Abstract
Wistar rats were intragastrically perfused with Chinese medicines used for tonifying the kidney. These included 0.180 g/mL of Herba Epimedii (Epimedium), Semen Cuscutae (Dodder Seed), or Herba Cistanches (Desertliving Cistanche), 0.04 mg/mL monoamine oxidase-B inhibitor selegiline, or distilled water for 14 consecutive days to prepare drug-containing serum or blank serum. MES23.5 cells in the logarithmic phase were cultured in media supplemented with 15% drug-containing serum for 24 hours, followed by incubation in culture solution containing 100 μmol/L H2O2 for 3 hours. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and flow tometry results showed that all drug-containing serums improved the survival rate of H2O2-injured MES23.5 cells, inhibited pro-apoptotic FasL and caspase-3 expression, promoted anti-apoptotic Bcl-2 expression. However, drug-containing serums had little influence on Fas expression in H2O2-injured MES23.5 cells. Enzyme-linked immunosorbent assay results showed that serum containing Herba Cistanches or Herba Epimedii increased the expression of nerve growth factor, brain-derived neurotrophic factor, and glial cell line-derived neurotrophic factor in injured MES23.5 cells; serum containing Semen Cuscutae only increased brain-derived neurotrophic factor expression; while expression of the above neurotrophic factors remained the same in cells treated with serum containing selegiline. These findings indicate that Chinese medicines used to tonify the kidney can protect nerve cells by regulating the expression of apoptosis-related factors and neuro-trophic factors in MES23.5 cells.
Keywords: neural regeneration, traditional Chinese medicine, drug-containing serum, MES23.5 dopaminergic nerve cells, neurotrophic factors, apoptosis factors, Parkinson's disease, neuroprotection
Research Highlights
(1) This study monitored apoptosis-related factors and neurotrophic factors to reveal the inhibitory effects of Chinese medicines for tonifying the kidney on apoptosis at the molecular level.
(2) Results showed that Chinese medicines for tonifying the kidney can protect nerve cells by gulating the expression of apoptosis-related factors and neurotrophic factors.
INTRODUCTION
Parkinson's disease is characterized by degeneration and loss of dopaminergic neurons in the substantia nigra, a region of the midbrain, as well as Lewy body formation[1,2]. The loss of dopaminergic neurons and Parkinson's disease are highly correlated with activation of the apoptotic pathway[3,4]. A variety of cytokines and neurotrophic factors have been used for neuroprotection[5,6]. Compound preparations of Chinese medicines that can tonify the kidney have been reported to significantly improve symptoms of Parkinson's disease. However, the mechanism of action remains unclear[7,8,9]. We have previously shown that Chinese medicines that tonify the kidney can protect dopaminergic neurons by regulating the expression of apoptosis-related factors and increasing neurotrophic factor content in an animal model of Parkinson's disease[10]. However, evidence from in vitro experiments is lacking.
The MES23.5 cell line is hybridized from midbrain cells from the rat embryo and the mouse neuroblastoma glioma cell line N18TG2. This cell line has properties of dopaminergic neurons and is easier to culture than midbrain nerve cells. Therefore, these cells have been extensively used for studying neurodegenerative diseases[11].
To investigate whether Chinese medicines that tonify the kidney can protect in vitro cultures of dopaminergic neurons, we induced oxidative damage in MES23.5 cells using H2O2, and treated the injured cells with serum containing Herba Epimedii (Epimedium), Semen Cuscutae (Dodder Seed), or Herba Cistanches (Desertliving Cistanche). Flow cytometry and enzyme-linked immunosorbent assay (ELISA) were utilized to detect the expression of apoptosis-related factors and neurotrophic factors. We aimed to explore the neuroprotective mechanism of Chinese medicines that can tonify the kidney for Parkinson's disease prevention. Selegiline and monoamine oxidase-B inhibitor were used as controls.
