Abstract
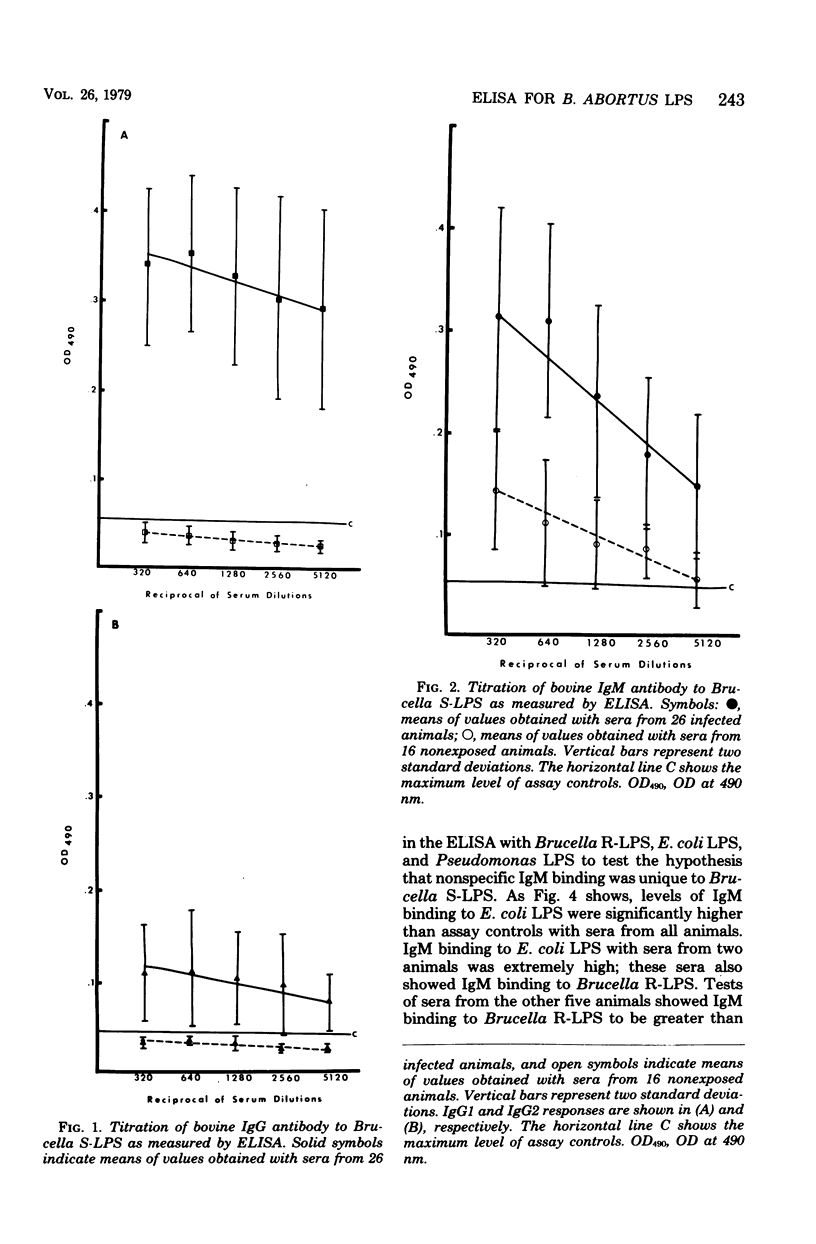
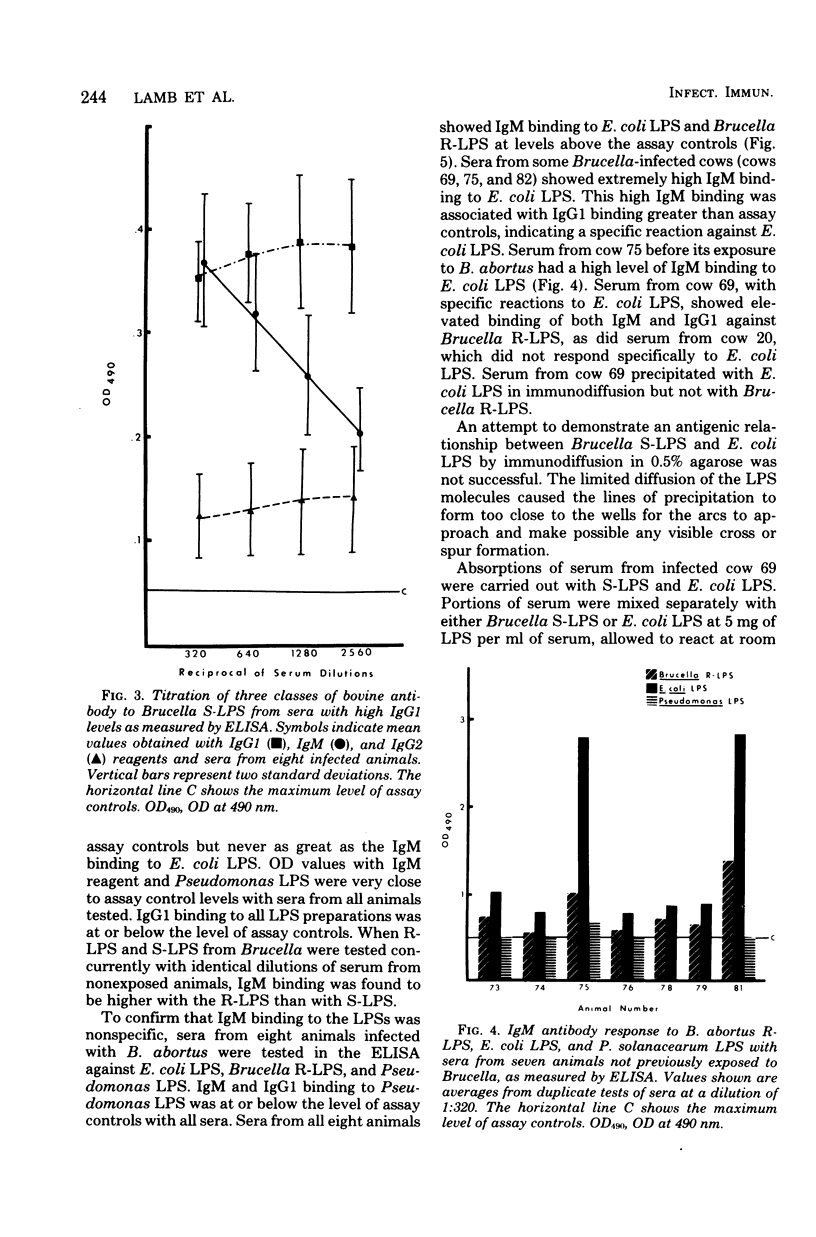
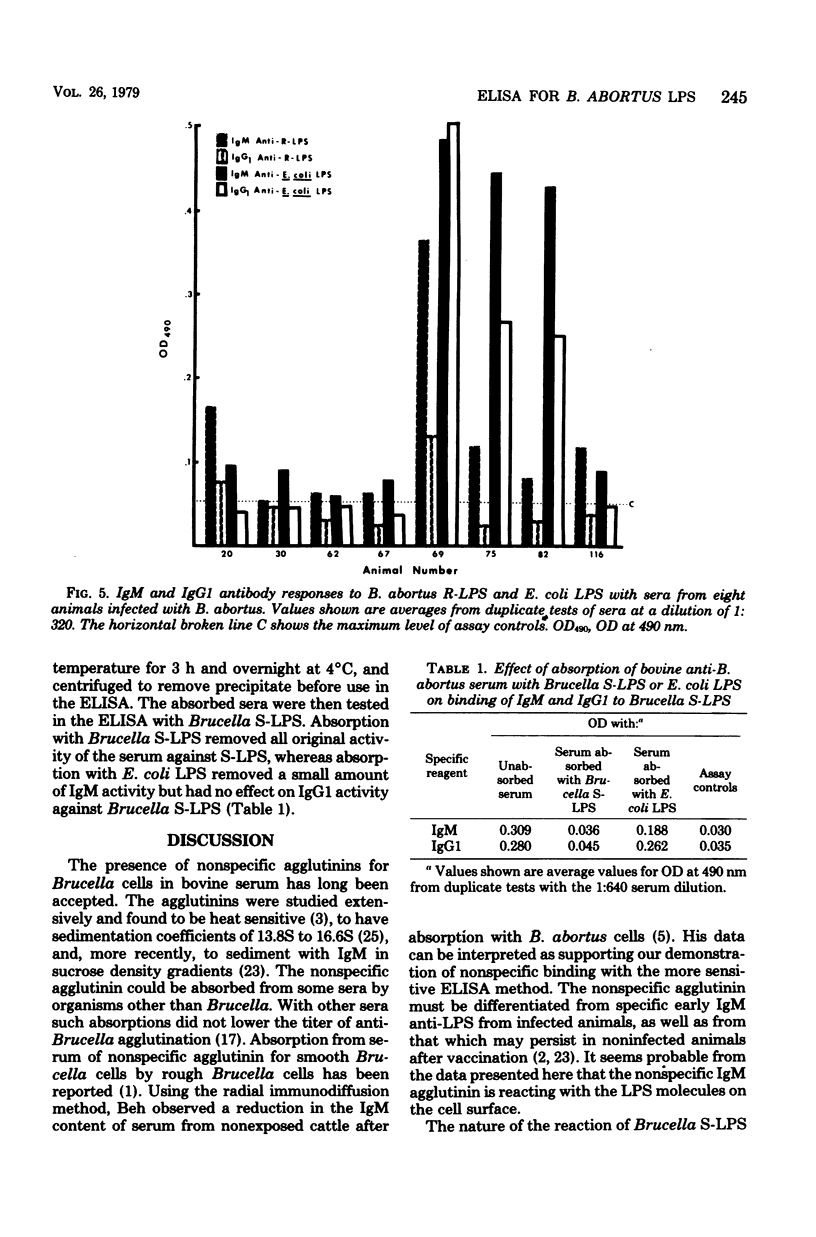
An enzyme-linked immunosorbent assay was developed to follow the bovine response, by immunoglobulin class and subclass, to defined smooth and rough lipopolysaccharides (LPS) of Brucella abortus. Binding to smooth LPS of immunoglobulin G1 (IgG1) and IgG2 in sera from Brucella-infected animals was significantly greater than binding in sera from normal uninfected animals. Competition or steric blocking among IgM, IgG1, and IgG2 for binding sites on smooth LPS was shown to occur. Binding of IgM to Brucella smooth LPS with sera from uninfected animals was elevated above the assay control levels, and attempts to eliminate this nonspecific IgM binding were not successful. The same levels of nonspecific IgM binding were also seen with Brucella rough LPS, Escherichia coli LPS, and Pseudomonas solanacearum LPS. Sera from some, but not all, Brucella-infected animals showed elevated binding of IgG1 and IgM to both E. coli LPS and Brucella rough LPS as well as to Brucella smooth LPS. This was interpreted as specific antibody. Cross-reactions between B. abortus smooth or rough LPS and E. coli LPS could not be shown by immunodiffusion.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMERAULT T. E., LAMBERT G., MANTHEI C. A. The heat stability of Brucella agglutinins in bovine serum. Am J Vet Res. 1962 Sep;23:1023–1026. [PubMed] [Google Scholar]
- AMERAULT T. E., MANTHEI C. A., GOODE E. R., Jr, LAMBERT G. A heat-inactivation test for differentiating specific and non-specific agglutination reactions for bovine brucellosis. Am J Vet Res. 1961 May;22:564–569. [PubMed] [Google Scholar]
