstructed, resistance to oxidative stress will diminish, leading to cell dysfunction or death. A previous study showed that the localization and levels of Nrf2 are dependent on a balance maintained by a nuclear localization sequence and a nuclear export sequence rich in leucine. This balance is broken during oxidative stress, leading to a decrease in Nrf2 levels[43]. The abnormal expression of Nrf2 is associated with diabetes. Accumulating data show that the Nrf2/ARE pathway can influence the pathogenesis of diabetes by regulating antioxidase expression. Zheng et al[44] showed that dietary therapy with activators of Nrf2 ameliorates the metabolic disorder in diabetic rats and alleviates renal damage caused by diabetes.
In the present study, we examined the effect of iron overload on Nrf2 expression in nerve cells under high glucose conditions, and tested whether iron overload aggravates oxidative stress in nerve cells by suppressing the Nrf2/ARE pathway. Results demonstrated that Nrf2 mRNA and protein expression in the high glucose concentration group was significantly lower than in the normal glucose concentration group, suggesting that the Nrf2/ARE signaling pathway is inhibited in nerve cells under high glucose conditions. This, in turn, increases intracellular oxidative stress injury. These findings demonstrate that there is a close relationship between the abnormal expression of Nrf2 and the development of diabetes. This study also suggests that with increasing iron concentration, the expression of both Nrf2 mRNA and protein diminish, and that Nrf2 levels increase after removing the iron, which shows a strong relationship between iron overload and Nrf2. Iron overload likely leads to reduced Nrf2 levels in cells, which enhances the sensitivity of PC12 cells to oxidative stress and leads to pathological damage and apoptosis. In short, iron overload in PC12 cells significantly increases oxidative stress, which results in cellular damage and apoptosis. Taken together, the results of this study provide support for a link between diabetic neuropathy, iron metabolic disorder, and oxidative stress. Our data suggest that iron overload may lead to diabetic peripheral neuropathy through oxidative stress, and that Nrf2 is involved in this process. Our findings provide insight into the mechanisms underlying diabetic peripheral neuropathy and should facilitate the development of novel therapeutic strategies (for example, iron-removal therapy) for this disease.
MATERIALS AND METHODS
Design
A comparative observational, controlled, molecular, in vitro study.
Time and setting
All experiments were performed at the Central Laboratory, Wuhan Central Hospital, China from May to December 2012.
Materials
PC12 cells were provided by Department of Neurobiology, College of Basic Medicine, Tongji Medical College, Huazhong University of Science and Technology, China. Ferric ammonium citrate was purchased from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China). Deferoxamine was purchased from Novartis (Basel, Switzerland).
Methods
PC12 cell culture
PC12 cells were cultured in Dulbecco's Modified Eagle Medium (DMEM; Hyclone, Logan, UT, USA) supplemented with 10% fetal bovine serum, 100 U/mL penicillin and 100 μg/mL streptomycin in a 5% CO2 incubator at 37°C and saturated humidity. The medium was changed every 3 days. After incubating for 24 hours, cells took on the characteristics of nerve cells and adhered to the culture flask. Cells were passaged at 80% confluence with 0.08% trypsin. All the cells used in this experiment were in the logarithmic phase of growth. To determine the appropriate experimental dose of ferric ammonium citrate, cells cultured under high glucose conditions were exposed to 12 different concentrations of ferric ammonium citrate—0, 12.5, 25, 50, 100, 200, 300, 400, 500, 600, 700 and 800 μmol/L—for 24 hours.
Grouping and treatments
PC12 cells were assigned to six different groups: (1) normal glucose concentration group: PC12 cells were incubated with a normal glucose concentration (5.6 mmol/L) in DMEM containing 10% fetal bovine serum for 24, 48 or 72 hours; (2) high glucose concentration group: PC12 cells were cultured in a high glucose concentration (25 mmol/L) in DMEM supplemented with 10% fetal bovine serum for 24, 48 or 72 hours; (3) 25, 100, or 400 μmol/L ferric ammonium citrate: PC12 cells were incubated with different concentrations of ferric ammonium citrate (25, 100 or 400 μmol/L) for 24, 48 or 72 hours after exposure to a high glucose concentration (25 mmol/L) culture medium for 24 hours; (4) ferric ammonium citrate + deferoxamine group: PC12 cells were incubated in a final concentration of 400 μmol/L ferric ammonium citrate and 200 μmol/L deferoxamine for 24, 48 or 72 hours after exposure to a high glucose concentration (25 mmol/L) culture medium for 24 hours.
