Abstract
Besides local neuronal damage caused by the primary insult, central nervous system injuries may secondarily cause a progressive cascade of related events including brain edema, ischemia, oxida-tive stress, excitotoxicity, and dysregulation of calcium homeostasis. Hypothermia is a beneficial strategy in a variety of acute central nervous system injuries. Mild hypothermia can treat high intra-cranial pressure following traumatic brain injuries in adults. It is a new treatment that increases sur-vival and quality of life for patients suffering from ischemic insults such as cardiac arrest, stroke, and neurogenic fever following brain trauma. Therapeutic hypothermia decreases free radical produc-tion, inflammation, excitotoxicity and intracranial pressure, and improves cerebral metabolism after traumatic brain injury and cerebral ischemia, thus protecting against central nervous system dam-age. Although a series of pathological and physiological changes as well as potential side effects are observed during hypothermia treatment, it remains a potential therapeutic strategy for central nervous system injuries and deserves further study.
Keywords: neural regeneration, reviews, brain injury, spinal cord injury, central nervous system injury, mild hypothermia, therapeutic hypothermia, traumatic brain injury, neuroregeneration
Research Highlights
-
(1)
Growing data support the use of mild hypothermia as a promising new treatment for brain and spinal cord injuries.
-
(2)
Mild hypothermia's protective effects, no matter whether they are positive or negative, on central nervous system injuries are summarized and analyzed through several aspects, in a broad attempt to explore the potential mechanisms and side effects of mild hypothermia as a new therapeutic strategy.
-
(3)
Although physiological and pathological changes, together with potential side effects, are observed during mild hypothermia treatment, it can increase survival and thus deserves clinical consideration.
INTRODUCTION
Injury to the adult central nervous system is devastating because of the inability of central neurons to regenerate axonal and dendritic connections. The consequences of injury are not just a break in communication between healthy neurons, but a cascade of events that can lead to neuronal degeneration and cell death[1]. Through improvements in surgical procedures, stabilization approaches, and critical care initiates, these individuals live relatively long lives with these devastating disabilities. At present, no therapeutic interventions have successfully improved patient outcomes following head injuries[2].
Hypothermia is a promising therapy, which targets multiple pathological effects of central nervous system injury[3]. For the past 20 years, various laboratories throughout the world have shown that mild or moderate hypothermia led to neuroprotection and improved functional outcomes in various models of brain and spinal cord injury[4]. In the early 1950s, profound levels of hypothermia were used in cardiac surgical procedures, as well as to treat acute trauma such as stroke, brain trauma, and spinal cord injury[5]. Even a modest reduction in temperature can have neuroprotective effects[6], suggesting the possibility that hypothermia affects pathways that extend beyond a decrease in cellular metabolism. Furthermore hypothermia is better than any known drugs in producing neuroprotection following blockage of blood to the brain[7,8]. Protracted hypothermia of a few Celsius can provide sustained behavioral and histological neuroprotection, whereas brief or very mild hypothermia only delays neuronal damage.
Nevertheless, a number of controversies still exist regarding hypothermic efficacy as well as the mechanisms by which mild hypothermia may protect the brain after trauma[9]. There is therefore a need to investigate the potentially beneficial effects of hypothermia in both patients with central nervous system injuries and experimental animal models.
Therapeutic hypothermia is a new treatment that increases survival and quality of life in patients suffering from ischemic insults such as stroke, cardiac arrest, and neurogenic fever following brain trauma[6,10]. This study was aimed to review and summarize experimental datum gained in different animal models and human clinical studies of brain and spinal cord injury demonstrating the beneficial effects of mild to moderate hypothermia.
In addition, the mechanisms underlying hypothermic protection and its physiological changes and side effects are also discussed.
HYPOTHERMIA IMPROVES OUTCOMES FOLLOWING SPINAL CORD INJURY
In early studies, local cooling was utilized to cool areas of the damaged spinal cord. In those investigations, relatively profound levels of hypothermia were shown to produce marked neurological and functional recovery after spinal cord trauma[9]. Mild hypothermia introduced after a traumatic or compressive spinal cord injury improved function and reduced histopathological damage[11,12,13,14]. Moderate hypothermia introduced after cervical spinal cord injury improved histopathological and behavioral outcomes[15]. Likewise, improved forelimb function, preservation of motor neurons, and decreased contusion volumes occur in rats cooled after cervical traumatic insult[15]. This evidence shows that mild to moderate hypothermia improves outcome in models of both cervical and thoracic spinal cord injury[14].
