Abstract
Forty-three patients with chronic spinal cord injury for over 6 months were transplanted with bryonic olfactory ensheathing cells, 2–4 × 106, into multiple sites in the injured area under the surgical microscope. The sympathetic skin response in patients was measured with an electromyography/evoked potential instrument 1 day before transplantation and 3–8 weeks after transtion. Spinal nerve function of patients was assessed using the American Spinal Injury Association impairment scale. The sympathetic skin response was elicited in 32 cases before olfactory ensheathing cell transplantation, while it was observed in 34 cases after transplantation. tantly, sympathetic skin response latency decreased significantly and amplitude increased cantly after transplantation. Transplantation of olfactory ensheathing cells also improved American Spinal Injury Association scores for movement, pain and light touch. Our findings indicate that factory ensheathing cell transplantation improves motor, sensory and autonomic nerve functions in patients with chronic spinal cord injury.
Keywords: neural regeneration, spinal cord injury, clinical practice, olfactory ensheathing cells, cell tation, olfactory ensheathing cell transplantation, sympathetic skin response, neurological function, autonomic nerve, paralysis, neuroregeneration
Research Highlights
(1) Recovery of autonomic nerve function is difficult in the treatment of spinal cord injury.
(2) Olfactory ensheathing cell transplantation improves somatic nerve function and sympathetic skin responses in patients with chronic spinal cord injury.
(3) Sympathetic skin response can objectively reflect the recovery of autonomic nerve function in patients with spinal cord injury after cell transplantation.
INTRODUCTION
Paralysis and other dysfunctions caused by chronic spinal cord injury are major concerns for scientists and clinicians. In recent animal experiments and clinical trials, olfactory ensheathing cell transplantation has been shown to improve neurological functioning, including motor, sensory and autonomic nerve functions (such as skin sweating), after spinal cord injury[1,2,3,4,5]. Neurological functions following spinal cord injury have generally been assessed using the American Spinal Injury Association (ASIA) impairment scale[6]. However, the scale primarily evaluates motor function, and little attention has been given to autonomic nerve functions, such as defecation, sweating or skin response.
The sympathetic skin response test is a simple, safe, noninvasive electrophysiological detection method, and can objectively assess autonomic nerve functions in patients with spinal cord injury[7,8].
