Abstract
Propagated sensation along the meridian can occur when acupoints are stimulated by acupuncture or electrical impulses. In this study, participants with notable propagated sensation along the dian were given electro-acupuncture at the Jianyu (LI15) acupoint of the large intestine meridian. When participants stated that the sensation reached the back of their hand, regular nervous system action discharge was examined using a physiological recording electrode placed on the superficial branch of the radial nerve. The topographical maps of brain-evoked potential in the primary cortical somatosensory area were also detected. When Guangming (GB37) acupoint in the lower limb and Hegu (LI4) acupoint in the upper limb were stimulated, subjects without propagated sensation along the meridian exhibited a high potential reaction in the corresponding area of the brain cortical so-matosensory area. For subjects with a notable propagated sensation along the meridian, the tion area was larger and extended into the face representative area. These electrophysiological measures directly prove the existence of propagated sensation along the meridian, and the pheral stimulated site is consistent with the corresponding primary cortical somatosensory area, which presents a high potential reaction.
Keywords: neural regeneration, electro-acupuncture, propagated sensation along meridian, expanding of central excitation, discharge of afferent nerve, topographical mapping of brain-evoked potential, peripheral nerve, central nerve, grants-supported paper, neuroregeneration
Research Highlights
-
(1)
Participants confirmed sensation along the meridian following acupuncture, suggesting that acupuncture induced a nerve action potential discharge.
-
(2)
We observed changes in functional activity of the cortical somatosensory area using topo-graphical mapping of brain-evoked potential in volunteers that reported notable propagated sensa-tion along the meridian and those that reported no propagated sensation. We also examined the mechanism of the propagated sensation along the meridian from the peripheral and central nervous system.
INTRODUCTION
In traditional Chinese medicine, the meridian is a complex network of 12 channels that cover the entire body. Inside the body, the meridian belongs to the viscus, while outside of the body, it connects the limb joints. The meridian promotes qi and blood, and nourishes yin and yang. It is a contact, regulation, and response system important for function of the body, and has been widely examined in clinical practice[1,2] and biomedical research.
Propagated sensation along the meridian is an important part of traditional Chinese medicine meridian. Numerous clinical trials and census data have shown that propagated sensation along the meridian is universal in humans[3,4,5,6,7,8,9,10,11]. Propagated sensation along the meridian has three characteristics that are effective in the clinic, which include propagated along meridian. Further, the therapeutic effects of propagated sensation along the meridian can be blocked. In acupuncture clinics, induction of propagated sensation along the meridian is associated with improved therapeutic effects. For example, propagated sensation along the meridian toward the affected region can produce marked health benefits, including a ‘single one needle effect’. The mechanism of propagated sensation along the meridian has been widely studied[12,13,14,15,16], and is thought to involve either ‘expansion of central excitation’ or ‘stimulation of peripheral reason’. Expansion of central excitation suggests that the propagated sensation along the meridian is caused by directed diffusion of the acupuncture-induced excitatory signal in the central nervous system (especially in the cerebral cortex). This signal circulates in the periphery, although the essence exists in the center of the body; this viewpoint is also termed ‘feeling in the central nervous system, circulation in the central nervous system’. For peripheral stimulation, some motivations (or effective processes) run along the channels when acupuncture is given, which stimulates nervous senses distributed along the meridian. This, in turn, causes nerve impulses to be successively transmitted to the central nervous system. Therefore, the propagated sensation along the meridian produces a subjective feeling in participants; this viewpoint is also termed ‘feeling in the central nervous system, circulation in the body surface’[17]. Although there are suggestive experimental data for these mechanisms, there is no direct evidence.
As such, in the present study we examined the hypothesis that integration of the periphery and the central nervous system occurs via domination of substantial processes that proceed along the meridians[18,19]. It should be emphasized that ‘periphery’ and ‘central nervous system’ are indivisible as a whole in the formation of propagated sensation along the meridian. There is an effective process of transmission along the channel in the periphery, whereas there is a functional connection in the central nervous system. Peripheral effective processes play an important role during the cooperative process between the periphery and the central nervous system. However, it remains unclear whether there is a motivation in the periphery that occurs during propagated sensation along the meridian. If the propagated sensation along the meridian transmits to some loci, the afferent discharge can be recorded from the sensory nerve that is located at that point[20,21]. It is well established that some motivations occur in the ‘surface’ during propagated sensation along the meridian, providing objective evidence for the existence of peripheral motivation. However, as the propagated sensation along the meridian is a subjective feeling, there is no direct evidence for its presence.