Histologically, the application of hypothermia after spinal cord injury significantly increased normal-appearing white matter (31% increase) and gray matter (38% increase) volumes, greater preservation (four-fold) of neurons immediately rostral and caudal to the injury epicenter, and enhanced sparing of axonal connections from retrogradely traced reticulospinal neurons (127% increase) compared with normothermic controls[14]. Case reports and clinical studies have provided encouraging results regarding the safety and efficacy of moderate hypothermia following severe spinal cord injury[16]. In compression injury models, hypothermia reduced blood flow to the focal area of the injured spinal cord[11,13]. Also, when used before decompressive surgeries, hypothermia can prevent neurological decline[17].
HYPOTHERMIA IMPROVES OUTCOMES FOLLOWING BRAIN INJURY
In the 1980s, it was first discovered neural that relatively small reductions in brain tissue temperature provided significant protection against ischemic and traumatic neuronal cell death[4]. In an important traumatic brain injury study, Hansebout and colleagues[18] reported that pre- and post-traumatic hypothermia improved beam walking in a rat model of fluid percussion brain injury. In another early traumatic brain injury investigation, 1-hour post-injury hypothermia (30°C) improved behavioral outcomes[19].
In addition to the neuroprotective and behavioral consequences of post-traumatic hypothermia, hypothermia has also been reported to reduce the degree of diffuse axonal damage in models of traumatic brain injury[20]. One of the advantages of hypothermia is that reduced temperature reduces several of the dominant injury mechanisms in the pathophysiology of traumatic brain injury[21]. Compared to short-term hypothermia (2–3 days), long-term hypothermia (5 days) significantly improved outcomes in patients with severe traumatic brain injury following cerebral contusions and elevated intracranial pressure without causing significant complications. The rate of rewarming following a prolonged hypothermic period is critical in producing the beneficial effects of post-traumatic hypothermia[21].
In other studies addressing transient global ischemia, a mere decrease of 2°C in body temperature provided 100% retention of neurons in the hippocampus CA1 region[22]. Although many investigators use whole body cooling to produce mild hypothermic effects, selective brain cooling during and after a global ischemic insult also protects the brain from histopathological damage[23]. In addition to histopathological assessments, mild hypothermia may improve functional outcomes following transient global ischemia[4]. Another factor that appears to be important in the beneficial effects of hypothermia is the duration of survival after ischemia.
Protective effects of hypothermia in models with either transient or complete permanent occlusions has been investigated, and the results show that hypothermia is the most protective against transient ischemia, and it may produce little or no protection in models of permanent occlusion[15]. Mild hypothermia is also protective in middle cerebral artery occlusion models when the hypothermic period is extended[24,25]. Induced hypothermia improves outcomes in patients who are comatose after resuscitation from out-of-hospital cardiac arrest[5]. A meta-analysis of randomized controlled trials found that therapeutic hypothermia with conventional cooling methods improved both survival and neurologic outcomes at hospital discharge for patients who experienced cardiac arrest[26].
MECHANISMS UNDERLYING THE PROTECTIVE EFFECTS OF HYPOTHERMIA
Numerous studies have investigated the underlying mechanisms by which small reductions in central nervous system temperature can improve outcomes in brain and spinal cord injury models[4,27]. The following will summarize the current thinking regarding basic mechanisms of hypothermic protection.
Reduced cerebral metabolism
When hypothermia was first used in the clinic, its protective effects were believed to purely because of slowing cerebral metabolism, leading to decreased glucose and oxygen consumption. Indeed, this assumption is not completely incorrect. Cerebral metabolism decreases by 6% to 10% for each 1°C reduction in body temperature during cooling[28,29]. However, reduced metabolic rates are only one of many mechanisms underlying hypothermia's protective effects[30].
