Abstract
Baicalin, a type of flavonoid extracted from the dried root of Scutellaria baicalensis georgi, has been shown to effectively inhibit cell apoptosis. Therefore, we assumed that baicalin would suppress co-listin sulfate-induced neuronal apoptosis. PC12 cells exposed to colistin sulfate (62.5–500 μg/mL) for 24 hours resulted in PC12 cell apoptosis. In addition, caspase-3 activity, lactate dehydrogenase level and free radical content increased in a dose-dependent manner. Subsequently, PC12 cells were pretreated with baicalin (25, 50 and 100 μg/mL), and exposed to 125 μg/mL colistin sulfate. Cell morphology markedly changed, and cell viability increased. Moreover, caspase-3 activity, tate dehydrogenase level and free radical content decreased. Results indicated that baicalin inhi-bited colistin sulfate-induced PC12 cell apoptosis by suppressing free radical injury, and reducing caspase-3 activity and lactate dehydrogenase activity.
Keywords: neural regeneration, traditional Chinese medicine, baicalin, colistin sulfate, PC12 cells, apoptosis, caspase-3, lactate dehydrogenase, grants-supported paper, neuroregeneration
Research Highlights
We are the first to report that the Chinese medicinal extract baicalin can inhibit colistin sulfate-induced PC12 cell apoptosis by inhibiting free radical damage, and reducing caspase-3 activity and lactate dehydrogenase level.
INTRODUCTION
Colistin sulfate, a polymyxin E sulfate, is a cyclic cationic polypeptide antibiotic that was first introduced in 1952. Since the 1980s, colistin sulfate has been used to treat infections caused by gram-negative bacteria, particularly those that are resistant to almost all classes of commercially available antibiotics[1,2,3,4]. However, colistin sulfate has been shown to have renal and neurological side effects. Through alteration of the outer membrane, colistin sulfate facilitates its own uptake and subsequently disrupts the bacterial cytoplasmic membrane. Resistance to colistin sulfate rarely occurs[5,6]. Although previous studies have shown that nephrotoxicity is the most common adverse effect of colistin sulfate (9–50%)[7,8,9,10,11] and that neurological toxicity is also an adverse effect, the role of colistin sulfate in inducing neuronal cell apoptosis remains unclear.
The PC12 cell line is derived from a tumor found in the rat adrenal gland, and has been widely used as in vitro model to investigate the neurotoxicity of various compounds and study mechanisms associated with neurodegenerative disorders[12,13]. The cells contain the enzymes required for synthesis and decomposition of dopamine such as tyrosine hydroxylase[8] and monoamine oxidase[14]. The membrane receptors and synthesized transmitters in PC12 cells are similar to dopaminergic neurons located in the midbrain. Therefore, PC12 cell lines have been used as a cellular model in Parkinsonism studies[15]. Polyphenols were chosen based on previous findings suggesting beneficial effects and decreased reactive oxygen species levels associated with delivery of polyphenols under oxidative stress conditions[16].
Baicalin, a type of flavonoid extracted from the dried root of Scutellaria baicalensis georgi[17], has a chemical formula of C21H18O11 and a relative molecular weight of 446.35. Previous studies have shown pharmacological effects of baicalin, such as antioxidation and scavenging of oxygen-free radicals[18,19]. Baicalin effectively protects against concanavalin A-induced liver cell apoptosis[20]. Baicalin pretreatment protects neonatal rats against hypoxic-ischemic brain damage, and reverses neonatal injuries[21]. Similar protective effects of baicalin have also been observed on oxidized low-density lipoprotein-induced apoptosis in vascular endothelial cells[22], and baicalin can inhibit amyloid-induced neuronal apoptosis[23]. However, little information exists on the role of baicalin in colistin sulfate-induced neuronal apoptosis. This study investigated the mechanisms of colistin sulfate-induced apoptosis in PC12 cells and the protective effect of baicalin in colistin sulfate-induced apoptosis of PC12 cells.
RESULTS
Baicalin increased cell viability in colistin sulfate-treated PC12 cells
The 3-[4,5-dimethylthiazol-2-yl]-2,5 diphen-yltetrazolium bromide (MTT) assay showed that compared with the control group, cell viability after treatment with colistin sulfate (62.5–500 μg/mL) was reduced from 94% to 27.2% in a concentration-dependent manner (P < 0.05 or P < 0.01) (Figure 1A). At the lower concentrations of colistin sulfate (125 μg/mL), cell viability reached 66.4%. This concentration was used for all subsequent experiments. As shown in Figure 1B, it was observed that pretreatment with baicalin (25, 50 and 100 μg/mL) significantly decreased colistin sulfate (125 μg/mL)-induced cell death (P < 0.01 or P < 0.05).
Figure 1.