- Avrameas S., Ternynck T. Biologically active water-insoluble protein polymers. I. Their use for isolation of antigens and antibodies. J Biol Chem. 1967 Apr 10;242(7):1651–1659. [PubMed] [Google Scholar]
- Beh K. J., Lascelles A. K. The use of the antiglobulin test in the diagnosis of bovine brucellosis. Res Vet Sci. 1973 Mar;14(2):239–244. [PubMed] [Google Scholar]
- Binaghi R. A., Oriol R., Boussac-Aron Y. Immunogenicity of heterologous Fc and Fab immunoglobulin fragments in rabbits, guinea-pigs and rats. Immunology. 1967 Jul;13(1):63–69. [PMC free article] [PubMed] [Google Scholar]
- Bruins S. C., Ingwer I., Zeckel M. L., White A. C. Parameters affecting the enzyme-linked immunosorbent assay of immunoglobulin G antibody to a rough mutant of Salmonella minnesota. Infect Immun. 1978 Sep;21(3):721–728. doi: 10.1128/iai.21.3.721-728.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson H. E., Hurvell B., Lindberg A. A. Enzyme-linked immunosorbent assay (ELISA) for titration of antibodies against Brucella abortus and Yersinia enterocolitica. Acta Pathol Microbiol Scand C. 1976 Jun;84(3):168–176. doi: 10.1111/j.1699-0463.1976.tb00016.x. [DOI] [PubMed] [Google Scholar]
- Chappel R. J., McNaught D. J., Bourke J. A., Allan G. S. Comparison of the results of some serological tests for bovine brucellosis. J Hyg (Lond) 1978 Jun;80(3):365–371. doi: 10.1017/s0022172400024815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz R., Levieux D. Rôle respiectif en sérologie de la brucellose bovine des antigènes et des immunoglobulines G 1 et G 2 dans les tests d'agglutination, de Coombs et au Rose Bengale ainsi que dans le phénomène de zone. C R Acad Sci Hebd Seances Acad Sci D. 1972 Mar 6;274(10):1593–1596. [PubMed] [Google Scholar]