In this study, we examined changes in sympathetic skin response and ASIA scores in spinal cord injury patients before and after olfactory ensheathing cell transplantation. Our aim was to provide insight into the effects of this therapeutic approach on neurological function in spinal cord injury patients.
RESULTS
Quantitative analysis of participants
Forty-three patients with chronic spinal cord injury were involved in the analysis.
Baseline data on participants
The baseline data for the 43 patients with chronic spinal cord injury are shown in Table 1.
Table 1.
Baseline information on patients with chronic spinal cord injury
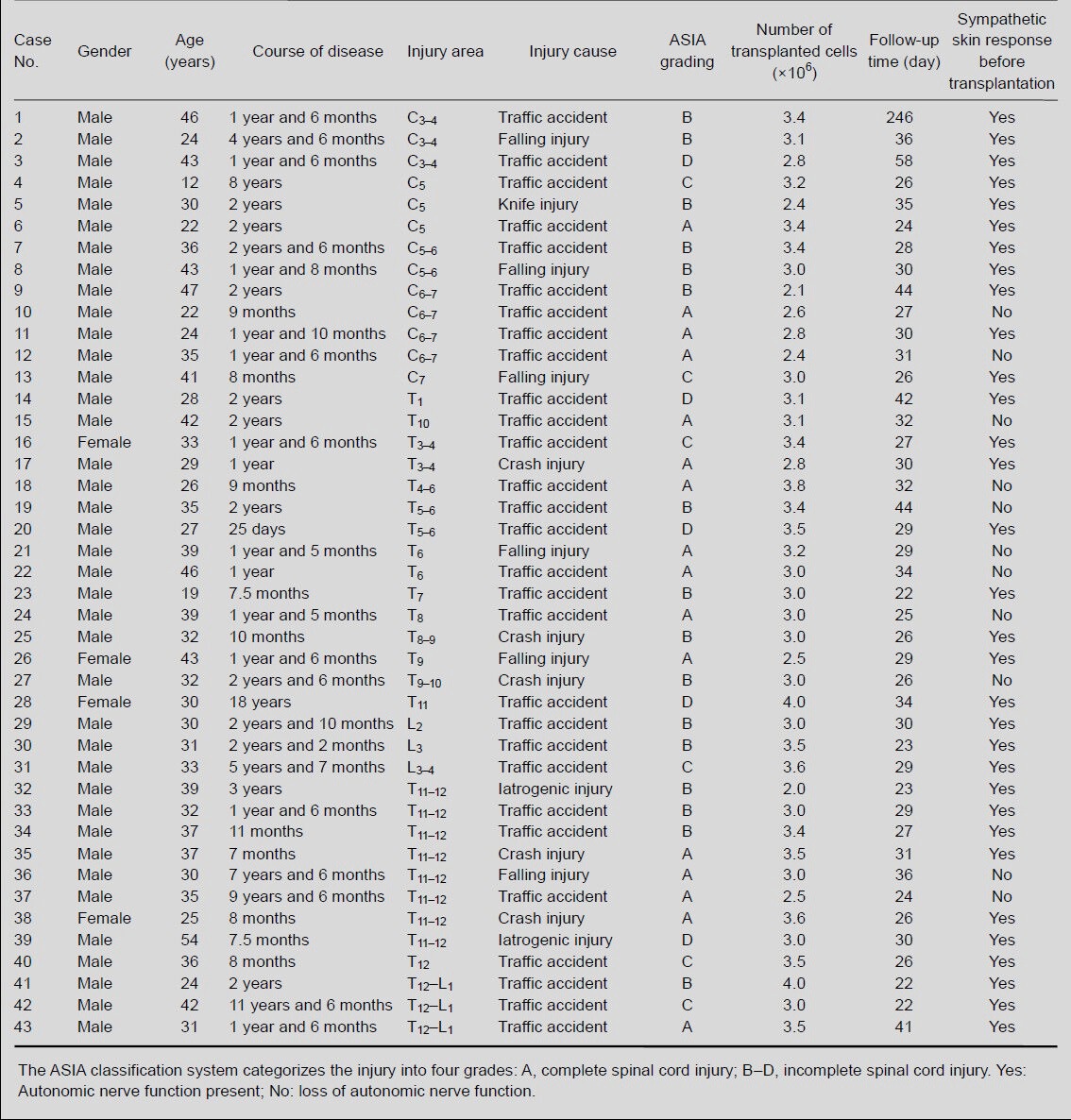
Olfactory ensheathing cell transplantation increased somatic nerve function in patients with chronic spinal cord injury
Somatic nerve function in 43 patients with spinal cord injury was evaluated using the ASIA scale at 3–8 weeks after olfactory ensheathing cell transplantation. After transplantation, the ASIA scores for pain, touch and motor functions increased, and patients showed improvements in sensory ability and motor power. Statistical analysis showed that, after transplantation, the pain scores in these patients increased significantly (P < 0.01), while the touch and motor scores did not increase significantly (P > 0.05; Table 2), compared with before transplantation.
Table 2.
Changes in pain, touch and motor scores using the American Spinal Injury Association scale in patients with spinal cord injury before and after olfactory ensheathing cell transplantation
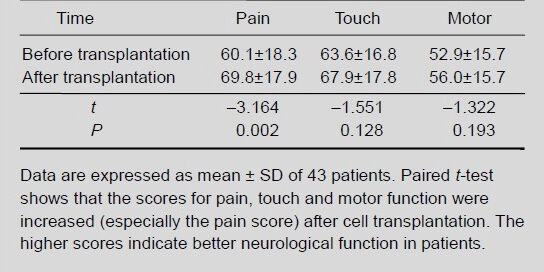
Olfactory ensheathing cell transplantation improved sympathetic skin response in patients with chronic spinal cord injury
After transplantation of olfactory ensheathing cells, the paraplegic patients exhibited increased skin sweating, a brighter skin tone, reduced desquamation, smoother stool, increased urine volume, and a reduction in residual urine volume. The sympathetic skin response was measured with an electromyography/evoked potential instrument. Among the 17 subjects with complete spinal cord injury, 8 cases showed a sympathetic skin response before transplantation and 10 cases showed a response after transplantation. Among the 26 subjects with incomplete spinal cord injury, a sympathetic skin response was observed in 24, both before and after transplantation (the number of cases was unchanged). In total, 32 patients were included in the statistical analysis. After transplantation, the sympathetic skin response amplitude significantly increased, while the latency period significantly decreased (both P < 0.01; Table 3).