MTT assay for PC12 cell viability
The cells were seeded in 96-well culture plates at a density of 1 × 108/L, 100 μL/well. Each group consisted of three wells. After PC12 cells were given the relevant drug treatment, MTT (5 g/L; Guge, Wuhan, Hubei Province, China) was added into each well and cells were incubated at 37°C for an additional 4 hours. Supernatant was discarded and dimethyl sulfoxide was added (100 μL/well) to dissolve the formazan product. Absorbance was measured at 570 nm with a microplate reader (Tecan Group Ltd, Männedorf, Switzerland). The cell viability was calculated as follows: (absorbance of experimental group–absorbance of blank group) / (absorbance of high glucose concentration group–absorbance of blank group) × 100%.
Annexin V-FITC/PI flow cytometry for PC12 cell apoptosis
Cells were harvested and resuspended at a density of 1 × 109 cells/L and incubated in 6-well culture plates. After 24 hours, PC12 cells adhered to the flask and the relevant drug treatments were provided to the six different experimental groups, and 48 hours later, cells were incubated in the dark with 5 μL Annexin V-FITC (Beyotime Institute of Biotechnology, Nanjing, Jiangsu Province, China) and 10 μL propidium iodide (Beyotime Institute of Biotechnology) at room temperature for 15 minutes[45]. The samples were analyzed with a flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA). Each group had three samples. A total of 1 × 104 cells were counted and analyzed using Win MDI2.8 software (Scripps Institute, West Lafayette, IN, USA).
Measurement of intracellular reactive oxygen species and malondialdehyde levels
To determine whether iron overload increased intracellular accumulation of reactive oxygen species and malondialdehyde, reactive oxygen species levels were estimated using 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA; Beyotime Institute of Biotechnology)[46]. In the presence of reactive oxygen species, 2′7′-dichlorofluorescin is rapidly oxidized to fluorescent 2′7′-dichlorofluorescein. Flow cytometry was used to measure the fluorescence intensity. The mean of the fluorescence intensity in cells reflects reactive oxygen species level. Malondialdehyde content was measured using the thiobarbituric acid assay according to the instructions in the kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, Jiangsu Province, China). The cells were incubated with DCFH-DA (10 μmol/L) in the dark at 37°C for 30 minutes, and then washed three times with PBS and resuspended in a final volume of 500 μL PBS. Subsequently, flow cytometry was used to measure the fluorescence intensity. Malondialdehyde index = (absorbancedetection tube – absorbancedetection blank tube)/(absorbancestandard tube – absorbancestandard blank tube). A total of 10 000 cells from each sample were measured. The excitation filter was set at 488 nm and the emission filter at 525 nm.
Real-time PCR for Nrf2 mRNA expression
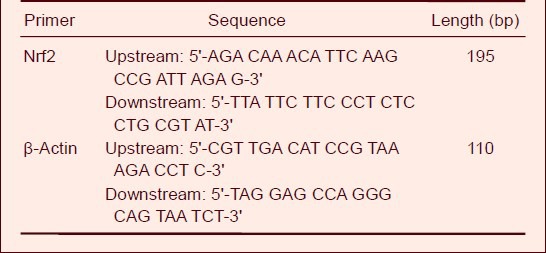
Cells were harvested from each group to extract total RNA. Total RNA was isolated from cells using Trizol Reagent (Invitrogen, Carlsbad, CA, USA). An ultraviolet spectrophotometer (Beckman, Brea, CA, USA) was used to measure the absorbance ratio (A260nm/280nm) which ranged between 1.80 and 2.00. According to the cDNA synthesis kit (Toyoba, Osaka, Japan), total mRNA was reverse transcribed into single-stranded cDNA, and the target gene and β-actin, used as an internal standard, were amplified. Reverse transcription reaction conditions were 42°C, 30 minutes; 80°C, 5 minutes. Primers for Nrf2 and β-actin were designed and synthesized (Invitrogen). The sequences are shown in Table 1. Real-time PCR conditions were as follows: pre-denaturation at 95°C for 1 minute; denaturation at 95°C for 15 seconds, annealing at 58 °C for 20 seconds and extension at 72°C for 20 seconds (40 cycles); extension at 72°C for 10 minutes. Finally, the solubility curve was drawn. Detection of each sample was repeated twice, and the relative content of Nrf2 mRNA was analyzed using the comparative threshold cycle method (2-ΔΔCt).