RESULTS
Influence of different concentrations of H2O2 on MES23.5 cell growth
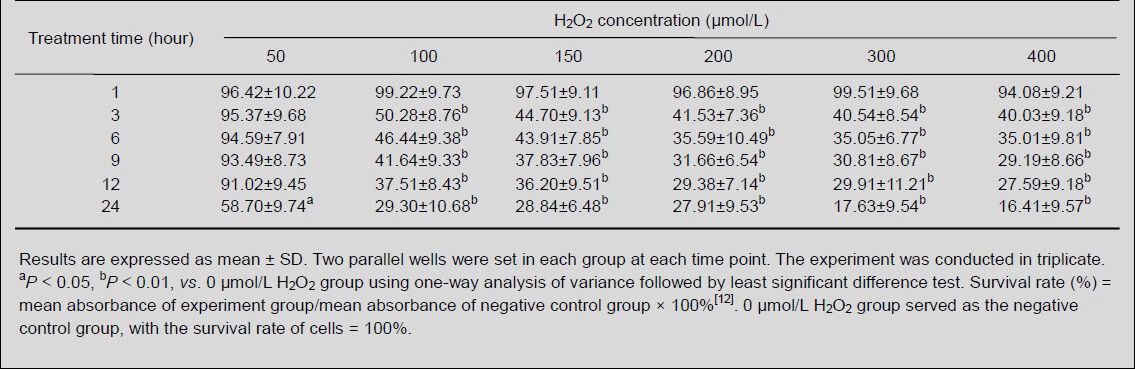
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) results showed that the survival rate of MES23.5 cells remained after exposure to 50, 100, 150, 200, 300 and 400 μmol/L H2O2 for 1 hour (P > 0.05), but significantly reduced after 3 hours, except the cells exposed to 50 μmol/L H2O2 (P < 0.01). Moreover, there was no significant difference in the survival rate among different H2O2 concentration groups, except 50 μmol/L H2O2, at each time point (P > 0.05). The survival rate of MES23.5 cells was not reduced until 50 μmol/L H2O2 treatment for 24 hours (P < 0.05). Thus, we treated MES23.5 cells with 100 μmol/L H2O2 for 3 hours (Table 1).
Table 1.
Survival rate (%) of MES23.5 cells treated with different concentrations of H2O2 over time
Influence of different concentrations of drug-containing serum on the growth of H2O2-induced MES23.5 cells
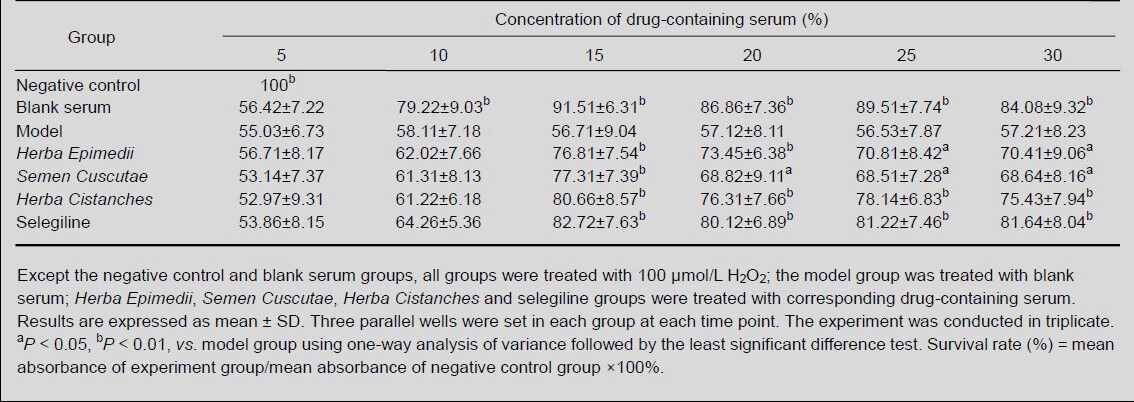
MTT results showed that no alteration was found in H2O2-induced MES23.5 cells pretreated with 5% (v/v) and 10% (v/v) drug-containing serum for 24 hours (P > 0.05). However, pretreatment with 15% (v/v), 20% (v/v), 25% (v/v) and 30% (v/v) drug-containing serum for 24 hours significantly improved the survival rate of H2O2-induced MES23.5 cells (P < 0.05 or P < 0.01), but no significant difference was observed among the concentrations (P > 0.05). Thus, 15% (v/v) drug-containing serum was used in subsequent experiments (Table 2).