Table 3.
Change in sympathetic skin response amplitude (mV) and latency (second) in spinal cord injury patients before and after olfactory ensheathing cell transplantation
DISCUSSION
Effective treatment of chronic spinal cord injury remains a medical challenge. Transplantation of olfactory ensheathing cells may promote neurological recovery in patients with chronic spinal cord injury[1,2,3,4]; however, the degree of recovery is limited[5]. While a great deal of attention has been given to the recovery of somatic nerve (sensory and motor) functions, autonomic nerve function has been neglected. Although the ASIA scale[6] and the Frankel grading system[9] are considered the most effective evaluation methods for spinal cord function and spinal cord injury, respectively, they do not include or reflect autonomic function. In addition, there is no effective method for the evaluation of spinal autonomic nerve function[10]. Furthermore, there is a biased and narrow perception of clinical effectiveness. For example, while no therapeutic effect may be indicated by the outcome index “standing and walking, bowel controlling”, it ignores other aspects of improved neurological functioning. The neurological functions of the spinal cord are complex, and functional abnormalities in the autonomic system manifest as the following[11]: (1) skin color, temperature and nutrition: skin cyanosis, chilling, drying, thinning, subcutaneous tissue slightly swelling, brittle nails, hair loss, trophic ulcer; (2) sphincter functional impairment: urinary retention or incontinence, constipation or incontinence; (3) circulation disorder: increased or lowered blood pressure; (4) peripheral vasomotor disorders: spastic and expanded; (5) sexual dysfunction; (6) hypohidrosis: no or reduced sweat after spinal cord injury.
Therefore, improvements in autonomic nerve functions can alleviate or prevent complications following spinal cord injury, and can greatly impact the patient's health and quality of life.
The sympathetic skin response is a skin sudomotor reflex involving the brain and spinal cord, and is recorded as an epidermal voltage change, which is related to sweat gland secretion[12,13,14]. It is a sympathetic polysynaptic reflex induced by exogenous and endogenous stimuli (including non-noxious)[15,16]. These stimuli recruit thick myelinated sensory nerve fibers or sympathetic sudomotor efferent fibers after integration in the acoustic nerve afferent center, which induces synchronous activity in the sweat glands. The electrical activity potential on the skin surface is recorded. This activity reflects the body-sympathetic nerve reflex, which involves the efferent nerve center composed of the medulla oblongata and the spinal cord. This pathway also involves the sympathetic postganglionic unmyelinated C fibers. Although the efferent central pathways in the human body are not entirely clear, the peripheral pathways include the sympathetic ganglia, preganglionic fibers, postganglionic fibers and skin sweat glands[17]. The degree of autonomic nerve functional impairment in spinal cord injury patients is associated with the severity of injury[7]. The measurement of the sympathetic skin response allows for the evaluation of sympathetic preganglionic fiber integrity, exclusive of supraspinal center abnormalities or sympathetic postganglionic fiber abnormalities[18]. It also shows high specificity and sensitivity in the detection of autonomic nerve function[19,20].