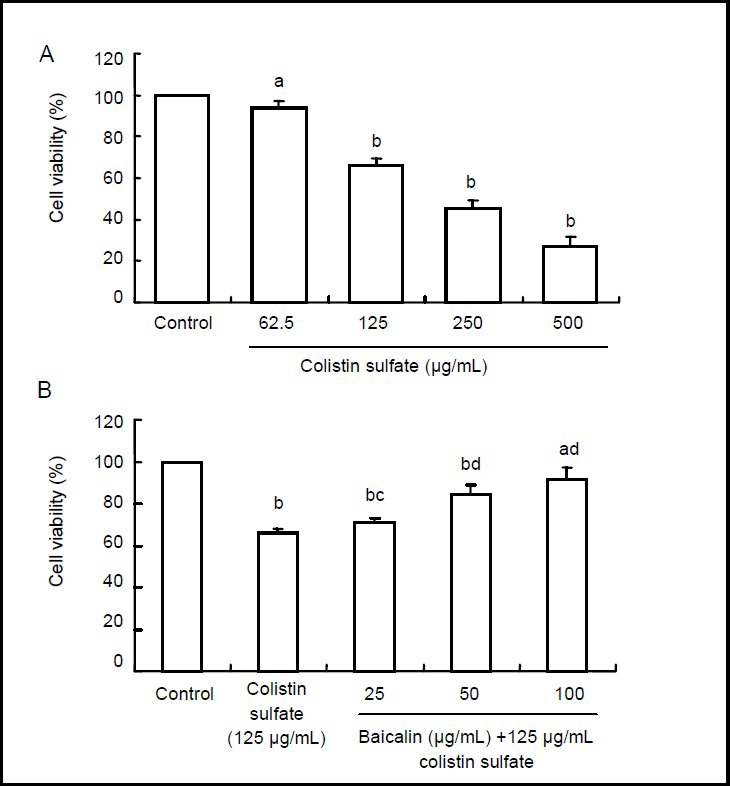
Colistin sulfate-induced cell death and a neuroprotective effect of baicalin on PC12 cells (MTT assay).
Cell viability = average absorbance value of experimental group/average absorbance value of control group × 100%. Data are expressed as an absorbance ratio to the control (untreated neurons). Data are expressed as mean ± SD. Significance of differences between means was determined by paired t-test. All experiments were repeated at least three times. aP < 0.05, bP < 0.01, vs. control group. cP < 0.05, dP < 0.01, vs. colistin sulfate group.
(A) Neurotoxic effect of colistin sulfate (62.5 to 500 μg/mL) at 24 hours.
(B) Neuroprotective effects of baicalin against colistin sulfate-induced neurotoxicity in PC12 cells (24 hours).
Baicalin improved morphologic changes in colistin sulfate-treated PC12 cells
As shown in Figure 2A, with an increased dose of colistin sulfate, cell morphology was significantly altered, the number of cells decreased, cells shrank and aggregated into clusters, axons decreased or were absent, and vacuoles and small spots were present when compared with the control group. A large number of cells died when treated with 500 μg/mL colostin (data not shown). As shown in Figure 2B, pretreatment with baicalin (25, 50 and 100 μg/mL) markedly improved the colistin sulfate-induced morphologic change of PC12 cells.
Figure 2.

Morphological evaluation of PC12 cells (inverted phase-contrast microscopy).
(A) Effect of different doses of colistin sulfate on PC12 cells (× 200). (a) Control group, (b–d) 62.5, 125 and 250 μg/mL. Black arrows: Cell shrinkage and aggregation into clusters; white arrows: vacuoles in cytoplasm. (a) Control group, normal cells, normal morphology, and obvious axons. (b) A few shrunken cells and clusters of aggregated cells. (c) A lot of shrunken cells and aggregated clusters, vacuoles in cytoplasm, and a reduction in axons. (d) Almost all cells were shrunken and aggregated into clusters, vacuoles were in cytoplasm, axons decreased or were absent.
(B) Neuroprotective effect of baicalin against colistin sulfate-induced neurotoxicity in PC12 cells (× 400). (a) Control group: normal cells, normal morphology, and obvious axons. (b) 125 μg/mL colistin sulfate group: abundant cells were shrunken and aggregated into clusters, vacuoles and small spots in cytoplasm, the axons almost absent. PC12 cells were pretreated with 25 (c), 50 (d), 100 μg/mL (e) baicalin, and induced with 125 μg/mL colistin sulfate. (c) Numerous cells exhibited vacuoles and small spots in the cytoplasm, and the axons began to appear. (d) Vacuoles and small spots in the cytoplasm decreased, and the axons of PC12 cells increased. (e) Cells showed normal morphology. Black arrows: Vacuoles in cytoplasm; white arrows: spots in cytoplasm.
Evaluation of the apoptotic morphology of PC12 cells using Hoechst 33258 staining was monitored using an inverted fluorescence microscope (Figure 3). Characteristics of colistin sulfate-induced apoptosis were associated with chromatin condensation. As shown in Figure 3A, an increased dose of colistin sulfate resulted in condensation and fragmentation of nuclei when compared with the control group. As shown in Figure 3B, pretreatment with baicalin (25, 50 and 100 μg/mL) markedly decreased condensation and fragmentation of nuclei in colistin sulfate-treated PC12 cells.
Figure 3.

Apoptotic morphology of PC12 cells after Hoechst 33258 staining (inverted fluorescence microscopy, × 200).
Arrows show condensed and fragmented nuclei.