Table 2.
Survival rate (%) of H2O2-induced MES23.5 cells following treatment with drug-containing serum at different concentrations
Influence of drug-containing serum on the expression of apoptosis-related factors in H2O2-induced MES23.5 cells
Flow cytometry showed that FasL and caspase-3 content significantly increased in the model group compared with the blank serum-treated group; P < 0.05). FasL and caspase-3 content significantly reduced in the Herba Epimedii, Semen Cuscutae, Herba Cistanches and selegiline groups when compared with the model group (P < 0.05). However, FasL and caspase-3 content in the Herba Epimedii group, as well as FasL content in the Herba Cistanches group, remained higher when compared with blank serum group (P < 0.05; Table 3).
Table 3.
FasL-, Fas-, caspase-3-, and Bcl-2-positive expression rate (%) in H2O2-induced MES23.5 cells following treatment with drug-containing serum

Changes in Bcl-2 content in cells from each group were opposite to FasL and caspase-3 levels. Bcl-2 content was significantly reduced in the model group when compared with blank serum group (P < 0.05); Bcl-2 content was significantly higher in the Herba Epimedii, Herba Cistanches and selegiline groups when compared with the model group (P < 0.05), but the increase in Bcl-2 content was not obvious in the Semen Cuscutae group (Table 3). No significant difference was found in Fas content among the groups (P > 0.05; Table 3).
Influence of drug-containing serum on the expression of neurotrophic factors in H2O2-induced MES23.5 cells
ELISA results revealed that nerve growth factor content significantly reduced in the model and selegiline groups when compared with the blank serum group (P < 0.05). However, nerve growth factor levels were greater in the Herba Epimedii, Semen Cuscutae, and Herba Cistanches groups when compared with the model group, but significant differences were only found between the Herba Cistanches and model groups (P < 0.05; Table 4).
Table 4.
Nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF) and glial cell line-derived neurotrophic factor (GDNF) content changes (absorbance) in H2O2-induced MES23.5 cells following treatment with drug-containing serum for 24 hours
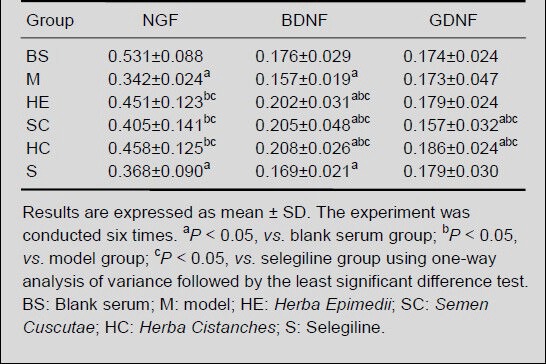
Brain-derived neurotrophic factor content was significantly reduced in the model and selegiline groups when compared with the blank serum group (P < 0.05), and was significantly greater in the Herba Epimedii, Semen Cuscutae, and Herba Cistanches groups when compared with the blank serum group (P < 0.05; Table 4).
Glial cell line-derived neurotrophic factor content was significantly greater in the Herba Cistanches group when compared with the blank serum, model, Semen Cuscutae and selegiline groups (P < 0.05), but no significant difference were found between the Herba Epimedii and Herba Cistanches groups (Table 4).
DISCUSSION
The pathological and neural biochemical changes in Parkinson's disease mainly include dopaminergic neuronal loss in the substantia nigra-corpus striatum, formation of eosinophilic Lewy bodies and reduction of dopamine transmitters in the corpus striatum[14]. Studies have shown that neuronal apoptosis is an important cause for the occurrence and progression of neurodegenerative diseases such as Parkinson's disease and Alzheimer's disease[15,16].