The sympathetic skin response can be used for the evaluation of autonomic nerve function in patients with spinal cord injury[21], especially to assess spinal cord core integrity[22]. The sympathetic skin response is not elicited in all patients with spinal cord injury; it is dependent on the degree and the position of the spinal cord injury, and it is affected by the site of electrical stimulation[18]. Complete transection of the spinal cord results in an absent sympathetic skin response[8,23]. Thus, the presence of an evoked skin response indicates intact sympathetic spinal cord functioning. Consequently, the ASIA grading system is not perfect because of its inability to fully assess autonomic nerve impairment. The sympathetic skin response is a supplementary tool for the detection of complete spinal cord injury and for assessing prognosis.
In this study, 17 patients with complete spinal cord injury were included. Before transplantation, 8 of these patients showed an evoked sympathetic skin response, while after transplantation, 10 patients showed a response. This suggests that spinal cord center functions were improved in two patients. A total of 32 subjects showed an improved sympathetic skin response after transplantation, indicating that olfactory ensheathing cell transplantation can improve the functioning of sympathetic spinal centers in patients with spinal cord injury.
Transplantation of olfactory ensheathing cells may promote the recovery of neurological function following spinal cord injury[24,25,26]. After transplantation of olfactory ensheathing cells, ASIA scores for movement, touch sensation and pain were significantly increased[27]. A total of 43 patients with spinal cord injury were included in the present study. After olfactory ensheathing cell transplantation, the patients showed improvements in sensory ability and motor force. While the ASIA scores for pain, touch sensation and motor function were increased, only the pain scores were significantly higher than before transplantation. This lack of statistical significance may be associated with the small sample size.
From the results of the somatic nerve function (sensory and motor) and autonomic nerve function assessment, we can conclude that olfactory ensheathing cell transplantation can improve neurological functioning in patients with chronic spinal cord injury. The sympathetic skin response can reflect changes in autonomic nerve function, and can be used to assess autonomic nerve functional recovery after transplantation of olfactory ensheathing cells. Consequently, it can be used as a supplement to ASIA scale evaluation.
SUBJECTS AND METHODS
Design
A self-controlled clinical case study.
Time and setting
Experiments were performed in the Department of Orthopedics, Taian Central Hospital, and the Department of Spine and Spinal Cord, Taian Rongjun Hospital, China between July 2009 and July 2010.
Subjects
Spinal cord injury patients who were candidates for olfactory ensheathing cell transplantation were selected from the Department of Orthopedics, Taian Central Hospital, and the Department of Spine and Spinal Cord, Taian Rongjun Hospital, China.
Inclusion criteria
(1) Clinical data are complete, the duration of spinal cord injury is more than 0.5 years, with no recovery of neurological functions after operation, medical treatment or acupuncture and moxibustion therapy (after understanding the potential of olfactory ensheathing cell transplantation, all patients volunteered for the study); (2) The patients can tolerate the cell transplantation operation, with no contraindications; (3) Spinal cord signals display anatomic continuity under MRI.
Exclusion criteria
(1) Clinical or electrophysiological evidence of peripheral nerve injury, other central nerve injury, diabetes or depression; (2) Receiving oral administration of anticholinesterase drugs and adrenaline to affect autonomic nerve activity.
A total of 43 patients with spinal cord injury were included in this study; 33 males and 10 females. No improvement was observed after operation, medication or acupuncture treatment. The spinal cord function was graded according to the ASIA classification system[7]: complete spinal cord injury (grade A) in 16 cases, incomplete spinal injury in 26 cases (grade B in 16 cases, grade C in 6 cases, grade D in 5 cases). The mean age of patients was 30.7 ± 10.3 years, and the average course of disease was 3.7 ± 3 years. The spinal cord injury was localized to the cervical segment in 16 cases, to the thoracic segment in 19 cases, and to the lumbar segment in eight cases. The cause of injury was traffic injury in 29 cases, falling injury in six cases, crush injury in five cases, iatrogenic injury in two cases, and wounds in 1 case. The patients and their relatives signed the informed consent, and the experiments met the Helsinki Declaration ethics requirements.
Methods
Obtainment of olfactory bulb
The olfactory bulb was obtained from embryos at 4–6 months through unintended voluntary delivery in the Taian Central Hospital and the Maternal and Child Health Hospital of Taian, China. Maternal health examinations had excluded infectious and genetic diseases. Pregnant women gave informed consent and signed a voluntary donation consent.