(A) Different doses of colistin sulfate-treated PC12 cells. (a) Control group, normal cells, without condensed and fragmented nuclei. (b) 62.5 μg/mL colistin sulfate group: condensed and fragmented nuclei are visible in several cells. (c) 125 μg/mL colistin sulfate group: condensed and fragmented nuclei are observed in many cells. (d) 250 μg/mL colistin sulfate group: condensed and fragmented nuclei are detectable in almost all cells.
(B) Neuroprotective effect of baicalin against colistin sulfate-induced neurotoxicity in PC12 cells. (a) Control group: normal cells, without condensed and fragmented nuclei. (b) 125 μg/mL colistin sulfate group: condensed and fragmented nuclei were detected in many cells. PC12 cells were pretreated with 25 (c), 50 (d), and 100 μg/mL (e) baicalin, and induced with 125 μg/mL colistin sulfate. (c, d) Compared with the colistin sulfate-induced group, condensation and fragmentation were significantly decreased. (e) Cells exhibited normal morphology.
Baicalin reduced lactate dehydrogenase release in colistin sulfate-treated PC12 cells
As shown in Figure 4, the levels of lactate dehydrogenase release significantly increased after incubation of PC12 cells with various concentrations of colistin sulfate (62.5–500 μg/mL) for 24 hours (P < 0.01 or P < 0.05; Figure 4A). When the cells were pretreated with baicalin (50 and 100 μg/mL) for 24 hours, lactate dehydrogenase release levels were significantly reduced in comparison to cells treated with colistin sulfate (125 μg/mL) (P < 0.01 or P < 0.05; Figure 4B).
Figure 4.
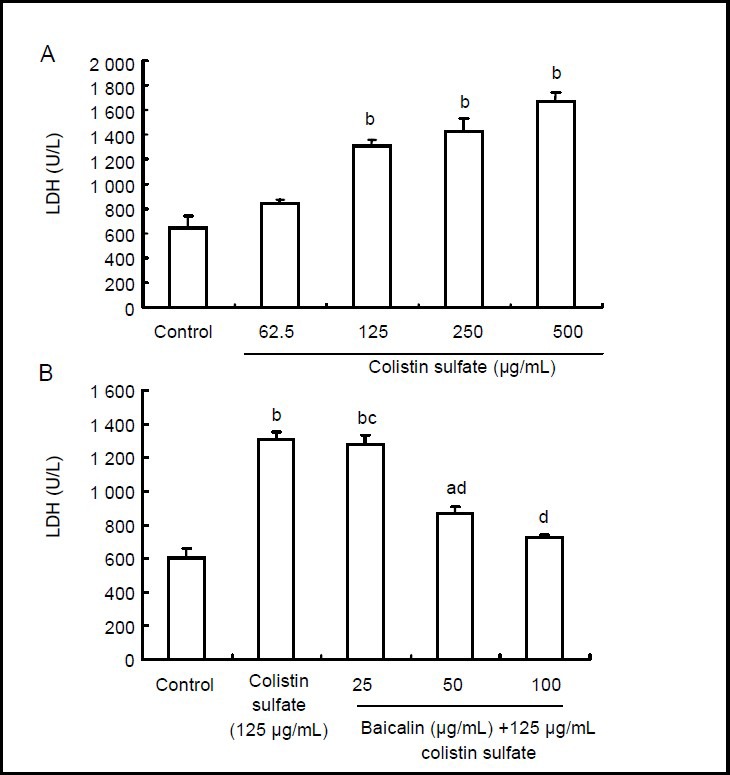
Effect of baicalin on colistin sulfate-induced neurotoxicity in PC12 cells.
Data are expressed as mean ± SD. Significant differences between means were determined using the paired t-test. All experiments were repeated at least three times. aP < 0.05, bP < 0.01, vs. control group. cP < 0.05, dP < 0.01, vs. colistin sulfate group.
(A) Neurotoxic effect of colistin sulfate at concentrations ranging from 62.5 to 500 μg/mL for 24 hours using the lactate dehydrogenase (LDH) assay kit.
(B) Neuroprotective effect of baicalin against colistin sulfate-induced neurotoxicity in PC12 cells detected using the LDH assay kit. The cells were preincubated with baicalin (final concentrations: 0, 25, 50, 100 μg/mL) for 24 hours, then the cells were exposed to 125 μg/mL colistin sulfate for 24 hours.
Effects of baicalin on caspase-3 activity in colistin sulfate-treated PC12 cells
Caspase-3 is an important regulatory protein expressed during apoptosis[23]. Caspase-3 was activated in PC12 cells by colistin sulfate treatment. Exposure to colistin sulfate for 24 hours caused an increase in caspase-3 activity in a dose-dependent manner (P < 0.05 or P < 0.01; Figure 5A). As shown in Figure 5B, when PC12 cells were treated with baicalin (25, 50 and 100 μg/mL) for 24 hours prior to exposing cells to colistin sulfate, caspase-3 activity was significantly reduced in comparison to cells treated with colistin sulfate (125 μg/mL) (P < 0.01 or P < 0.05). The above-described results showed that baicalin suppressed caspase-3 activity in a dose-dependent manner.
Figure 5.