In the present study, we used electrophysiological methods to examine whether afferent nervous discharges could be recorded when transmission arrived at the sensory nerve during acupuncture-induced propagated sensation. In addition, we determined whether the propagated sensation along the meridian actually exists using an objective electrophysiological index, and elucidated the mechanism of propagated sensation along the meridian using topographical mapping of evoked potentials in the primary cortical somatosensory area.
RESULTS
Quantitative analysis of experimental subjects
Thirty volunteers participated in this experiment; 19 with marked propagated sensation along the meridian, and 11 without propagated sensation along the meridian. There were no significant differences between the two groups of participants in sex ratio, age, height, and body mass (P > 0.05, Table 1). All participants completed the experiments.
Table 1.
General information of participants
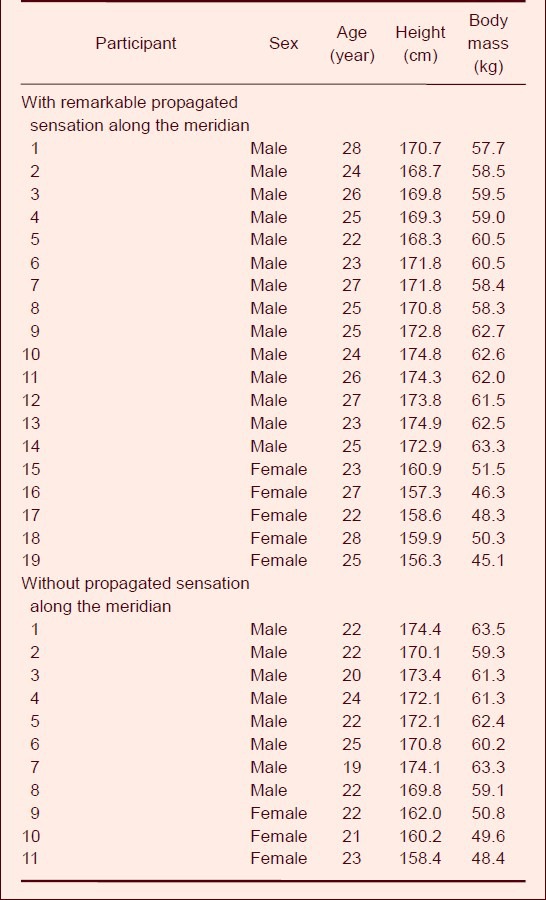
Comparison of the discharge between the afferent nerve of the superficial branch of the radial nerve and electromyography
An electromyographic signal is a complex biological electrical wave formed when a muscle contracts. This signal is related to the physiological characteristics of muscle tissue and the neural control system. Changes in electromyographic signals are regulated by the number and activity patterns of motor units and metabolic conditions. Electromyographic signals can be used to accurately reflect the state of muscle activity and function accurately in real time[22], and can be easily distinguished from nerve action potentials. To confirm that the potentials recorded in this study were from the superficial branch of the radial nerve, we performed a control experiment on the afferent nerve discharge of the superficial branch of the radial nerve using electromyography. We found that the afferent nerve discharge of the superficial branch of the radial nerve was clearly observed when the receptive field on the back of the second knuckle of the index finger was stimulated by pulling; a typical three-phase action potential is shown on the third line in Figure 1.
Figure 1.
The afferent nerve discharge of the superficial branch of the radial nerve.
The nerve action potential was detected using a guide electrode, and was input into a VC-10 storage phase oscilloscope and stored in a tape recorder.
(A) Nervous discharge (downward arrow represents stimulation onset, upward arrow represents stimulation end).
(B) Control record of the subcutaneous electrode located 1 cm lateral to the superficial branch of the radial nerve.
(C) Nervous system discharge recordings after amplification. The receptive field was the dorsal part of the second forefinger knuckle.
When tactual stimulation was stopped, the action potential disappeared. However, no electromyographic responses could be recorded in the control electrode located 1 cm beside the superficial branch of the radial nerve. By contrast, when the participant moved the index finger voluntarily, a series of electromyographic signals of different sizes and in a dense array was observed in both recording and reference electrodes. Larger ranges of the finger movement triggered an increasing frequency and size of the electromyography signal. Overall, these data validate that the electrode recordings represent afferent discharges induced by the stimulation of the receptive fields (Figure 1).