Culture and identification of olfactory ensheathing cells
Olfactory bulbs were stripped and cut into pieces or small tissue blocks, which were then cultured in Dulbecco's modified Eagle's medium/F12 medium containing 10% fetal bovine serum (Gibco, Carlsbad, CA, USA)[1,2]. The cells were digested and prepared into a cell suspension at 1 × 1010 cells/L. Low-affinity neurotrophin receptor (p75) immunofluorescence staining showed that the cultured cells were olfactory ensheathing cells (Figure 1).
Figure 1.
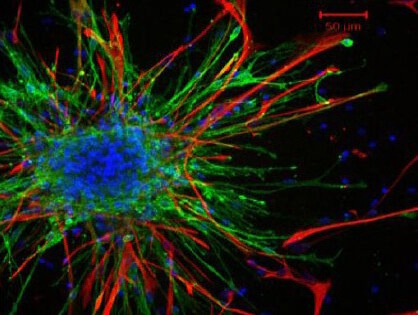
Olfactory ensheathing cells isolated from the olfactory bulb of embryos expressed low-affinity neurotrophin receptor (p75).
Red by immunofluorescence staining. Scale bar: 50 μm.
Cell transplantation
Intramedullary transplantation of olfactory ensheathing cells was performed. In brief, patients under general anesthesia were fixed in the prone position, and using a posterior median approach, the dura mater was cut to expose the injured spinal cord. The cultured olfactory ensheathing cell suspension was transplanted into the injured spinal cord under the surgical microscope (Möller, Nuremberg, Germany). There were 2–5 injection sites located at the ends of the injured area. A 50-μL volume of cell suspension, containing 2–4 × 106 cells, was injected at each site. After transplantation, the wound dressing was changed, and rehabilitation exercise was given. No immunosuppressive or neurotrophic drugs were given.
Evaluation of neurological function
Patients were evaluated with the ASIA scale 1 day before transplantation and 3 weeks–2 months after transplantation[6,27]. The scale is composed of three parts: movement, sensation and pain. The motor function was assessed as grade 1–5 according to the muscle force, for a total of 10 muscles and a total of 100 points. Sensation and pain were assessed as follows: 2 points for normal, 1 point for abnormal, and 0 point for no feeling. A total of 112 points were assessed: 56 pain points and 56 sensory points (including 28 key sensory points). Higher scores indicate better neurological function.
Determination of sympathetic skin response
The sympathetic skin response was measured in a quiet and bright room at 24–26°C. The patients were lying in the supine position, with a palm and foot temperature > 31°C. Using the Keypoint-4 electromyography/evoked potential system (Medtronic; Minneapolis, Minnesota, USA), sympathetic skin responses were evoked by electrical stimulation (0.2 ms duration, 1 Hz frequency, 0.5 mV/D sensitivity). The stimulus intensity was initially 40 mA, and then increased to 60 mA. The left tibial nerve was stimulated using a saddle electrode in the left medial malleolus. All patients were given four stimuli until a stable waveform was observed. Each stimulation was given for 15 minutes, and the interval between stimuli was 1 minute. The recording electrode was a disposable surface electrode located on the right foot. The reference electrode was placed on the foot back, corresponding to the recording electrode, and the grounding electrode was a ring electrode located between the left heel and the left ankle. The sympathetic skin response latency and amplitude were recorded 1 day before transplantation and 3 weeks to 2 months after transplantation.
Statistical analysis
Data were analyzed using SPSS 13.0 software (SPSS, Chicago, IL, USA) and were expressed as mean ± SD. Differences before and after transplantation were compared with the paired t-test. A P-value less than 0.05 was considered significant.
Acknowledgments
We are grateful to Professor Huang HY from the Department of Neurosurgery, Beijing Rehabilitation Center, and Professor Wang DJ from the International Spinal Injury Association, as well as the Taian Maternal and Child Healthcare Hospital and the Taian Central Hospital in China for support.
Footnotes
Conflicts of interest: None declared.