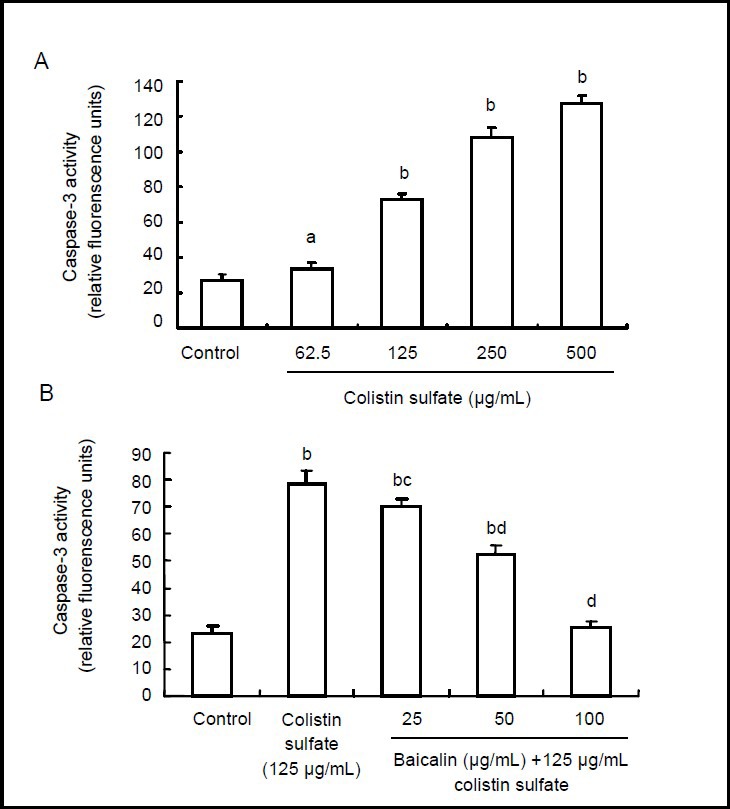
Effect of baicalin on caspase-3 expression after colistin sulfate induction (fluorometric analysis).
Caspase-3 levels were expressed as relative fluorescence units. One unit is the amount of enzyme that will cleave 1.0 nmol of the colorimetric substrate Ac-DEVD-pNA per hour at 37°C under saturated substrate concentrations. Data are expressed as mean ± SD. Significance of differences between means was determined by paired t-test. All experiments were repeated at least three times. aP < 0.05, bP < 0.01, vs. control group. cP < 0.05, dP < 0.01, vs. colistin group.
(A) Neurotoxic effect of colistin sulfate at concentrations ranging from 0 to 500 μg/mL for 24 hours.
(B) Neuroprotective effect of baicalin against colistin sulfate-induced neurotoxicity in PC12 cells. Cells were preincubated with baicalin (final concentrations: 0, 25, 50, 100 μg/mL) for 24 hours, and then they were cultured in 125 μg/mL colistin sulfate for 24 hours.
Intracellular accumulation of oxygen-free radicals in PC12 cells
We next determined if baicalin attenuated caspase-3 activity by its antioxidant property or whether suppression of free radical production was sufficient to prevent apoptosis. To evaluate dose-dependent reactive oxygen species generation, dichloro-fluorescein oxidation was measured fluorometrically after 24 hours of cell incubation in the presence of colistin sulfate.
Fluorescence significantly increased from 36% to 137% of the control without colistin sulfate (P < 0.01 or P < 0.05; Figure 6A). Pretreatment with baicalin (50 and 100 μg/mL) for 24 hours significantly suppressed the generation of reactive oxygen species in comparison to cells treated with colistin sulfate (125 μg/mL) (P < 0.01; Figure 6B).
Figure 6.

Baicalin prevented oxidative stress induced by colistin sulfate in PC12 cells (fluorometric assay).
Reactive oxygen species (ROS) level = (average absorbance value of experimental group/average absorbance value of control group) × 100%. Results are expressed as the % absorbance of the control (untreated neurons). Data are expressed as mean ± SD. Significant differences between means were determined by paired t-test. All experiments were repeated at least three times. aP < 0.05, bP < 0.01, vs. control group. cP < 0.01, vs. colistin group.
(A) Neurotoxic effect of colistin sulfate at concentrations ranging from 0 to 500 μg/mL for 24 hours.
(B) Neuroprotective effect of baicalin against colistin sulfate-induced neurotoxicity in PC12 cells. Cells were preincubated with baicalin (final concentrations: 0, 25, 50, 100 μg/mL) for 24 hours, and then they were cultured in 125 μg/mL colistin sulfate for 24 hours.
DISCUSSION
Neuronal apoptosis is an important pathological mechanism and a key event for memory loss. Therefore, disrupting the apoptotic pathway and inhibiting apoptosis have become novel targets[24]. The present study demonstrated that cell survival was significantly reduced following colistin sulfate injury. Further tests showed that colistin sulfate significantly induced cell apoptosis in a dose-dependent manner.