Discharge records of the afferent nerve when the propagated sensation along the meridian reached the superficial branch of the radial nerve
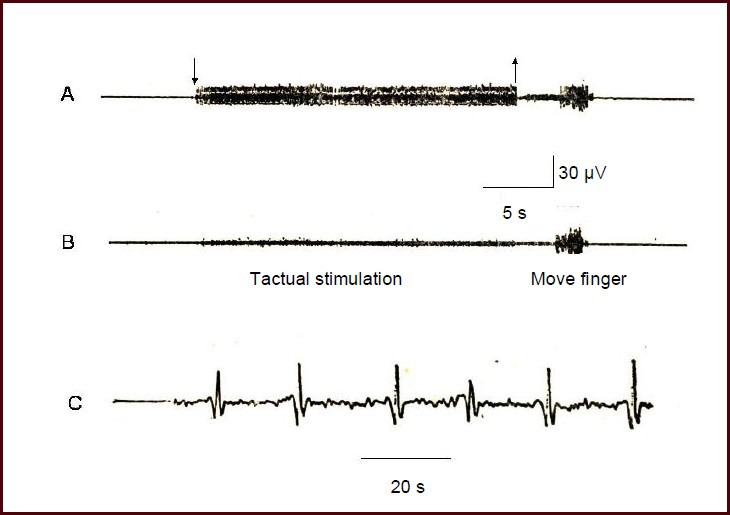
Nineteen volunteers with significant propagated sensation along the meridian were stimulated on Jianyu (LI15) acupoint in the large intestine meridian. A regular discharge was recorded at the superficial branch of the radial nerve in 18 volunteers when the propagated sensation along the meridian reached the back of hand and the dorsal part of the index finger. These results were stable and could be repeated. One volunteer exhibited a propagated sensation along the meridian that only reached the elbow joint, but did not transmit down to the finger.
Figure 2 shows a representative example of the experimental records from a participant. After electro-acupuncture at Jianyu, participants could feel one or multiple senses of aching, swelling, and numbness, which were slowly transmitted bi-directionally through the line of the large intestine meridian (rate of approximately 2–5 cm/s). When participants clearly expressed that the propagated sensation along the meridian had reached the elbow and wrist, the guide electrode positioned on the superficial branch of radial nerve failed to record any response. By contrast, when participants felt the propagated sensation at the back of the hands, the regular neuromotor discharge appeared on the guide electrode. When electro-acupuncture stimulation stopped, the propagated sensation along the meridian and the discharge both disappeared. Once electro-acupuncture was stimulated again, the action potentials reappeared. When the afferent discharge was amplified, a typical three-phase action potential was observed.
Figure 2.
The afferent nerve discharge when the propagated sensation along the meridian reached the superficial branch of the radial nerve.
The nerve action potential was detected using a guide electrode, and was input into a VC-10 storage phase oscilloscope and stored in a tape recorder.
(A) Time mark (seconds). (B) Mark of the location reached by the propagated sensation along the meridian. (C) Records of the afferent nervous discharge. (D) Nervous system discharge after amplification.
Acupuncture point: Jianyu acupoint; recording location: dorsal branch of the ipsilateral radial nerve; receptive field: dorsal part of the second forefinger knuckle.
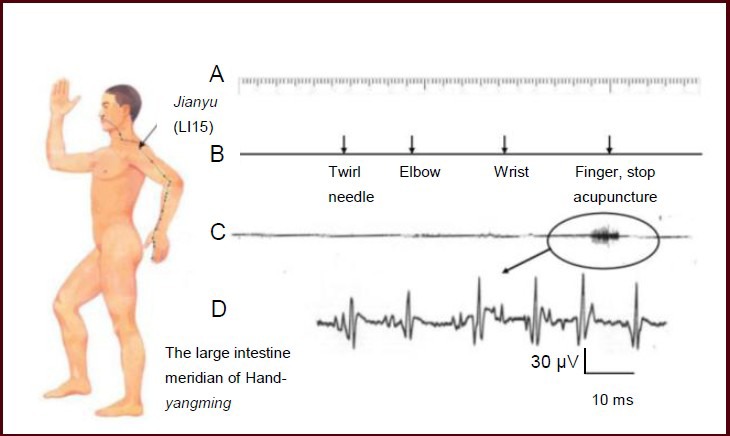
To confirm that this afferent discharge was the action potential of the superficial branch of the radial nerve triggered during propagated sensation along the meridian, rather than electromyography interference, a control electrode was placed on the Shousanli (LI10) acupoint, which was located along the meridian where the propagated sensation passed (Figure 3). We observed a regular afferent discharge on the superficial branch of the radial nerve when the propagated sensation along the meridian was transmitted down to the index finger. However, there was no evidence of any discharge at the electrode at Shousanli acupoint. These data support that the rhythmical discharge that occurred along the meridian was not electromyographic, but was rather the afferent discharge caused by the stimulation of the receptive field in the back of the index finger.