Table 1.
Primer sequences
Western blot assay for Nrf2 protein expression
Total protein from PC12 cells was extracted using the Protein Extraction Kit (Invitrogen). Protein concentrations were determined using the Bradford method[47], employing a Universal Microplate Reader (Bio-Rad, Hercules, CA, USA) at 595 nm. A total of 25 μg protein from each sample was resolved with sodium dodecyl sulfate polyacrylamide gel electrophoresis, transferred to a polyvinylidene difluoride membrane (Millipore, Billerica, MA, USA), blocked, incubated in primary rabbit anti-Nrf2 monoclonal antibody (1:400; ab54364, Abcam, Cambridge, UK) and goat anti-β-actin polyclonal antibody (1:500; ab8229, Abcam) overnight at 4°C. After washing with PBS three times, the membrane was incubated with horseradish peroxidase-conjugated secondary goat anti-rabbit IgG (1:3 000; AB21-K, Millipore) or rabbit anti-goat IgG (1:5 000; SC-2768, Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) for 30 minutes at room temperature. The antibody-reactive bands were visualized with X-ray film, and band absorbance values were calculated with Alpha Software 5.0 software (Alpha Technologies Inc., Bellingham, WA, USA).
Immunofluorescence
PC12 cells were fixed for 10 minutes with 4% paraformaldehyde/PBS and blocked in 4% normal goat serum (NGS)/PBS/0.1% Triton-X for 30 minutes at room temperature, then incubated with Nrf2 antibody diluted in 4% NGS/PBS overnight at 4°C. Cells were washed three times with PBS and then incubated with Alexa 488-conjugated secondary antibody (Jackson ImmunoResearch, Lancaster, Pennsylvania, United States; 1:500) diluted in 4% NGS/PBS for 30 minutes at 4°C, and then subjected to immunofluorescence microscopy. Light micrographs were taken using a Zeiss microscope (Axiovert 200 MOT, Oberkochen, Germany).
Statistical analysis
All experimental data were provided as mean ± SD and analyzed using SPSS 17.0 software (SPSS, Chicago, IL, USA). One-way analysis of variance was adopted for multiple-group comparisons. Two-tailed Student's t-test was used for intergroup comparison. A level of P < 0.05 was considered statistically significant.
Research background: Numerous studies have shown that iron overload is strongly associated with diabetes mellitus and its chronic complications. Oxidative stress and inflammatory factors play an important role in this relationship.
Research frontiers: If iron steady-state levels are perturbed, excessive free iron may lead to progressive damage to the body.
Clinical significance: Our results indicate that iron overload aggravates oxidative stress injury in neural cells, and this damage can be alleviated by expelling excess iron from PC12 cells. The interaction between iron overload and high glucose may play a major role in the pathogenesis of diabetic neuropathy.
Academic terminology: Iron overload is a serious chronic condition that develops when the body absorbs too much iron and excess iron builds up in organs, tissues and/or cells.
Peer review: The authors in this paper analyzed the role of iron toxicity in the presence of high glucose, which is very interesting. After analyzing the impact on cell survival and apoptosis, the authors went on to analyze markers of oxidative stress, and they investigated the underlying mechanisms. This study adds new knowledge to the field of diabetic peripheral neuropathy.
Acknowledgments
The authors would like to thank Xu ZH from the Department of Endocrinology, Wuhan Central Hospital, Wuhan, Hubei Province, China for assisting with paper editing and data discussion.
Footnotes
Funding: The study was supported by the Natural Science Foundation of Hubei Province, No. 2010CDB09001.
Conflicts of interest: None declared.