- Duncan J. R., Wilkie B. N., Hiestand F., Winter A. J. The serum and secretory immunoglobulins of cattle: characterization and quantitation. J Immunol. 1972 Apr;108(4):965–976. [PubMed] [Google Scholar]
- Eisen H. N., Little J. R., Steiner L. A., Simms E. S., Gray W. Degeneracy in the secondary immune response: stimulation of antibody formation by cross-reacting antigens. Isr J Med Sci. 1969 May-Jun;5(3):338–351. [PubMed] [Google Scholar]
- Engvall E., Perlmann P. Enzyme-linked immunosorbent assay (ELISA). Quantitative assay of immunoglobulin G. Immunochemistry. 1971 Sep;8(9):871–874. doi: 10.1016/0019-2791(71)90454-x. [DOI] [PubMed] [Google Scholar]
- HESS W. R. Studies on a nonspecific Brucella-agglutinating substance in bovin serum. I. The differentiation of the specific and nonspecific agglutinins by heat treatment. Am J Vet Res. 1953 Apr;14(51):192–194. [PubMed] [Google Scholar]
- Hebert G. A. Ammonium sulfate fractionation of sera: mouse, hamster, guinea pig, monkey, chimpanzee, swine, chicken, and cattle. Appl Microbiol. 1974 Feb;27(2):389–393. doi: 10.1128/am.27.2.389-393.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leong D., Diaz R., Milner K., Rudbach J., Wilson J. B. Some structural and biological properties of Brucella endotoxin. Infect Immun. 1970 Feb;1(2):174–182. doi: 10.1128/iai.1.2.174-182.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno E., Pitt M. W., Jones L. M., Schurig G. G., Berman D. T. Purification and characterization of smooth and rough lipopolysaccharides from Brucella abortus. J Bacteriol. 1979 May;138(2):361–369. doi: 10.1128/jb.138.2.361-369.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mylrea P. J., Fraser G. C. The use of supplementary tests in the serological diagnosis of bovine brucellosis. Aust Vet J. 1976 Jun;52(6):261–266. doi: 10.1111/j.1751-0813.1976.tb00103.x. [DOI] [PubMed] [Google Scholar]