Apoptosis, calpain-mediated proteolysis, and mitochondrial dysfunction
Hypothermia can interrupt the apoptotic pathway, thereby preventing cellular-injury-induced apoptosis[31,32]. Effects of hypothermia include inhibition of caspase enzyme activation[32,33], prevention of mitochondrial dysfunction[31], modification of intracellular ion concentrations, and reduce overload of excitatory neurotransmitters[30]. The c-Jun NH2-terminal kinase pathway mediates traumatic injury-induced apoptosis in astrocytes. Prolonged hyperthermia as a secondary insult worsens apoptosis by increasing c-Jun NH2-terminal kinase activation[33]. Hypothermia protects against traumatic injury via early suppression of c-Jun NH2-terminal kinase activation and subsequent prevention of apoptosis. Furthermore hypothermia markedly reduces ischemia/reperfusion-induced endothelial cell apoptosis and the expression of cleaved caspase-3 and poly(ADP-ribose) polymerase[34]. Moreover, hypothermia inhibits c-Jun NH2-terminal kinase 1/2 activation via induction of protein kinase phosphatase-1. These studies indicate that apoptotic cell death is another important target by which temperature may affect long-term outcome in various models of central nervous system injury.
Ion pumps and neuroexcitotoxicity
Reperfusion and ischemia interrupt the delicate balance between calcium influx and sequestration at the cellular level. Various animal experiments have clearly demonstrated that key destructive processes of the neuroexcitatory cascade—such as calcium influx, accumulation of glutamate, and the release of its coagonist glycine—can be prevented, interrupted, or mitigated by hypothermia[35,36,37]. Even a relatively small decrease in temperature can significantly improve ion homeostasis, whereas the occurrence of fever can trigger and stimulate these destructive processes[30].
Immune and inflammatory responses
Attenuation of inflammation is a major mechanism by which hypothermia provides beneficial effects following central nervous system injury[4]. Numerous animal experiments and clinical studies have shown that hypothermia suppresses ischemia-induced inflammatory reactions and the release of proinflammatory cytokines[38]. Hypothermia may block ischemic damage by blocking cytochrome c release or caspase activity after both transient focal and global ischemia[39,40,41]. It also prevents or mitigates reperfusion-related DNA damage, lipid peroxidation, and leukotriene production, and decreases the production of nitric oxide, which is a key agent in the development of post-ischemic brain injury[36]. Moreover, the proinflammatory response of stimulated microglial cells is significantly reduced after moderate hypothermia[42].
Free radical production
Free radicals can oxidize and damage numerous cellular components. Under hypothermic conditions, significantly fewer free radicals are generated, even though free radical production is not completely prevented[43,44]. This allows the endogenous antioxidative (protective) mechanisms to better cope with free radicals that are being released, thereby preventing or significantly mitigating oxidative damage.
Vascular permeability, blood-brain barrier disruption, and edema formation
Mild hypothermia significantly reduces blood-brain barrier disruptions[45,46] and also decreases vascular permeability following ischemia-reperfusion, further decreasing edema formation. Furthermore, hypothermia has been used to treat brain edema and reduce intracranial pressure in a wide range of neurological injuries[47,48].
Intracellular and extracellular acidosis and cellular metabolism
The diminished integrity of cell membranes, the failure of various ion pumps, development of mitochondrial dysfunction, inappropriate activation of numerous enzyme systems with cellular hyperactivity, and the disruption of various other intracellular processes all contribute to the development of intracellular acidosis, a factor that powerfully stimulates the abovementioned destructive processes[49]. Ischemia-reperfusion also leads to substantial rises in cerebral lactate levels[50]. All of these factors can be significantly attenuated by hypothermia[49,50]. In addition, brain glucose utilization is affected by ischemia-reperfusion, and hypothermia can improve brain glucose metabolism; i.e., the ability of the brain to utilize glucose[51,52]. Similar observations have been made in severe traumatic brain injury, for which an initial increase in cerebral glucose metabolism after trauma is followed by a deep and persistent decrease in metabolic rate, with a depression of mitochondrial oxidative phosphorylation and glucose utilization that can last for several weeks[53,54]. Hypothermia during or after reperfusion increases the speed of metabolic recovery, with a better preservation of high-energy phosphates and reduced accumulation of toxic metabolites[53,54].
Coagulation activation and formation of microthrombi
Cardiopulmonary arrest and resuscitation are accompanied by a marked activation of coagulation, which can lead to intravascular fibrin formation with blockage of the microcirculation in the brain and heart[55]. Hypothermia has some anticoagulatory effects. Mild platelet dysfunction occurs at temperature ≤ 35°C, and inhibition of the coagulation cascade develops at temperature ≤ 33°C; platelet count can also decrease during cooling[56]. In theory, this anticoagulation effect may constitute yet another neuroprotective mechanism. This remains speculative given that no studies directly addressing this issue have been performed.