Modern pharmacological studies have addressed that the Chinese medicines Herba Epimedii, Semen Cuscutae, and Herba Cistanches, which can tonify the kidney, are neuroprotective. Herba Epimedii, an herbaceous plant of berberidaceae family, can protect nerve cells against amyloid beta peptide (25–35) injury and reduce apoptosis[17]. Semen Cuscutae is the dried mature seed of dodder. It can regulate immunity, delay aging, reduce free radical production, promote superoxide dismutase generation, increase Ca2+ content in serum, inhibit L-type calcium channels, control calcium influx, regulate osteoporosis and suppress nerve cell apoptosis[18,19]. Herba Cistanches is a chylocaulous of scale leaf in dry areas. It can prolong life span, inhibit oxidation, and improve central neurotransmitter content[20,21]. Selegiline, monoamine oxidase-B inhibitor, can also protect nerve cells by several pathways that include inhibition of monoamine oxidase-B activity, reduction of endogenous or exogenous dopamine degradation, maintenance of dopamine concentration in the synaptic terminal, reduction of monoamine oxidase-B, promotion of antioxidase activity, slow oxidation, decrease hydroxyl radical generation, and protect dopaminergic neurons[22].
Fas, FasL, and the caspase and Bcl-2 protein family play important roles in regulating cell apoptosis[23,24]. Previous animal experiments from our group have shown that Chinese medicines that tonify the kidney can reduce caspase-3 and FasL content in the substantia nigra-corpus striatum of mice with Parkinson's disease[11]. This indicates important roles for Chinese medicines that tonify the kidney by downregulating apoptotic factors to protect neurons in mice with Parkinson's disease. This study used cultured cells in vitro and exposed them to drug-containing serum to directly observe the effects of Chinese medicines. Moreover, this method minimized the interference of direct exposure of cells to medicines. Results showed that Herba Epimedii and Herba Cistanches upregulated Bcl-2 expression, and Herba Epimedii, Semen Cuscutae, and Herba Cistanches reduced caspase-3 and FasL expression. This indicates that regulation of Bcl-2 and caspase-3 expression by Herba Epimedii and Herba Cistanches plays an important role in nerve cell apoptosis.
Neurotrophic factors are a type of polypeptide or protein released by organisms. Studies have shown that nerve growth factor, brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor can offer a nutritive microenvironment for the nervous system and play critical roles in maintaining normal development of the nervous system, neuronal survival and nervous system regulation[25,26,27]. Nerve growth factor is mainly responsible for the development, differentiation and survival of dopaminergic neurons, as well as repair of injured dopaminergic neurons[28,29]. Brain-derived neurotrophic factor can serve as an antioxidant and protect nerve cells by synergizing glial cell line-derived neurotrophic factor[30,31]. Glial cell line-derived neurotrophic factor has specific trophic actions on dopaminergic neurons and can nourish nerve cells by altering the volume of nerve cells, length of processes and influence specific protein production[32]. Our previous study showed that Chinese medicines that can tonify the kidney can upregulate the endogenous content of nerve growth factor, brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor in the substantia nigra-corpus striatum of mice with Parkinson's disease and effectively reduce nerve cell apoptosis[11]. Results from this study indicated that Herba Cistanches increased nerve growth factor and glial cell line-derived neurotrophic factor content, and that Herba Epimedii, Semen Cuscutae, and Herba Cistanches increased brain-derived neurotrophic factor content in MES23.5 cells.
In summary, Chinese medicines used for tonifying the kidney, such as Herba Epimedii and Herba Cistanches, can protect nerve cells by regulating the expression of apoptosis-related factors and neurotrophic factors. The action of Semen Cuscutae is slow and mild, so its effects on cell apoptosis are not obvious. However, we only monitored the apoptotic inhibitory effects of these medicines. Further studies are needed to investigate the neuroprotective mechanisms of these kidney tonifying Chinese medicines.
MATERIALS AND METHODS
Design
In vitro comparative observation of serum pharmacology.
Time and setting
The experiments were conducted in the Laboratory of Cytobiology, Fujian University of Traditional Chinese Medicine, China from 2010 to 2011.