Figure 3.
Comparison of the recordings at the superficial branch of the radial nerve and the subcutaneous part of Shousanli (LI10) acupoint.
The nerve action potential was detected using a guide electrode, and was input into a VC-10 storage phase oscilloscope and stored in a tape recorder. (A) Time mark (seconds). (B) Mark of the location reached by the propagated sensation along the meridian. (C) Discharge record of the afferent nervous system. (D) Nervous system discharge after amplification. (E) Records from the subcutaneous electrode at Shousanli acupoint. The acupuncture point was the Jianyu acupoint.
Overall, these data indicate that a clear afferent nervous discharge could be recorded in the superficial branch of the radial nerve when Jianyu of the large intestine meridian was stimulated and the propagated sensation along the meridian was transmitted down to the index finger.
Performance of topographical mapping of evoked potential in the cerebral cortical somatosensory area I during propagated sensation along the meridian
We typically observed high potential reactions in the representative area of the lower and upper limbs on the topographical map of evoked potential in the cortical somatosensory area after stimulation of Guangming and Hegu acupoints in volunteers that reported no propagated sensation along the meridian. Volunteers with a significant propagated sensation along the meridian exhibited a high potential reaction in the lower and upper limbs representative area, as well as in the meridian route along where the propagated sensation passed, when Guangming and Hegu acupoints were stimulated. When the propagated sensation along the meridian was blocked by stress and was not transmitted to the head and face, the high potential reaction in the representative area disappeared (Figure 4). Thus, the high potential reaction in the representative area was consistent with the sensory reaction induced by peripheral acupuncture.
Figure 4.
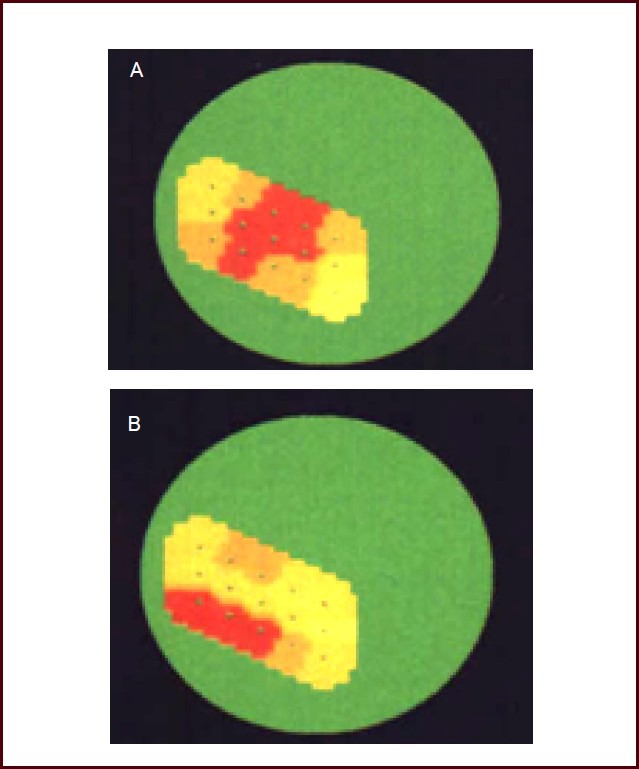
Volunteer's topographical map of evoked potential in the cortical somatosensory area I.
(A) Topographical map of volunteers without propagated sensation along the meridian. A clear high potential reaction was confined to the representative area of the upper limb (red section).
(B) Topographical map of volunteers with significant propagated sensation along the meridian. A high potential reaction covered the representative area of the upper limb, and extended to the face representative area (red area reflects the representative area of the upper limbs, head, and face).
The color from blue, green, light yellow, yellow, red, to dark red reflects the reaction process of the cortical potential from low to high.
DISCUSSION
Xie and Lin[23] analyzed the meridian phenomenon in the spinal cord at the neurobiological level, and found that the anterior horn motor neurons of the spinal cord exhibited a propagated sensation along the meridian in response to peripheral afferent stimuli in cats, rats, and monkeys. Further, following injection of cholera toxin B subunit-conjugated horseradish peroxidase into the acupoints along the stomach, gallbladder, and bladder meridian, every meridian located on the anterior horn of the spinal cord was visualized as a columnar motor neuron chain in a longitudinal array. Thus, meridian activity likely reflects a functional representation for the reflex activity of muscle groups with a collaborative dominative function and motor neurons with specific spatial association. Using transganglionic cholera toxin B subunit-conjugated horseradish peroxidase tracing in rabbits, Lin and colleagues[24] also suggested the presence of a neural network chain with a specific distribution corresponding to the stomach and bladder meridians in the spinal cord and brainstem substantia gelatinosa.