(Reviewed by Patel B, Robens J, Chen YS, Li XF)
(Edited by Mu WJ, Qiu Y, Li CH, Song LP, Liu WJ, Zhao M)
REFERENCES
- [1].Farmer KL, Li C, Dobrowsky RT. Diabetic peripheral neuropathy: should a chaperone accompany our therapeutic approach? Pharmacol Rev. 2012;64(4):880–900. doi: 10.1124/pr.111.005314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Singh B, Xu Y, McLaughlin T, et al. Resistance to trophic neurite outgrowth of sensory neurons exposed to insulin. J Neurochem. 2012;121(2):263–276. doi: 10.1111/j.1471-4159.2012.07681.x. [DOI] [PubMed] [Google Scholar]
- [3].Cameron NE, Cotter MA, Jack AM, et al. Protein kinase C effects on nerve function, perfusion, Na(+), K(+)-ATPase activity and glutathione content in diabetic rats. Diabetologia. 1999;42(9):1120–1130. doi: 10.1007/s001250051280. [DOI] [PubMed] [Google Scholar]
- [4].Eckersley L. Role of the Schwann cell in diabetic neuropathy. Int Rev Neurobiol. 2002;50:293–321. doi: 10.1016/s0074-7742(02)50081-7. [DOI] [PubMed] [Google Scholar]
- [5].Stavniichuk R, Drel VR, Shevalye H, et al. Baicalein alleviates diabetic peripheral neuropathy through inhibition of oxidative-nitrosative stress and p38 MAPK activation. Exp Neurol. 2011;230(1):106–113. doi: 10.1016/j.expneurol.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Figueroa-Romero C, Sadidi M, Feldman EL. Mechanisms of disease: the oxidative stress theory of diabetic neuropathy. Rev Endocr Metab Disord. 2008;9(4):301–314. doi: 10.1007/s11154-008-9104-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Vincent AM, Brownlee M, Russell JW. Oxidative stress and programmed cell death in diabetic neuropathy. Ann N Y Acad Sci. 2002;959:368–383. doi: 10.1111/j.1749-6632.2002.tb02108.x. [DOI] [PubMed] [Google Scholar]
- [8].Finch C. Regulators of iron balance in humans. Blood. 1994;84(6):1697–1702. [PubMed] [Google Scholar]
- [9].Schneider SA, Bhatia KP. Excess iron harms the brain: the syndromes of neurodegeneration with brain iron accumulation (NBIA) J Neural Transm. 2013;120(4):695–703. doi: 10.1007/s00702-012-0922-8. [DOI] [PubMed] [Google Scholar]
- [10].Simcox JA, McClain DA. Iron and diabetes risk. Cell metabolism. 2013;17(3):329–341. doi: 10.1016/j.cmet.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Turlin B, Mendler MH, Moirand R, et al. Histologic features of the liver in insulin resistance-associated iron overload. A study of 139 patients. Am J Clin Pathol. 2001;116(2):263–270. doi: 10.1309/WWNE-KW2C-4KTW-PTJ5. [DOI] [PubMed] [Google Scholar]
- [12].Cooksey RC, Jones D, Gabrielsen S, et al. Dietary iron restriction or iron chelation protects from diabetes and loss of beta-cell function in the obese (ob/ob lep-/-) mouse. American journal of physiology. Am J Physiol Endocrinol Metab. 2010;298(6):E1236–1243. doi: 10.1152/ajpendo.00022.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ashourpour M, Djalali M, Djazayery A, et al. Relationship between serum ferritin and inflammatory biomarkers with insulin resistance in a Persian population with type 2 diabetes and healthy people. Int J Food Sci Nutr. 2010;61(3):316–323. doi: 10.3109/09637480903555150. [DOI] [PubMed] [Google Scholar]
- [14].Andrews M, Arredondo M. Hepatic and adipocyte cells respond differentially to iron overload, hypoxic and inflammatory challenge. Biometals. 2012;25(4):749–759. doi: 10.1007/s10534-012-9543-9. [DOI] [PubMed] [Google Scholar]
- [15].Pollak Y, Mechlovich D, Amit T, et al. Effects of novel neuroprotective and neurorestorative multifunctional drugs on iron chelation and glucose metabolism. J Neural Transm. 2013;120(1):37–48. doi: 10.1007/s00702-012-0795-x. [DOI] [PubMed] [Google Scholar]