Ethical approval: The project was approved by the ethics committee of Taian Rongjun Hospital and Taian Center Hospital in China.
(Reviewed by Patel B, Norman C, Pan Y, Ma HH, Xiu B)
(Edited by Wang LM, Yang Y, Li CH, Song LP, Liu WJ, Zhao M)
REFERENCES
- [1].DeLucia TA, Conners JJ, Brown TJ, et al. Use of a cell line to investigate olfactory ensheathing cell-enhanced axonal regeneration. Anat Rec B New Anat. 2003;271(1):61–70. doi: 10.1002/ar.b.10014. [DOI] [PubMed] [Google Scholar]
- [2].Keyvan-Fouladi N, Raisman G, Li Y. Functional repair of the corticospinal tract by delayed transplantation of olfactory ensheathing cells in adult rats. J Neurosci. 2003;23(28):9428–9434. doi: 10.1523/JNEUROSCI.23-28-09428.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Chen L, Chen D, Xi H, et al. Olfactory ensheathing cell neurorestorotherapy for amyotrophic lateral sclerosis patients: benefits from multiple transplantations. Cell Transplant. 2012;21(Suppl 1):S65–77. doi: 10.3727/096368912X633789. [DOI] [PubMed] [Google Scholar]
- [4].Huang H, Wang H, Chen L, et al. Influence factors for functional improvement after olfactory ensheathing cell transplantation for chronic spinal cord injury. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2006;20(4):434–438. [PubMed] [Google Scholar]
- [5].Radtke C, Sasaki M, Lankford KL, et al. CNPase expression in olfactory ensheathing cells. J Biomed Biotechnol 2011. 2011 doi: 10.1155/2011/608496. 608496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ditunno JF, Jr, Young W, Donovan WH, et al. The international standards booklet for neurological and functional classification of spinal cord injury. American Spinal Injury Association. Paraplegia. 1994;32(2):70–80. doi: 10.1038/sc.1994.13. [DOI] [PubMed] [Google Scholar]
- [7].Atlanta, GA: American Spinal Injury Association: International Stan-dards for Neurological Classification of Spinal Cord Injury, Revised 2011; 2011. [Google Scholar]
- [8].Burton AR, Brown R, Macefield VG. Selective activation of muscle and skin nociceptors does not trigger exaggerated sympathetic responses in spinal-injured subjects. Spinal Cord. 2008;46(10):660–665. doi: 10.1038/sc.2008.33. [DOI] [PubMed] [Google Scholar]
- [9].Maynard FM, Jr, Bracken MB, Creasey G, et al. International Standards for Neurological and Functional Classification of Spinal Cord Injury. American Spinal Injury Association. Spinal Cord. 1997;35(5):266–274. doi: 10.1038/sj.sc.3100432. [DOI] [PubMed] [Google Scholar]
- [10].Grangeon M, Charvier K, Guillot A, et al. Using sympathetic skin responses in individuals with spinal cord injury as a quantitative evaluation of motor imagery abilities. Phys Ther. 2012;92(6):831–840. doi: 10.2522/ptj.20110351. [DOI] [PubMed] [Google Scholar]
- [11].Krassioukov A, Biering-Sørensen F, Donovan W, et al. International standards to document remaining autonomic function after spinal cord injury. J Spinal Cord Med. 2012;35(4):201–210. doi: 10.1179/1079026812Z.00000000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Emad R, Zafarghasempour M, Roshanzamir S. Sympathetic skin response in incomplete spinal cord injury with urinary incontinence. Ann Indian Acad Neurol. 2013;16(2):234–238. doi: 10.4103/0972-2327.112479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Macefield VG, James C, Henderson LA. Identification of sites of sympathetic outflow at rest and during emotional arousal: Concurrent recordings of sympathetic nerve activity and fMRI of the brain. Int J Psychophysiol. doi: 10.1016/j.ijpsycho.2013.06.002. in press. [DOI] [PubMed] [Google Scholar]