In the present study, we examined the superficial branch of the radial nerve, which is a sensory nerve branch that does not contain any motor nerve fibers, but controls the back of the hand, half of the skin in the dorsal radius of the thumb, index finger, and middle finger, and the subcutaneous tissue[22]. The superficial branch of the radial nerve was easy to locate near the styloid process of the radius, and the electrode was simple to insert. Furthermore, this was a convenient location that allowed volunteers to maintain a comfortable body position during experiments.
The action potential recorded in a sensory nerve is likely to reflect the afferent discharge induced by stimulation on a peripheral receptive field[25,26,27,28,29]. In our study, the recording reflected the afferent nerve discharge, rather than an alternative potential. When we inserted the guide electrode, a strong feeling of radiation to the tip of the index finger was observed, with a clear receptive field, and a stable response afferent discharge was recorded when the receptive field was stimulated by pressure or touch. When the stimulation was stopped, the afferent discharge disappeared immediately. These data support that the recorded action potential recorded arose from the afferent discharge of certain receptive fields.
The records collected in the present study were not electromyographic, as we used completely quiet experimental conditions and a determinate receptive field of the afferent discharge. Nerve muscle units, also known as motor units, comprise an anterior horn motor neuron and its innervated muscle fiber. Muscle fibers exist in a polarized state when normal motor units are at rest. When the muscle is stressed or voluntarily moves, nerve impulses are then transmitted to muscle fibers to trigger muscle fiber depolarization, and generation of action potentials. A slight change in the electrode locations causes the afferent discharge to disappear. If another electrode is inserted into the site at 1 cm lateral to the superficial branch of the radial nerve, the amplitude of afferent discharge significantly dropped or could not be recorded completely. By contrast, the muscle reaction of the flexor was almost identical in the recordings at the superficial branch of the radial nerve and at the subcutaneous part of Shousanli acupoint. Overall, these data indicate that the action potential recorded in our study was not electromyographic. Furthermore, the interference electromyography and rhythmic afferent nerve discharge exhibited significantly different types of curves. Thus, the potential recorded in our experiment was an afferent impulse of the sensory nerve induced when the propagated sensation along the meridian reached a receptive field.
We also compared the topographical maps of evoked potential in the cortical somatosensory area between volunteers with and without propagated sensation along the meridian. We found that the two groups exhibited different reaction areas in response to electro-acupuncture stimulation. The reaction area in the volunteers without propagated sensation along the meridian was generally limited to the representative area of the upper limb, with no invasion to adjacent area. By contrast, the reaction area in the volunteers with propagated sensation was larger and usually extended into the representative area of the face. The reaction characteristics of the topographical mapping of evoked potential in the cortical somatosensory area in volunteers with propagated sensation along the meridian corresponded with the region that the propagated sensation along the meridian passed through when the upper limb acupoints were stimulated. Overall, these data prove that there was a ‘stimulative motivation in the periphery’ (or a peripheral substantive process) on the body surface during propagated sensation along the meridian, and suggest a potential mechanism underlying propagated sensation[30,31,32,33,34,35].
SUBJECTS AND METHODS
Design
A clinical controlled neural electrophysiological experiment.
Time and setting
The experiment was completed in the Class III Laboratory of Acupuncture Physiology, Fujian Meridian Institute of Traditional Chinese Medicine, Key Unit of the Propagated Sensation along the Meridian of State Administration of Traditional Chinese Medicine, China from January 2009 to December 2011.
Subjects
We called for volunteers to participate in the experiment by oral solicitation and advertising from Fujian University of Traditional Chinese Medicine Research Interest Group and Fujian University of Traditional Chinese Medicine, China. Nineteen volunteers (11 males, eight females), aged 25 ± 3 years, who exhibited marked propagated sensation along the meridian, were recruited from the Outpatient Department of Fujian People's Hospital, China. When the Jianyu acupoint of the large intestine meridian was acupunctured, the propagated sensation along the meridian reached the Shangyang acupoint in all these subjects. The route was clear and stable, the transmission speed was 10–15 cm/s[3], and the route length was 50–60 cm[36]. Eleven volunteers (eight males, three females; aged 22 ± 3 years) without propagated sensation along the meridian were recruited from students at Fujian University of Traditional Chinese Medicine in China. All volunteers signed the informed consent forms.