Vasoactive mediators
Hypothermia affects local secretion of vasoactive substances such as endothelin, thromboxane A2, and prostaglandin I2 in the brain and other organs. Endothelin and thromboxane A2 are powerful vasoconstrictive agents, whereas prostaglandin I2 is a vasodilator[30]. Thromboxane A2 also stimulates platelet aggregation[57]. Thromboxane A2 and prostaglandin I2 play important roles in regulating local cerebral blood flow, and a balance between the two is required to maintain homeostasis[57]. This local homeostasis can be disrupted following an ischemic or traumatic event, with a relative increase in production of thromboxane A2[58]. This disruption in equilibrium can lead to vasoconstriction, hypoperfusion, and thrombogenesis in injured areas of the brain. The predominance of local vasoconstrictors can be corrected or modified by hypothermia[25,59,60].
Improved tolerance to ischemia
Hypothermia improves tolerance for ischemia in various animal models[13,61,62]. It is widely used in major vascular surgery, neurosurgical interventions, and cardiothoracic surgery[47].
Suppression of epileptic activity
Growing evidence shows that hypothermia can suppress epileptic activity. Small case series report successful use of cooling to treat grand mal seizures[63]. External warming increases the extent of epilepsy-induced brain injury, prevention or prompt treatment of fever reduces these injuries, and induction of hypothermia further decreases seizure-induced brain injuries[64].
Spreading depression-like depolarizations
Neuronal damage in different types of neurological injury can be significantly increased by the so-called spreading of depression-like depolarizations[37]. Hypothermia can suppress spreading depressions in various types of neurological injury[37,65].
Influence on genetic expression
Hypothermia increases the expression of immediate early genes, which are a part of the protective cellular stress response to injury, and stimulates the induction of cold-shock proteins, which can protect the cell from ischemic and traumatic injury[66]. Figure 1 summarizes the protective effects of hypothermia.
Figure 1.
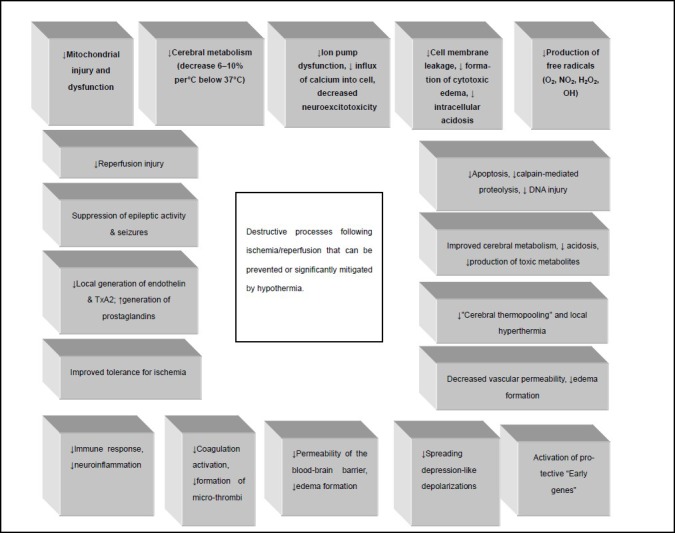
Schematic depiction of the protective mechanisms underlying mild to moderate hypothermia[70].
TxA2: Thromboxane A2; ↓: inhibition; ↑: promotion; black lettering: early mechanisms; gray lettering: late mechanisms.
PHYSIOLOGICAL CHANGES AND SIDE EFFECTS OF MILD HYPOTHERMIA
Various changes throughout the body are induced by hypothermia. As separate articles are devoted to this topic[4,30,66,67,68], it is discussed only briefly here.
Circulatory system
One side effects of hypothermia is the induction of cold diuresis, which causes hypotension.