Materials
Animals
A total of 60 male Wistar rats, 8 weeks old, weighing 200–220 g, were purchased from Slac Laboratory Animal Co., Ltd., Shanghai, China (license No. SCXK (Hu) 2007-0005). They were housed in the Laboratory Animal Center of Fujian University of Traditional Chinese Medicine in China at 20–22°C with a humidity of 40–60% and illumination from 7:00 a.m. to 7:00 p.m. Food was provided by the Laboratory Animal Center of Fujian University of Traditional Chinese Medicine. All experimental procedures were in accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals, formulated by the Ministry of Science and Technology of China[33].
Drugs
Herba Epimedii, the aerial part of the Epimedium sagittatum Maxim was from Sichuan Province, China; Semen Cuscutae, the dry mature seed of Cuscuta chinensis Lam., was from Shandong Province, China; and Herba Cistanches, the chylocaulous of scale leaf of Cistanche deserticola Y.C.Ma, was from Xinjiang Uygur Autonomous Region. All medicines were provided by Fujian Pharmaceutical Co., Ltd., Fujian Province, China. Herba Epimedii, Semen Cuscutae, and Herba Cistanches, 97.2 g each, were immersed in a decoction vessel containing 1 500 mL distilled water, rapidly heated, boiled, maintained at 100°C to allow the active components of the drugs to dissolve and condensed to 540 mL. The crude amount of drug in the drug solution was 0.180 g/mL. The solution was filtered, placed in a bottle, sealed, sterilized and stored at 4°C. Selegiline, 5 mg × 10 tablets per kit, was purchased from Nanjing Sike Pharmaceutical Co., Ltd. (Jiangsu Province, China). Selegiline, 21.6 mg, was dissolved in double distilled water to prepare a 540 mL water solution. The concentration of the drug solution was 0.04 mg/mL. The drug solution was placed in a bottle, sealed, sterilized and stored at 4°C.
Methods
Preparation of drug-containing serum
The Herba Epimedii, Semen Cuscutae, Herba Cistanches and selegiline groups were intragastrically perfused with drug-containing serum containing 0.180 g/mL Herba Epimedii, Semen Cuscutae, or Herba Cistanches, or 0.04 mg/mL selegiline, twice a day, at a dose of 0.1 mL/kg, for 14 consecutive days. The blank serum group was administrated an equal volume of distilled water. The animals were deprived of food and water for 12 hours following the final administration. The rats were anesthetized by intraperitoneal injection with 0.3 mL/100 g ketamine hydrochloride. Blood was harvested from the abdominal aorta, and centrifuged at 1 000 r/min for 15 minutes. The supernatant was harvested, deactivated in a water bath at 56°C for 30 minutes, filtered through a 0.22 μm microporous membrane and stored at –20 °C.
Preparation of 50×Sato's solution
A mixture of 60 mL DMEM/F12 (Gibco, Carlsbad, CA, USA), 15 mg bovine insulin (Sigma, St. Louis, MO, USA), 15 mg transferrin (Sigma), 145.8 mg sodium pyruvate (Biosharp, Korea), 12 mg putrescine (Solarbio, Beijing, China), 25 μL sodium selenate (1 mg/mL, 100 mg/100 mL H2O; Sigma), and 100 μL progesterone (0.315 mg/mL, 15.75 mg/50 mL; Biosharp) was subjected to ultrasound in an ice bath for 1.5 hours, filtered through 0.22 μm filter, aliquoted and stored at –20°C.
Cell culture
Cells were seeded in DMEM/F12 supplemented with 5% (v/v) fetal bovine serum (Gibco), 1% (v/v) glutamine (Sigma), 2% (v/v) 50×Sato's solution, and 2% (v/v) penicillin/streptomycin. Cells were then incubated in 5% (v/v) CO2 and maintained at saturated humidity at 37°C. Cells were trypsinized with 0.25% (w/v) trypsin and passaged. The cells in the logarithmic phase were collected for subsequent experiments.