- [14].Xia JD, Han YF, Zhou LH, et al. Sympathetic skin response in patients with primary premature ejaculation. Int J Impot Res. doi: 10.1038/ijir.2013.23. in press. [DOI] [PubMed] [Google Scholar]
- [15].Dolu N, Keloglan S, Bitiktas S, et al. The effects of the enriched environment on sympathetic skin response in pentylenetetrazol-kindled rats. Biomed Environ Sci. 2013;26(5):394–397. doi: 10.3967/0895-3988.2013.05.009. [DOI] [PubMed] [Google Scholar]
- [16].Jiang H, Wang L, Wang X, et al. Comparison of skin sympathetic reaction in patients with generalized anxiety disorder and with major depression disorder. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2013;42(2):192–196. doi: 10.3785/j.issn.1008-9292.2013.02.010. [DOI] [PubMed] [Google Scholar]
- [17].Vetrugno R, Liguori R, Cortelli P, et al. Sympathetic skin response: basic mechanisms and clinical applications. Clin Auton Res. 2003;13(4):256–270. doi: 10.1007/s10286-003-0107-5. [DOI] [PubMed] [Google Scholar]
- [18].Kumru H, Vidal J, Perez M, et al. Sympathetic skin responses evoked by different stimuli modalities in spinal cord injury patients. Neurorehabil Neural Repair. 2009;23(6):553–558. doi: 10.1177/1545968308328721. [DOI] [PubMed] [Google Scholar]
- [19].Giza E, Katsarou Z, Georgiadis G, et al. Sympathetic skin response in Parkinson's disease before and after mental stress. Neurophysiol Clin. 2012;42(3):125–131. doi: 10.1016/j.neucli.2011.11.002. [DOI] [PubMed] [Google Scholar]
- [20].Al-Moallem MA, Zaidan RM, Alkali NH. The sympathetic skin response in diabetic neuropathy and its relationship to autonomic symptoms. Saudi Med J. 2008;29(4):568–572. [PubMed] [Google Scholar]
- [21].Curt A, Weinhardt C, Dietz V. Significance of sympathetic skin response in the assessment of autonomic failure in patients with spinal cord injury. J Auton Nerv Syst. 1996;61(2):175–180. doi: 10.1016/s0165-1838(96)00080-x. [DOI] [PubMed] [Google Scholar]
- [22].Prévinaire JG, Mathias CJ, El Masri W, et al. The isolated sympathetic spinal cord: Cardiovascular and sudomotor assessment in spinal cord injury patients: A literature survey. Ann Phys Rehabil Med. 2010;53(8):520–532. doi: 10.1016/j.rehab.2010.06.006. [DOI] [PubMed] [Google Scholar]
- [23].Ogura T, Kubo T, Lee K, et al. Sympathetic skin response in patients with spinal cord injury. J Orthop Surg (Hong Kong) 2004;12(1):35–39. doi: 10.1177/230949900401200108. [DOI] [PubMed] [Google Scholar]
- [24].Huang H, Xi H, Chen L, et al. Long-term outcome of olfactory ensheathing cell therapy for patients with complete chronic spinal cord injury. Cell Transplant. 2012;21(Suppl 1):S23–31. doi: 10.3727/096368912X633734. [DOI] [PubMed] [Google Scholar]
- [25].Salehi M, Pasbakhsh P, Soleimani M, et al. Repair of spinal cord injury by co-transplantation of embryonic stem cell-derived motor neuron and olfactory ensheathing cell. Iran Biomed J. 2009;13(3):125–135. [PubMed] [Google Scholar]
- [26].Huang H, Chen L, Wang H, et al. Safety of fetal olfactory ensheathing cell transplantation in patients with chronic spinal cord injury. A 38-month follow-up with MRI. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2006;20(4):439–443. [PubMed] [Google Scholar]
- [27].Zheng ZC, Wei KB, Liu F, et al. Clinical verification of olfactory ensheathing cell transplantation in treatment of spinal cord injury. Zhongguo Zuzhi Gongcheng Yanjiu yu Linchuang Kangfu. 2010;14(27):5119–5122. [Google Scholar]