- Nakane P. K., Kawaoi A. Peroxidase-labeled antibody. A new method of conjugation. J Histochem Cytochem. 1974 Dec;22(12):1084–1091. doi: 10.1177/22.12.1084. [DOI] [PubMed] [Google Scholar]
- Patterson J. M., Deyoe B. L., Stone S. S. Identification of immunoglobulins associated with complement fixation, agglutination, and low pH buffered antigen tests for brucellosis. Am J Vet Res. 1976 Mar;37(3):319–324. [PubMed] [Google Scholar]
- ROSE J. E., ROEPKE M. H. An acidified antigen for detection of nonspecific reactions in the plate-agglutination test for bovine brucellosis. Am J Vet Res. 1957 Jul;18(68):550–555. [PubMed] [Google Scholar]
- ROSE J. E., ROEPKE M. H., BRIGGS D. R. PHYSICOCHEMICAL PROPERTIES OF NONSPECIFIC BOVINE SEROAGGLUTININS FOR BRUCELLA ABORTUS. Am J Vet Res. 1964 Jan;25:118–121. [PubMed] [Google Scholar]
- Saunders G. C., Clinard E. H., Bartlett M. L., Sanders W. M. Application of the indirect enzyme-labeled antibody microtest to the detection and surveillance of animal diseases. J Infect Dis. 1977 Oct;136 (Suppl):S258–S266. doi: 10.1093/infdis/136.supplement_2.s258. [DOI] [PubMed] [Google Scholar]
- Vos J. G., Buys J., Hanstede J. G., Hagenaars A. M. Comparison of enzyme-linked immunosorbent assay and passive hemagglutination method for quantification of antibodies to lipopolysaccharide and tetanus toxoid in rats. Infect Immun. 1979 Jun;24(3):798–803. doi: 10.1128/iai.24.3.798-803.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]