- [16].Fernandez-Real JM, Lopez-Bermejo A, Ricart W. Cross-talk between iron metabolism and diabetes. Diabetes. 2002;51(8):2348–2354. doi: 10.2337/diabetes.51.8.2348. [DOI] [PubMed] [Google Scholar]
- [17].Zhang WF, Xu YY, Xu KP, et al. Inhibitory effect of selaginellin on high glucose-induced apoptosis in differentiated PC12 cells: role of NADPH oxidase and LOX-1. Eur J Pharmacol. 2012;694(1-3):60–68. doi: 10.1016/j.ejphar.2012.08.011. [DOI] [PubMed] [Google Scholar]
- [18].Bournival J, Francoeur MA, Renaud J, et al. Quercetin and sesamin protect neuronal PC12 cells from high-glucose-induced oxidation, nitrosative stress, and apoptosis. Rejuvenation Res. 2012;15(3):322–333. doi: 10.1089/rej.2011.1242. [DOI] [PubMed] [Google Scholar]
- [19].Salvador GA, Oteiza PI. Iron overload triggers redoxsensitive signals in human IMR-32 neuroblastoma cells. Neurotoxicology. 2011;32(1):75–82. doi: 10.1016/j.neuro.2010.11.006. [DOI] [PubMed] [Google Scholar]
- [20].Vanotti A, Osio M, Mailland E, et al. Overview on pathophysiology and newer approaches to treatment of peripheral neuropathies. CNS drugs. 2007;21(Suppl 1):3. doi: 10.2165/00023210-200721001-00002. [DOI] [PubMed] [Google Scholar]
- [21].Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414(6865):813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- [22].Jomova K, Valko M. Importance of iron chelation in free radical-induced oxidative stress and human disease. Curr Pharm Des. 2011;17(31):3460–3473. doi: 10.2174/138161211798072463. [DOI] [PubMed] [Google Scholar]
- [23].Pu YM, Wang Q, Qian ZM. Effect of iron and lipid peroxidation on development of cerebellar granule cells in vitro. Neuroscience. 1999;89(3):855–861. doi: 10.1016/s0306-4522(98)00384-4. [DOI] [PubMed] [Google Scholar]
- [24].Cameron NE, Cotter MA. Effects of an extracellular metal chelator on neurovascular function in diabetic rats. Diabetologia. 2001;44(5):621–628. doi: 10.1007/s001250051669. [DOI] [PubMed] [Google Scholar]
- [25].Cameron NE, Cotter MA. Neurovascular dysfunction in diabetic rats. Potential contribution of autoxidation and free radicals examined using transition metal chelating agents. J Clin Invest. 1995;96(2):1159–1163. doi: 10.1172/JCI118104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kaeidi A, Esmaeili-Mahani S, Abbasnejad M, et al. Satureja khuzestanica attenuates apoptosis in hyperglycemic PC12 cells and spinal cord of diabetic rats. J Nat Med. 2013;67(1):61–69. doi: 10.1007/s11418-012-0646-y. [DOI] [PubMed] [Google Scholar]
- [27].Malliopoulou V, Xinaris C, Mourouzis I, et al. High glucose protects embryonic cardiac cells against simulated ischemia. Mol Cell Biochem. 2006;284(1-2):87–93. doi: 10.1007/s11010-005-9018-1. [DOI] [PubMed] [Google Scholar]
- [28].Ren Y, Tian H, Li X, et al. Elevated serum ferritin concentrations in a glucose-impaired population and in normal glucose tolerant first-degree relatives in familial type 2 diabetic pedigrees. Diabetes Care. 2004;27(2):622–623. doi: 10.2337/diacare.27.2.622. [DOI] [PubMed] [Google Scholar]
- [29].Jiang R, Manson JE, Meigs JB, et al. Body iron stores in relation to risk of type 2 diabetes in apparently healthy women. JAMA. 2004;291(6):711–717. doi: 10.1001/jama.291.6.711. [DOI] [PubMed] [Google Scholar]
- [30].Silva M, de Brito Magalhaes CL, de Paula Oliveira R, et al. Differential expression of iron metabolism proteins in diabetic and diabetic iron-supplemented rat liver. J Biochem Mol Toxicol. 2012;26(3):123–129. doi: 10.1002/jbt.20418. [DOI] [PubMed] [Google Scholar]