Data from this study showed that treatment with colistin sulfate resulted in a significant increase in reactive oxygen species levels in a dose-dependent manner. Generation of reactive oxygen species in the cytoplasm of cells may increase mitochondrial hydrogen peroxide production and lipid peroxidation of the mitochondrial membrane, resulting in the loss of membrane integrity and cell necrosis or apoptosis. This was consistent with our hypothesis addressing the neurological toxicity mechanism of colistin sulfate. In addition, colistin sulfate-injured cells shrank and aggregated into clusters; axons reduced, vacuoles appeared, and the number of cells with condensed and fragmented nuclei increased. Moreover, lactate dehydrogenase activity in the supernatant significantly increased in colistin sulfate-injured PC12 cells, caspase-3 activity increased and PC12 cell survival decreased. The above-mentioned data showed that colistin sulfate could induce apoptosis in PC12 cells.
Baicalin, a flavonoid with a glucuronide structure, produces baicalin and glucuronic acid following hydrolysis. Many phenolic hydroxyl groups exist in the chemical structural formula of baicalin, and the A ring comprises a catechol structure. The number of phenolic hydroxyl groups is strongly associated with free radical scavenging activity[25]. A greater number of phenolic hydroxyl groups and a greater amount of hydrogen atoms that integrate with free radicals result in more effective free radical scavengers[25]. Pharmacological studies have revealed a strong effect of baicalin to remove hydroxyl, superoxide, and alkyl radicals, as well as to prevent and reverse cellular apoptosis and aging mechanisms[18]. The present study used baicalin to inhibit colistin sulfate-induced apoptosis in PC12 cells and increase cell viability in a dose-dependent manner. This study confirmed that baicalin was effective at protecting PC12 cells against colistin sulfate injury. This study further showed that baicalin, not only suppressed the generation of reactive oxygen species and decreased lactate dehydrogenase level, but also attenuated caspase-3 activity, and eventually protected against colistin sulfate-induced apoptosis. Our findings indicate that baicalin may act on reactive oxygen species to inhibit apoptosis following colistin sulfate-induced neurotoxicity. This study especially demonstrated a significant decrease in lactate dehydrogenase level, caspase-3 activity and reactive oxygen species production in PC12 cells preincubated with baicalin following colistin sulfate exposure. Previous studies have used other oxidants such as tert-butylhydroperoxide and H2O2 as oxidative stressors because they have been shown to efficiently increase the activity of endogenous enzymes in cultured cells[26,27]. Plant polyphenolic compounds such as panduratin A and sylibin, as well as plant extracts, which have high antioxidant activity, have been shown to reverse the glutathione-depleting effect of tert-butylhydroperoxide in hepatocyte systems[28]. Our research data also suggests that baicalin may have antioxidant properties.
In conclusion, our findings, in conjunction with data reported by other investigators, clearly indicate that colistin sulfate can induce PC12 cell apoptosis and that baicalin effectively inhibits colistin sulfate-induced apoptosis in PC12 cells. In addition, mechanistic studies showed that baicalin protected neurons and regulated key steps of cellular apoptosis, such as excessive inhibition of free radical injury, caspase-3 expression, and lactate dehydrogenase activity. When colistin is used to control multidrug resistant bacterial infections in hospitals, we suggest that a concentration of 50 μg/mL baicalin be added in colistin sulfate injections to protect against colistin-induced neurotoxicity. Simultaneously, baicalin in conjunction with colistin can be used to control bacterial infections and increase the clinical curative effect. Overall, our study provides the basis and reference for long-term reasonable use of colistin in a clinical setting.
MATERIALS AND METHODS
Design
An in vitro, comparative, cell biology study.
Time and setting
The experiment was performed from July 2011 to March 2012 at the Veterinary Pharmacology Laboratory, College of Veterinary Medicine, Northeast Agricultural University, China.
Materials
Cells
PC12 cells were obtained from the Chinese Academy of Sciences (Shanghai, China).
Drugs
Baicalin (lot No. 110715-200514) was bought from the Chinese Veterinary Medicine Supervision Institute and stored in a dark and dry place at room temperature.
Colistin sulfate (CAS No.1 264-72-8, purity ≥ 99.9%) was purchased from Sigma-Aldrich (St. Louis, MO, USA) and stored in a dark and dry place at 2–8°C.
Colistin sulfate and baicalin were dissolved in Dulbecco's modified Eagle's medium (DMEM).
Methods
PC12 cell culture
PC12 cells were cultured in DMEM (Gibco, New York, USA) supplemented with 10% (v/v) fetal calf serum (Gibco) in 25 cm2 flasks. Cells were maintained in a humidified atmosphere containing 5% (v/v) CO2 at 37°C and were seeded at a density of 2 × 105 cells/mL. After 24 hours, cells were grown in flasks, or on 6- or 96-well plates.
Exposure to baicalin and colistin sulfate
For colistin sulfate exposure, PC12 cells were incubated with colistin sulfate at doses of 62.5–250 μg/mL for 24 hours. For baicalin protective experiments, cells were preincubated with baicalin (final concentrations: 0, 25, 50, 100 μg/mL) for 24 hours, and then exposed to 125 μg/mL colistin sulfate for 24 hours. In all experiments, control groups received DMEM.