Methods
Propagated sensation along the meridian following electro-acupuncture at Jianyu acupoint
When subjects entered the laboratory, they were asked to lie quietly for 30 minutes and maintain a stable mood to acclimatize to the laboratory environment. The Jianyu acupoint of the large intestine meridian in the upper limbs was then stimulated. The stimulating electrodes were a pair of stainless steel needles. The active electrode needle was inserted perpendicularly and quickly into the Jianyu acupoint, and then gently lifted and thrust again. When the feeling of aching, swelling, and numbness was reported, another needle was inserted into the route of the gall bladder meridian (1 cm below the Jianyu acupoint, depth 0.5 cm). The distance between the two needles was 5 mm to form an electrical stimulation circuit. A constant voltage stimulation was given to the participants via an isolator (Nihon Kohden Corporation, Tokyo, Japan) with a rectangular wave of 0.2 ms pulse width, a stimulation frequency of 1.5 s/pulse, and a stimulus intensity of 2–3 V. When participants began to accurately express every location of the propagated sensation along the meridian (such as propagated sensation along the meridian arriving at the elbow, wrist, and back of the hand), and when the propagated sensation along the meridian was stable at the back of the hand, we started to record the afferent nervous discharge. Three tests were performed per day per person.
Action potential recordings
The recording electrode was a tungsten semi-microelectrode (0.2 mm diameter) equipped with a conical tip (10 μm diameter). The tungsten was insulated by insulation varnish, with only a 200 μm tip exposed. The electrode was placed at the superficial branch of the radial nerve at 10–15 cm from the thumb. The reference electrode, a 2.5 cm long stainless needle, was inserted subcutaneously at 5 cm lateral to the recording electrode. The potential signals were monitored using a storage oscilloscope VC-10 (Nihon Kohden), and were stored by a tape recorder (type RMG-5204; Nihon Kohden). The nervous discharge was recorded (type RMG-6200; Nihon Kohden) following stimulation of the skin by gently touching the receptive field of dorsal index finger with swabs.
Recordings of the brain electrical topographic map
A 4400 electroencephalography (Nihon Kohden) and an ND-1 brain electrical topographic mapping instrument (Beijing Institute of New Technology Application) were used. For recording somatosensory evoked potentials, we cleaned the skin surface with ethanol to remove grease and dust, and the impedance of the skin was maintained below 10 kΩ. A disc electrode of silver chloride (7 mm diameter; Nihon Kohden) was adhered to the scalp of the cortical somatosensory area using electrode paste (Nihon Kohden); the main components were sodium chloride, carboxymethyl cellulose, and medical electrical paste. The cortex evoked potential was recorded with a 15-electrode-composed matrix, with the electrodes arranged into three rows, and a center electrode distance of 15 mm. Every row was made up of five electrodes that stimulated the representative area from the foot to the face of the somatosensory cortex, and the center distance of the electrodes was 20 mm. A reference electrode was placed into the ipsilateral earlobe, and the contralateral earlobe was connected to the ground. The amplifier was used with a high cutoff frequency 1 000 Hz, 256 times superposition, and 200 millisecond analysis time. The peak-peak value of C4–5 composition was measured, and the distribution characteristics of the evoked reaction in the brain electrical topographic map were observed[37,38,39,40].
Statistical analysis
SPSS 13.0 software (SPSS, Chicago, IL, USA) was used for statistical analyses. A two-sample t-test was used to compare differences between the groups. A P value less than 0.05 was considered statistically significant.
Footnotes
Funding: This study was supported by the General Project of the National Natural Science Foundation of China, No. 30973720; the Natural Science Foundation of Fujian Province in China, No. 2011J01192; and Free Topics of Fujian Provincial Science & Technology Ministry in China, No. 2012fjzyyk-6.
Conflicts of interest: None declared.