- [31].de Oliveira Otto MC, Alonso A, Lee DH, et al. Dietary intakes of zinc and heme iron from red meat, but not from other sources, are associated with greater risk of metabolic syndrome and cardiovascular disease. J Nutr. 2012;142(3):526–533. doi: 10.3945/jn.111.149781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Jiang F, Sun ZZ, Tang YT, et al. Hepcidin expression and iron parameters change in Type 2 diabetic patients. Diabetes Res Clin Pract. 2011;93(1):43–48. doi: 10.1016/j.diabres.2011.03.028. [DOI] [PubMed] [Google Scholar]
- [33].Fernandez-Real JM, Penarroja G, Castro A, et al. Blood letting in high-ferritin type 2 diabetes: effects on vascular reactivity. Diabetes Care. 2002;25(12):2249–2255. doi: 10.2337/diacare.25.12.2249. [DOI] [PubMed] [Google Scholar]
- [34].Beutler E, Hoffbrand AV, Cook JD. Iron deficiency and overload. Hematology Am Soc Hematol Educ Program. 2003:40–61. doi: 10.1182/asheducation-2003.1.40. [DOI] [PubMed] [Google Scholar]
- [35].Cai L, Kang YJ. Oxidative stress and diabetic cardiomyopathy: a brief review. Cardiovasc Toxicol. 2001;1(3):181–193. doi: 10.1385/ct:1:3:181. [DOI] [PubMed] [Google Scholar]
- [36].Chyun DA, Young LH. Diabetes mellitus and cardiovascular disease. (viii-ix).Nurs Clin North Am. 2006;41(4):681–695. doi: 10.1016/j.cnur.2006.07.007. [DOI] [PubMed] [Google Scholar]
- [37].Van Campenhout A, Van Campenhout C, Lagrou AR, et al. Iron-binding antioxidant capacity is impaired in diabetes mellitus. Free Radic Biol Med. 2006;40(10):1749–1755. doi: 10.1016/j.freeradbiomed.2006.01.010. [DOI] [PubMed] [Google Scholar]
- [38].Finck BN, Lehman JJ, Leone TC, et al. The cardiac phenotype induced by PPARalpha overexpression mimics that caused by diabetes mellitus. J Clin Invest. 2002;109(1):121–130. doi: 10.1172/JCI14080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].de Mello VD, Zelmanovitz T, Perassolo MS, et al. Withdrawal of red meat from the usual diet reduces albuminuria and improves serum fatty acid profile in type 2 diabetes patients with macroalbuminuria. Am J Clin Nutr. 2006;83(5):1032–1038. doi: 10.1093/ajcn/83.5.1032. [DOI] [PubMed] [Google Scholar]
- [40].Youdim MB, Ben-Shachar D, Riederer P. Is Parkinson's disease a progressive siderosis of substantia nigra resulting in iron and melanin induced neurodegeneration? Acta neurologica Scandinavica. Acta Neurol Scand Suppl. 1989;126:47–54. doi: 10.1111/j.1600-0404.1989.tb01782.x. [DOI] [PubMed] [Google Scholar]
- [41].Jiang T, Huang Z, Lin Y, et al. The protective role of Nrf2 in streptozotocin-induced diabetic nephropathy. Diabetes. 2010;59(4):850–860. doi: 10.2337/db09-1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Kang KW, Lee SJ, Kim SG. Molecular mechanism of nrf2 activation by oxidative stress. Antioxid Redox Signal. 2005;7(11-12):1664–1673. doi: 10.1089/ars.2005.7.1664. [DOI] [PubMed] [Google Scholar]
- [43].Jain AK, Bloom DA, Jaiswal AK. Nuclear import and export signals in control of Nrf2. J Biol Chem. 2005;280(32):29158–29168. doi: 10.1074/jbc.M502083200. [DOI] [PubMed] [Google Scholar]
- [44].Zheng H, Whitman SA, Wu W, et al. Therapeutic potential of Nrf2 activators in streptozotocin-induced diabetic nephropathy. Diabetes. 2011;60(11):3055–3066. doi: 10.2337/db11-0807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Pelicano H, Feng L, Zhou Y, et al. Inhibition of mitochondrial respiration: a novel strategy to enhance drug-induced apoptosis in human leukemia cells by a reactive oxygen species-mediated mechanism. J Biol Chem. 2003;278(39):37832–37839. doi: 10.1074/jbc.M301546200. [DOI] [PubMed] [Google Scholar]
- [46].Iuvone T, De Filippis D, Esposito G, et al. The spice sage and its active ingredient rosmarinic acid protect PC12 cells from amyloid-beta peptide-induced neurotoxicity. J Pharmacol Exp Ther. 2006;317(3):1143–1149. doi: 10.1124/jpet.105.099317. [DOI] [PubMed] [Google Scholar]
- [47].Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]