Effect of H2O2 on the growth of MES23.5 cells
Cells were seeded in 96-well culture plates (5 × 105 cells/well), incubated with 50, 100, 150, 200, 300, and 400 μmol/L H2O2 culture solution (Shanghai Shiyi Chemicals Reagent Co., Ltd., Shanghai, China) after 24 hours for 1, 3, 6, 9, 12, 24 hours, followed by MTT (Sigma) for 4 hours. The solution in the wells was discarded, and 150 μL dimethyl sulfoxide (Sigma) was added. The culture plate was shaken for 10 minutes, and the absorbance of samples at 570 nm was determined using an automatic microplate reader (EXL800; Biotek, Winooski, VT, USA). Freshly prepared DMEM/F12 culture solution was used as a negative control. Two parallel wells were set at each time point. The experiments were conducted in triplicate.
Influence of drug-containing serum at different concentrations on the growth of H2O2-treated MES23.5 cells
Cells were seeded and exposed to drug-containing serum containing 5, 10, 15, 20, 25, and 30% (v/v) Herba Epimedii, Semen Cuscutae, Herba Cistanches and selegiline or blank serum for 24 hours. Cells were further cultured with culture solution containing 100 μmol/L H2O2 for 3 hours, followed by MTT for 4 hours. Three parallel wells were set in each group. Freshly prepared DMEM/F12 culture solution was used as a negative control. Cells cultured in culture solution containing 100 μmol/L H2O2 and blank serum served as the model group, and cells cultured in blank serum alone served as the blank serum group. The experiments were conducted in triplicate. The mean absorbance value of each group was calculated as the survival rate of cells.
Flow cytometry for FasL, Fas, caspase-3 and Bcl-2 expression in MES23.5 cells
Cells were seeded and treated as before. Cells cultured in 100 μmol/L H2O2 alone were regarded as the model group. The phycoerythrin-labeled FasL antibody and FasL isotype control (5 μL each), were added into two tubes (BD, San Jose, CA, USA), mixed evenly with cells, stored at room temperature in the dark for 15 minutes, centrifuged at 1 000 r/min for 5 minutes after addition of 2 mL PBS. The supernatant was discarded, and cells were resuspended using 500 μL PBS. The wavelength of the laser device for flow cytometry (Facscalibur; BD) was 488 nm. The expression rate of FasL-positive cells was analyzed and calculated by Cell Quest software (BD). The expression rate of Fas-, caspase-3-, and Bcl-2-positive cells was detected using the same method.
ELISA for content of nerve growth factor, brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor in cells
Cells were seeded and treated as described previously. The supernatant was harvested, and nerve growth factor, brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor content was measured according to the manufacturer's instructions (BD). The absorbance was measured at 450 nm using an automatic microplate reader (EXL800; Biotek, Winooski, VT, USA).
Statistical analysis
Results were expressed as mean ± SD. Data were analyzed using SPSS 18.0 software (SPSS, Chicago, IL, USA). One-way analysis of variance was conducted followed by multiple comparison least significant difference test. A value of P < 0.05 was considered statistically significant.
Acknowledgments
We thank Chen B, the Laboratory of Neurobiology, Capital Medical University, for graciously providing MES23.5 dopaminergic nerve cells.
Footnotes
Funding: This study was supported by the Developmental Fund of Chen Keji Integrative Medicine, No. CKJ2010025 and the Key Foundation of Society Development in Fujian Province, No. 2013Y0059.
Conflicts of interest: None declared.
Ethical approval: This study received permission from the Animal Ethics Committee of Fujian University of Traditional Chinese Medicine, China.