Analysis of cell viability
The MTT assay was used to assess cell viability. Cells were seeded onto 96-well plates at a density of 1 × 105 cells/well. After cells were incubated for 24 hours, the supernatant was discarded, and each well was treated with 5 mg/mL MTT (Sigma-Aldrich) for 4 hours. The reaction was terminated in 150 μL anhydrous dimethyl sulfoxide (DMSO; Sigma-Aldrich) with shaking for 10 minutes. The absorbance at 570 nm was measured on a Sunrise enzyme immunoassay instrument (Tecan, Sweden)[29,30]. Cell viability was calculated using the following equation: average absorbance value of experimental group/average absorbance value of control group × 100%.
Examination of nuclear morphology detected by Hoechst 33258
Hoechst 33258 was employed to label both intact and apoptotic nuclei. Cells were seeded onto 96-well plates at a density of 1 × 105 cells/well. Following treatment, PC12 cells were washed in ice-cold phosphate buffered saline (PBS; pH 7.4), fixed with 4% (w/v) paraformaldehyde and incubated with 1 μg/mL Hoechst 33258 (Sigma-Aldrich) for 3 minutes at room temperature. Condensed and fragmented nuclei were evaluated by intercalation of the fluorescent probe into nuclear DNA. Visualization was conducted at an excitation wavelength of 480 nm and an emission wavelength of 520 nm using the Olympus IMT-2 fluorescence microscope (Tokyo, Japan).
Lactate dehydrogenase release assay
Cytotoxicity was quantitatively assessed by measuring the activity of lactate dehydrogenase released from damaged cells into the cell culture medium[30]. At the end of the treatments, PC12 cells and the media which contained detached cells were collected and centrifuged at 1 000 r/min at 4°C for 5 minutes. The supernatant was used to measure lactate dehydrogenase activity. Enzyme activity was determined using an assay kit according to the manufacturer's protocol (Nanjing Jiancheng Biotech, Nanjing, Jiangsu Province, China). The absorbance of the samples was read at 450 nm. Lactate dehydrogenase release was proportional to the number of damaged PC12 cells. Reagent blanks were subtracted.
Fluorometric analysis for caspase-3 activity
Caspase-3 activity was measured with an ApoAlert Caspase Assay Kit (Clontech Laboratories, USA). Briefly, following treatment, cells were washed with PBS and collected into tubes. The tubes were centrifuged and the supernatant was removed. Cell pellets were suspended in lysis buffer (10 mmol/L Tris, 1% (v/v) Triton X-100 in PBS, pH 7.5), placed on ice for 20 minutes and centrifuged (30 minutes, 12 000 × g) at 4°C. Aliquots of cell lysates (25 μg protein) were dissolved in 175 μL protease assay buffer (20 mmol/L 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES; pH 7.5), 2 mmol/L dithiothreitol and 10% (v/v) glycerol), followed by addition of 25 μL 7-amino-4-methylcoumarin-N-acetyl-L-aspartyl-L-gltamyl-L-valyl-L-aspartic acid amide (AC-DEVD-AMC; Sigma-Aldrich) (15 μmol/L). After 1 hour incubation at 37°C in the dark, substrate cleavage was measured fluorometrically in a black 96-well plate at an excitation wavelength of 355 nm and an emission wavelength of 460 nm. Fluorescence values of cell-free wells containing 15 μmol/L AC-DEVD-AMC in the assay buffer were subtracted from the fluorescence values of cell samples. Cell lysates containing cytosolic fractions were collected and total protein concentration was determined using the BCA™ protein assay kit (Boster Biotech, Wuhan, Hubei Province, China). The microplate spectrofluorometer reader (Perkin Elmer, Vernon Hills, USA) output corresponding to caspase-3 activity was corrected for total protein. Caspase-3 activity was expressed as relative fluorescence units.
Evaluation of intracellular reactive oxygen species generation
To measure reactive oxygen species generation, a fluorometric assay using intracellular oxidation of DCFH2-DA was performed as reported previously with slight modifications[31]. DCFH2-DA is a nonfluorescent compound, and can be enzymatically converted to the highly fluorescent compound DCF in the presence of reactive oxygen species. After treatment, PC12 cells were incubated with 200 μL medium containing 2 μL of a 20 mmol/L stock solution of DCFH2-DA (Sigma-Aldrich) dissolved in ethanol. Cells were left in the dark for 30 minutes at 37°C in a 5% (v/v) CO2 atmosphere. After loading, cells were washed twice with normal medium and DCF fluorescence was measured using the Wallac Victor Multilabel Counter (Perkin Elmer, Vernon Hills, USA) with excitation and emission wavelengths of 485 and 530 nm, respectively[32,33]. Results were expressed as a percentage of controls.
Statistical analysis
Data are presented as mean ± SD and were analyzed by SPSS 16.0 software (SPSS, Chicago, IL, USA). A paired t-test was used to compare between two groups and analysis of variance was used for multiple comparisons. A value of P < 0.05 or P < 0.01 was considered statistically significant.