(Reviewed by Dean J, Norman C, Chang XR, Wang XM)
(Edited by Yu J, Yang Y, Li CH, Song LP, Liu WJ, Zhao M)
REFERENCES
- [1].Hu Y, Yang CB. Preliminary discussion on phenomenon of transmission along channels. Shijie Zhongyiyao. 2010;5(5):349–350. [Google Scholar]
- [2].Geng LQ. Preliminary analysis on influencing factors of transmission along channels. Gansu Zhongyi. 2010;23(8):45–46. [Google Scholar]
- [3].Hu XL, Bao JZ, Ma TF. Beijing: People's Medical Publishing House; 1990. Meridian Modern Research. [Google Scholar]
- [4].Ding SS, Shang XK. The conduction of moxibustion sensation on zusanli acupoint. Zhenjiu Linchuang Zazhi. 2011;27(12):24–26. [Google Scholar]
- [5].Zheng SH, Chen GD, Lin WM, et al. 3D Virtual simulation research and implementation of human sensation conduction along meridian. Jisuanji Fangzhen. 2008;25(11):251–255. [Google Scholar]
- [6].Sun WG. Hypothesis of nerve-body fluid-low hydraulic resistance of mechanism of sensation conduction along meridians. Henan Zhongyi. 2008;28(7):13–15. [Google Scholar]
- [7].Chen RX, Kang MF. Acupoint heat-sensitization and its clinical significance. Zhongyi Zazhi. 2006;47(12):905–906. [Google Scholar]
- [8].Hu XL. Research on meridians in China. Zhenjiu Tuina Yixue. 2008;6(5):257–258. [Google Scholar]
- [9].Hu XL. Exploration on the phenomenon of channel blocking. Keji Chengguo Guanli yu Yanjiu. 2008;12:67–74. [Google Scholar]
- [10].Shen JZ, Chen X. Research of locating and imaging system in human skin meridian. Jisuanji Gongcheng yu Ying-yong. 2012;48(6):60–62. [Google Scholar]
- [11].Zhuo LS. Rethink on “experiment of progated sensation along meridians”. Zhongguo Zhenjiu. 2011;31(11):1045–1048. [PubMed] [Google Scholar]
- [12].Wang YZ. Enlightenment and thinking on deqi (arrival of needling sensation) by abdominal acupuncture. Zhongguo Zhenjiu. 2011;31(2):183–185. [PubMed] [Google Scholar]
- [13].Xu RZ, Wang MP. A research on the relativity between “acupuncture needle reaches disease location” and “Qi reaches disease location”. Zhonghua Zhongyiyao Xuekan. 2009;27(3):627–630. [Google Scholar]
- [14].Chen M, Wu ZX, Hu XL, et al. Experimental observation of acupuncture effect on partial oxygen pressure and microcirculatory blood perfusion of deep tissues along large intestine meridian in 30 normal volunteer subjects. Huanqiu Zhongyiyao. 2011;4(3):186–189. [Google Scholar]
- [15].Lan CL, Pan XH, Xu JS, et al. Comparison between governor meridian and its bilateral control points in heat transmission stimulated by moxibustion. Fujian Zhongyiyao Daxue Xuebao. 2011;21(3):4–5. [Google Scholar]
- [16].Hu XL. Jingluo: a vitality strong ancient doctrine. Keji Zhongguo. 2008;8):42–47. [Google Scholar]
- [17].Pan XH, Zheng SX, Xu JS, et al. Diffusion rate of skin temperature along Ren meridian during local heating. Fujian Zhongyiyao Daxue Xuebao. 2011;21(5):1–3. [Google Scholar]
- [18].Pan XH, Hu XL, Xu JS, et al. Elicitation of infrared radiant track along Ren meridian courses by local heating. Huanqiu Zhongyiyao. 2010;3(5):352–354. [Google Scholar]
- [19].Hu XL, Wu BH. A hypothesis on the mechanism underlying the formation of PSC—integration of “Periphery” and CNS under the domination of substantial process proceeding along the channels. Zhenci Yanjiu. 1987;12(S1):1–8. [PubMed] [Google Scholar]
- [20].Hu W, Wang YX, Liu D, et al. Volume conduction in the electrophysiological assessment of acute sciatic nerve crush in rats. Nantong Daxue Xuebao: Yixue Ban. 2012;32(1):7–12. [Google Scholar]
- [21].Lan YF, Wen Y, Fang Z, et al. Age-related increase of early afterdepolarization in calsequestrin-2 knock-in mouse cardiomycyte. Laonian Xinzangbing Xue Zazhi. 2011;8(3):147–158. [Google Scholar]
- [22].Zhu CG. Beijing: People's Medical Publishing House; 2009. Neuroanatomy. [Google Scholar]