(Reviewed by Diwakarla S, Norman C, Cheng HY, Wang JM)
(Edited by Wang LM, Su LL, Li CH, Song LP, Liu WJ, Zhao M)
REFERENCES
- [1].Zhang ZX, Roman GC, Hong Z, et al. Parkinson's disease in China: prevalence in Beijing, Xian, and Shanghai. Lancet. 2005;365(9459):595–597. doi: 10.1016/S0140-6736(05)17909-4. [DOI] [PubMed] [Google Scholar]
- [2].He JC, Wang WW. Effect of Tianma Gouteng Yin on apoptosis of dopaminergic neurons in Parkinson's disease model rats. Zhongyi Zazhi. 2010;51(11):1024–1027. [Google Scholar]
- [3].Perier C, Bové J, Vila M. Mitochondria and programmed cell death in Parkinson's disease: apoptosis and beyond. Antioxid Redox Signal. 2012;16(9):883–895. doi: 10.1089/ars.2011.4074. [DOI] [PubMed] [Google Scholar]
- [4].Gallagher DA, Schapira AH. Etiopathogenesis and treatment of Parkinson's disease. Curr Top Med Chem. 2009;9(10):860–868. [PubMed] [Google Scholar]
- [5].Rangasamy SB, Soderstrom K, Bakay RA, et al. Neurotrophic factor therapy for Parkinson's disease. Prog Brain Res. 2010;184:237–264. doi: 10.1016/S0079-6123(10)84013-0. [DOI] [PubMed] [Google Scholar]
- [6].Zuccato C, Cattaneo E. Brain-derived neurotrophic factor in neurodegenerative diseases. Nat Rev Neurol. 2009;5(6):311–322. doi: 10.1038/nrneurol.2009.54. [DOI] [PubMed] [Google Scholar]
- [7].Cai DF, Chen XQ, Gao Y. Effect of bushen yanggan recipe on nigrostriatal function in parkinsonian model rats after long-term levodopa treatment. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2002;22(1):43–46. [PubMed] [Google Scholar]
- [8].Yang MH, Wang HM, Liu Y. Effect of Bushen Huoxue Decoction on the orphan receptor and tyrosine hydroxylase in the brain of rats with Parkinson's disease. Chin J Integr Med. 2011;17(1):43–47. doi: 10.1007/s11655-011-0618-1. [DOI] [PubMed] [Google Scholar]
- [9].Li SD, Liu Y, Yang MH. Effects of bushen huoxue yin on brain NF-kB and NO content in the Parkinson's disease model mouse. J Tradit Chin Med. 2012;32(1):67–70. doi: 10.1016/s0254-6272(12)60034-x. [DOI] [PubMed] [Google Scholar]
- [10].Tian Y, Cai J, Chen XZ, et al. Protective effects of Chinese nourishing kidney herbs on neurons of striatal neurons in Parkinson's disease model mouse. Zhongguo Laonian Xue Zazhi. 2011;31(3):440–443. [Google Scholar]
- [11].Yu H, Li YH, Feng XL, et al. Identification and activity assay of the tyrosine hydroxylase in MES23.5 cells. Zhongguo Shengwu Huaxue yu Fenzi Shengwu Xuebao. 2004;20(1):95–100. [Google Scholar]
- [12].Wang XR. Beijing: People's Medical Publishing House; 2007. Toxicology Experimental Methods and Technology. [Google Scholar]
- [13].Lv SJ. Beijing: China Medical Science Press; 2010. Clinical Immunology Test. [Google Scholar]
- [14].Eriksen JL, Wszolek Z, Petrucelli L. Molecular pathogenesis of Parkinson disease. Arch Neurol. 2005;62(3):353–357. doi: 10.1001/archneur.62.3.353. [DOI] [PubMed] [Google Scholar]
- [15].Christen Y. Oxidative stress and Alzheimer disease. Am J Clin Nutr. 2000;71(2):621S–629S. doi: 10.1093/ajcn/71.2.621s. [DOI] [PubMed] [Google Scholar]
- [16].Blandini F, Armentero MT, Fancellu R, et al. Neuroprotective effect of rasagiline in a rodent model of Parkinson's disease. Exp Neurol. 2004;187(2):455–459. doi: 10.1016/j.expneurol.2004.03.005. [DOI] [PubMed] [Google Scholar]
- [17].Zhang XN, Wang HH, Wang ZQ, et al. Protective effect of icaritin on apoptosis of primarily cultured rat neurons induced by Aâ25-35 peptide. Zhejiang Daxue Xuebao: Yixue Ban. 2007;36(3):224–228. doi: 10.3785/j.issn.1008-9292.2007.03.003. [DOI] [PubMed] [Google Scholar]