Footnotes
Funding: The work was supported by the National Natural Science Foundation of China, No. 31201951 and 31272613; the Scientific and Technological Innovation Talent Scientific Research Foundation for the Returned Overseas Chinese Scholars by State Education Ministry and Heilongjiang Province in China, No. 2012RFLXN005 and LC201018; the Youth Science and Technology Foundation of Liaoning Medical University in China, No. Y2012Z023; and the Science and Technology Department of Liaoning Provincial Foundation Programs, No. 2011214001.
Conflicts of interest: None declared.
(Reviewed by Diwakarla S, Robens J, Bao YL, Chen X)
(Edited by Yu J, Qiu Y, Li CH, Song LP, Liu WJ, Zhao M)
REFERENCES
- [1].Falagas ME, Kasiakou SK. Toxicity of polymyxins: a systematic review of the evidence from old and recent studies. Crit Care. 2006;10(1):R27. doi: 10.1186/cc3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Michalopoulos A, Falagas ME. Colistin and polymyxin B in critical care. Crit Care Clin. 2008;24(2):377–391. doi: 10.1016/j.ccc.2007.12.003. x. [DOI] [PubMed] [Google Scholar]
- [3].Michalopoulos AS, Tsiodras S, Rellos K, et al. Colistin treatment in patients with ICU-acquired infections caused by multiresistant Gram-negative bacteria: the renaissance of an old antibiotic. Clin Microbiol Infect. 2005;11(2):115–121. doi: 10.1111/j.1469-0691.2004.01043.x. [DOI] [PubMed] [Google Scholar]
- [4].Zavascki AP, Goldani LZ, Li J, et al. Polymyxin B for the treatment of multidrug-resistant pathogens: a critical review. J Antimicrob Chemother. 2007;60(6):1206–1215. doi: 10.1093/jac/dkm357. [DOI] [PubMed] [Google Scholar]
- [5].Conway SP, Brownlee KG, Denton M, et al. Antibiotic treatment of multidrug-resistant organisms in cystic fibrosis. Am J Respir Med. 2003;2(4):321–332. doi: 10.1007/BF03256660. [DOI] [PubMed] [Google Scholar]
- [6].Littlewood JM, Koch C, Lambert PA, et al. A ten year review of colomycin. Respir Med. 2000;94(7):632–640. doi: 10.1053/rmed.2000.0834. [DOI] [PubMed] [Google Scholar]
- [7].Cheng CY, Sheng WH, Wang JT, et al. Safety and efficacy of intravenous colistin (colistin methanesulphonate) for severe multidrug-resistant Gram-negative bacterial infections. Int J Antimicrob Agents. 2010;35(3):297–300. doi: 10.1016/j.ijantimicag.2009.11.016. [DOI] [PubMed] [Google Scholar]
- [8].Betrosian AP, Frantzeskaki F, Xanthaki A, et al. Efficacy and safety of high-dose ampicillin/sulbactam vs. colistin as monotherapy for the treatment of multidrug resistant Acinetobacter baumannii ventilator-associated pneumonia. J Infect. 2008;56(6):432–436. doi: 10.1016/j.jinf.2008.04.002. [DOI] [PubMed] [Google Scholar]
- [9].Furtado GH, d’Azevedo PA, Santos AF, et al. Intravenous polymyxin B for the treatment of nosocomial pneumonia caused by multidrug-resistant Pseudomonas aeruginosa. Int J Antimicrob Agents. 2007;30(4):315–319. doi: 10.1016/j.ijantimicag.2007.05.017. [DOI] [PubMed] [Google Scholar]
- [10].Holloway KP, Rouphael NG, Wells JB, et al. Polymyxin B and doxycycline use in patients with multidrug-resistant Acinetobacter baumannii infections in the intensive care unit. Ann Pharmacother. 2006;40(11):1939–1945. doi: 10.1345/aph.1H353. [DOI] [PubMed] [Google Scholar]
- [11].Oliveira MS, Prado GV, Costa SF, et al. Ampicillin/sulbactam compared with polymyxins for the treatment of infections caused by carbapenem-resistant Acinetobacter spp. J Antimicrob Chemother. 2008;61(6):1369–1375. doi: 10.1093/jac/dkn128. [DOI] [PubMed] [Google Scholar]
- [12].Meng H, Li C, Feng L, et al. Effects of Ginkgolide B on 6-OHDA-induced apoptosis and calcium over load in cultured PC12. Int J Dev Neurosci. 2007;25(8):509–514. doi: 10.1016/j.ijdevneu.2007.09.010. [DOI] [PubMed] [Google Scholar]
- [13].Sasaki N, Toda T, Kaneko T, et al. Flavonoids suppress the cytotoxicity of linoleic acid hydroperoxide toward PC12 cells. Biol Pharm Bull. 2002;25(8):1093–1096. doi: 10.1248/bpb.25.1093. [DOI] [PubMed] [Google Scholar]
- [14].Qu L, Chen H, Liu X, et al. Protective effects of flavonoids against oxidative stress induced by simulated microgravity in SH-SY5Y cells. Neurochem Res. 2010;35(9):1445–1454. doi: 10.1007/s11064-010-0205-4. [DOI] [PubMed] [Google Scholar]