- [23].Xie YK, Li HQ. The neurobiology characteristic of meridians and propagated sensation along meridian. Zhongguo Kexue. 1995;7:721–732. [Google Scholar]
- [24].Lin WZ, Xu MH, Fan L, et al. Foot Yangming stomach spinal cord brainstem neural networks: HRP retrograde and the cross-ganglion tracking study on Jiexi, Zusanli. Shanghai Zhenjiu Zazhi. 1994;5:23–26. [Google Scholar]
- [25].Hallin RG, Torebjörk HE. Single unit sympathetic activity in human skin nerves during rest and various maneuvers. Acta Physiol Scand. 1974;92(3):303–317. doi: 10.1111/j.1748-1716.1974.tb05749.x. [DOI] [PubMed] [Google Scholar]
- [26].Sato A, Schmidt RF. Somatosympathetic reflexes: afferent fibers, central pathways, discharge characteristics. Physiol Rev. 1973;53(4):916–947. doi: 10.1152/physrev.1973.53.4.916. [DOI] [PubMed] [Google Scholar]
- [27].Bagust J, Forsythe ID, Kerkut GA. An investigation of the dorsal root reflex using an in vitro preparation of the hamster spinal cord. Brain Res. 1985;331(2):315–325. doi: 10.1016/0006-8993(85)91557-4. [DOI] [PubMed] [Google Scholar]
- [28].Hensel H, Boman KK. Afferent impulses in cutaneous sensory nerves in human subjects. J Neurophysiol. 1960;23:564–578. doi: 10.1152/jn.1960.23.5.564. [DOI] [PubMed] [Google Scholar]
- [29].Kaeser HE. Microelectrode recording from human peripheral nerves (microneurography) In: Remond A, editor. Handbook of electroencephalography and neurophysiology. New York: Elsevier; 1975. [Google Scholar]
- [30].Xu JS, Pan XH, Zheng SX, et al. Comparison of topographical mapping of cortical somatosensory evoked response during propagated sensation along meridian and imitating propagated sensation along meridian. Fujian Zhongyi Xueyuan Xuebao. 2010;20(3):4–6. [Google Scholar]
- [31].Xu JS, Pan XH, Hu XL, et al. Effect of electroacupuncture of different acupoints on electroretinogram and cerebral visual evoked potentials in healthy subjects. Zhenci Yanjiu. 2010;35(1):47–51. [PubMed] [Google Scholar]
- [32].Xu JS, Pan XH, Hu XL, et al. Influence of external stimulation on topographical mapping of cortical somatosensory evoked potentials during acupuncture. Zhonghua Zhongyiyao Zazhi. 2010;25(6):841–844. [Google Scholar]
- [33].Zheng SX, Xu JS, Pan XH, et al. Influences of electroacupuncture in different acupoints on evoked potential map of primary somatosensory area of cerebral cortex. Fujian Zhongyiyao Daxue Xuebao. 2011;21(3):1–3. [Google Scholar]
- [34].Liang XC, Wu BH, Su ZF, et al. Correlation between brain evoked potential in Jing-Luo sensation-people and running fractal mapping. Tongji Yike Daxue Xuebao. 2000;29(6):489–492. [Google Scholar]
- [35].Wu BH, Hu XL, Xu JS, et al. The observation of the evoked potential graph in cortical deep sensory area during the meridian transmission. Zhongguo Yiyao Xuebao. 2001;16(6):15–17. [Google Scholar]
- [36].Wang PQ, Hu XL, Wu BH. Displaying of the infrared radiant track along meridians on the back of human body. Zhenci Yanjiu. 1993;18(2):90–93. [PubMed] [Google Scholar]
- [37].Xu JS, Wu BH, Hu XL, et al. Topographical feature of somatosensory cortical evoked potential with augmented blocking of the sensation transmission along meridians. Zhenjiu Tuina Yixue. 2009;7(4):239–242. [Google Scholar]
- [38].Yang HQ, Xie SS, Hu XL, et al. Appearance of human meridian-like structure and acupoints and its time correlation by infrared thermal imaging. Am J Chin Med. 2007;35(2):231–240. doi: 10.1142/S0192415X07004771. [DOI] [PubMed] [Google Scholar]
- [39].Xu JS, Hu XL, Wang PQ, et al. Influence of electroacupuncture on infrared radiant track along meridian courses over human body surface. Zhongguo Linchuang Kangfu. 2005;9(29):251–253. [Google Scholar]
- [40].Chen M, Wu ZX, Hu XL, et al. Effect of acupuncture on partial oxygen pressure in different depths of tissues along governor vessel in 61 normal volunteer subjects. Zhonghua Zhongyiyao Zazhi. 2011;26(8):1856–1858. [Google Scholar]