- [18].Wang LH, Bu PC, Bao YM. Neuroprotective effect of cuscuta chinensis Lam extract on the neuronal differentiated PC12 cells damage induced by reactive oxygen species. Xibao Shengwu Xue Zazhi. 2005;27:69–72. [Google Scholar]
- [19].Sun SL, Wang B, Peng HS. Inhibition of Dodder extract to cardiac myocyte apoptosis in aging mice. Zhongguo Laonian Xue Zazhi. 2011;31:642–644. [Google Scholar]
- [20].Yang JH, Hu JP, Rena K, et al. Structure-activity relationships of phenylethanoid glycosides in plants of Cistanche salsa on antioxidative activity. Zhong Yao Cai. 2009;32(7):1067–1069. [PubMed] [Google Scholar]
- [21].Wu Y, Li L, Wen T, et al. Protective effects of echinacoside on carbon tetrachloride-induced hepatotoxicity in rats. Toxicology. 2007;232(1-2):50–56. doi: 10.1016/j.tox.2006.12.013. [DOI] [PubMed] [Google Scholar]
- [22].Cheng SD. Beijing: People's Medical Publishing House; 2006. Parkinson's Disease. [Google Scholar]
- [23].Yang T. Beijing: People's Medical Publishing House; 2010. Cell Biology. [Google Scholar]
- [24].Baba N, Koji T, Itoh M, et al. Reciprocal changes in the expression of Bcl-2 and Bax in hypoglossal nucleus after axotomy in adult rats: possible involvement in the induction of neuronal cell death. Brain Res. 1999;827(1-2):122–129. doi: 10.1016/s0006-8993(99)01315-3. [DOI] [PubMed] [Google Scholar]
- [25].Zuccato C, Cattaneo E. Brain-derived neurotrophic factor in neurodegenerative diseases. Nat Rev Neurol. 2009;5(6):311–322. doi: 10.1038/nrneurol.2009.54. [DOI] [PubMed] [Google Scholar]
- [26].Peterziel H, Unsicker K, Krieglstein K. TGFbeta induces GDNF responsiveness in neurons by recruitment of GFRalpha1 to the plasma membrane. J Cell Biol. 2002;159(1):157–167. doi: 10.1083/jcb.200203115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hong M, Mukhida K, Mendez I. GDNF therapy for Parkinson's disease. Expert Rev Neurother. 2008;8(7):1125–1139. doi: 10.1586/14737175.8.7.1125. [DOI] [PubMed] [Google Scholar]
- [28].Chaturvedi RK, Shukla S, Seth K, et al. Nerve growth factor increases survival of dopaminergic graft, rescue nigral dopaminergic neurons and restores functional deficits in rat model of Parkinson's disease. Neurosci Lett. 2006;398(1-2):44–49. doi: 10.1016/j.neulet.2005.12.042. [DOI] [PubMed] [Google Scholar]
- [29].Kavanagh ET, Loughlin JP, Herbert KR, et al. Functionality of NGF-protected PC12 cells following exposure to 6-hydroxydopamine. Biochem Biophys Res Commun. 2006;351(4):890–895. doi: 10.1016/j.bbrc.2006.10.104. [DOI] [PubMed] [Google Scholar]
- [30].Baquet ZC, Bickford PC, Jones KR. Brain-derived neurotrophic factor is required for the establishment of the proper number of dopaminergic neurons in the substantia nigra pars compacta. J Neurosci. 2005;25(26):6251–6259. doi: 10.1523/JNEUROSCI.4601-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hung SY, Liou HC, Fu WM. The mechanism of heme oxygenase-1 action involved in the enhancement of neurotrophic factor expression. Neuropharmacology. 2010;58(2):321–329. doi: 10.1016/j.neuropharm.2009.11.003. [DOI] [PubMed] [Google Scholar]
- [32].Duarte EP, Curcio M, Canzoniero LM, et al. Neuroprotection by GDNF in the ischemic brain. Growth Factors. 2012;30(4):242–257. doi: 10.3109/08977194.2012.691478. [DOI] [PubMed] [Google Scholar]
- [33].The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006 Sep 30; [Google Scholar]