- [15].Shafer TJ, Atchison WD. Transmitter, ion channel and receptor properties of pheochromocytoma (PC12) cells: a model for neurotoxicological studies. Neurotoxicology. 1991;12(3):473–492. [PubMed] [Google Scholar]
- [16].Crispo JA, Ansell DR, Piche M, et al. Protective effects of polyphenolic compounds on oxidative stress-induced cytotoxicity in PC12 cells. Can J Physiol Pharmacol. 2010;88(4):429–438. doi: 10.1139/y09-137. [DOI] [PubMed] [Google Scholar]
- [17].China Pharmacopoeia Committee. Beijing: Chemical Industry Press; 2005. Pharmacopoeia of the People's Republic of China. [Google Scholar]
- [18].Huang Y, Tsang SY, Yao X, et al. Biological properties of baicalein in cardiovascular system. Curr Drug Targets Cardiovasc Haematol Disord. 2005;5(2):177–184. doi: 10.2174/1568006043586206. [DOI] [PubMed] [Google Scholar]
- [19].Song LL, Meng QG. Study development on pharmacodynamics effect of scutellaria. Zhonghua Zhongyiyao Xuekan. 2008;26(8):29. [Google Scholar]
- [20].Liu LL, Gong LK, Wang H, et al. Baicalin protects mouse from Concanavalin A-induced liver injury through inhibition of cytokine production and hepatocyte apoptosis. Liver Int. 2007;27(4):582–591. doi: 10.1111/j.1478-3231.2007.01450.x. [DOI] [PubMed] [Google Scholar]
- [21].Lin XJ, Yang YJ, Qi BX, et al. Protective effects of baicalin pretreatment on hypoxic-ischemic brain damage in neonatal rats. Zhongguo Dang Dai Er Ke Za Zhi. 2006;8(3):221–224. [PubMed] [Google Scholar]
- [22].Gao AD, Shen XJ. Protective effect of baicalin on apoptosis of vascular endothelial cells induced by oxidized low density lipoprotein. Shijie Zhongyiyao. 2009;4(5):44. [Google Scholar]
- [23].Geng M, Chen H, Wang J, et al. Protective effects of baicalin on amyloid beta 25-35-induced apoptosis in human neuroblastoma SH-SY5Y cells. Neural Regen Res. 2010;5(22):1739–1744. [Google Scholar]
- [24].Camins A, Pallas M, Silvestre JS. Apoptotic mechanisms involved in neurodegenerative diseases: experimental and therapeutic approaches. Methods Find Exp Clin Pharmacol. 2008;30(1):43–65. doi: 10.1358/mf.2008.30.1.1090962. [DOI] [PubMed] [Google Scholar]
- [25].Ng TB, Liu F, Wang ZT. Antioxidative activity of natural products from plants. Life Sci. 2000;66(8):709–723. doi: 10.1016/s0024-3205(99)00642-6. [DOI] [PubMed] [Google Scholar]
- [26].Alía M, Ramos S, Mateos R, et al. Response of the antioxidant defense system to tert-butyl hydroperoxide and hydrogen peroxide in a human hepatoma cell line (HepG2) J Biochem Mol Toxicol. 2005;19(2):119–128. doi: 10.1002/jbt.20061. [DOI] [PubMed] [Google Scholar]
- [27].Chen X, Zhang N, Zou HY. Protective effect of baicalin on mouse with Parkinson's disease induced by MPTP. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2007;27(11):1010–1012. [PubMed] [Google Scholar]
- [28].Sohn JH, Han KL, Lee SH, et al. Protective effects of panduratin A against oxidative damage of tert-butylhydroperoxide in human HepG2 cells. Biol Pharm Bull. 2005;28(6):1083–1086. doi: 10.1248/bpb.28.1083. [DOI] [PubMed] [Google Scholar]
- [29].Ahmadian S, Barar J, Saei AA, et al. Cellular toxicity of nanogenomedicine in MCF-7 cell line: MTT assay. J Vis Exp. 2009;26 doi: 10.3791/1191. pii: 1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Peng Q, Wei Z, Lau BH. Fructus corni attenuates oxidative stress in macrophages and endothelial cells. Am J Chin Med. 1998;26(3-4):291–300. doi: 10.1142/S0192415X98000336. [DOI] [PubMed] [Google Scholar]
- [31].Lee YM, Park SH, Shin DI, et al. Oxidative modification of peroxiredoxin is associated with drug-induced apoptotic signaling in experimental models of Parkinson disease. J Biol Chem. 2008;283(15):9986–9998. doi: 10.1074/jbc.M800426200. [DOI] [PubMed] [Google Scholar]
- [32].Li Y, Shi W, Li Y, et al. Neuroprotective effects of chlorogenic acid against apoptosis of PC12 cells induced by methylmercury. Environ Toxicol Pharmacol. 2008;26(1):13–21. doi: 10.1016/j.etap.2007.12.008. [DOI] [PubMed] [Google Scholar]
- [33].Zhao Y, Bu Q, Zhou Y, et al. Mechanism study of Aconitum-induced neurotoxicity in PC12 cells: involvement of dopamine release and oxidative damage. Neurotoxicology. 2010;31(6):752–757. doi: 10.1016/j.neuro.2010.06.005. [DOI] [PubMed] [Google Scholar]