Cardiovascular system
Mild hypothermia decreases cardiac output by about 25%, and leads to an increase in vascular resistance and central venous pressure. Hypothermia may induce changes in heart rhythms and electrocardiograms. When body temperatures begin to decrease, patients will initially develop sinus tachycardia. As temperature decreases further (below 35.5°C), sinus bradycardia occurs. While temperature remains higher than 30°C, the risk of developing clinically significant arrhythmias is very low. However, these risks increase significantly if core temperature decreases below 28–30°C, and may be increased further if electrolyte disorders develop. Usually the first observed form of arrhythmia is atrial fibrillation, which can be followed by severe arrhythmia, including ventricular tachycardia and ventricular fibrillation as temperature decreases further. Once arrhythmia develops, these effects are much more difficult to treat because the myocardium becomes less responsive to defibrillation and cardiac drugs during hypothermia. Thus great care should be taken to keep temperature above 30°C. Further studies will be required to settle these issues.
Metabolism
Metabolic effects of mild hypothermia have been shown using nuclear magnetic resonance spectroscopy, where the metabolic effects of different levels of hypothermia were reported[60]. Thus, hypothermia lowers metabolic and energy demands, which can have beneficial effects on cytoplasmic adenosine triphosphate stores and maintain normal transmembrane ion and neurotransmitter gradients. The preservation of adenosine triphosphate depends on both the hypothermic level and the insult severity. Although cerebral hypothermia may not prevent eventual depletion of adenosine triphosphate or lactate accumulation during a prolonged period of ischemia, hypothermia could certainly slow adenosine triphosphate depletion during ischemia[71].
Electrolyte disorders
A combination of hypothermia-induced intracellular shifts and tubular dysfunction can increase renal excretion of electrolytes and lead to depletion of magnesium, potassium, and phosphate during cooling[31]. These electrolyte disorders can increase the risk for arrhythmias and other potentially harmful complications[72]. Therefore, electrolyte levels should be kept in the high-normal range during and after hypothermia treatment. Extra care should be taken during rewarming because hyperkalemia may develop during this phase because of release of potassium sequestered to intracellular compartments during induction of hypothermia[56].
Hyperglycaemia
Another side effect of hypothermia is hyperglycemia caused by insulin resistance. Hyperglycemia is associated with increased infection rates, higher incidences of renal failure, and critical illness neuropathy. The amount of insulin required to maintain glucose levels within the normal range (4.44–6.10 mmol/L) is usually higher than expected because of insulin resistance[73].
Shivering
In awake patients, shivering induces unwanted effects such as increased oxygen consumption and metabolic rates, excess work of breathing, and increased heart rates with increased myocardial oxygen consumption[56,74]. The effects of the shivering response decrease with age. Thus, the doses of drugs required to suppress shivering are usually lower in older patients. Warm-air applied to the skin can be used as an accessory method to lower the shivering threshold, and to reduce drug doses required to prevent shivering[3,75]. All patients should be closely monitored for shivering during all phases of hypothermia treatment.
Skin injuries or bedsores
Direct exposure of skin to ice packs can cause burns, which can be exacerbated by vasoconstriction.
Infection
Hypothermia inhibits proinflammatory responses through inhibition of leukocyte migration and phagocytosis and reduced synthesis of proinflammatory cytokines[56]. Indeed, suppression of harmful neuroinflammation is one of the protective mechanisms of hypothermia. A side effect of this protective mechanism is an increased risk of infections. Strategies to deal with increased risk of infection include antibiotic prophylaxis. Patients should be closely monitored for signs of infection during therapeutic temperature management. Some normal signs of infection are absent or suppressed during hypothermia, including fever, increased C-reactive protein levels, and elevated white blood cell counts. Many treatment protocols include daily blood surveillance cultures to screen for bacteremia. The threshold for antibiotic treatment should be low.
In patients developing infections after hypothermia treatment, fever should be treated symptomatically, to prevent new or additional neurological injuries[47]. Additional attention should be paid to catheter and central line insertion sites, as local infections are also more likely to develop[30].
Clotting disorders
Mild hypothermia can prompt mild coagulopathy[56]. Temperatures below 35°C can cause platelet dysfunction and a mild reduction in platelet counts. At 33°C, other steps in the coagulation cascade, such as the synthesis and kinetics of clotting enzymes and plasminogen activator inhibitors, may also be affected[56].
Clinical observations suggest that the risk for severe bleeding connected with therapeutic cooling is comparatively small[47,56]. It has been argued that bleeding risks should not be viewed as a reason to withhold hypothermia treatment. Very mild hypothermia (35°C) does not affect coagulation, and can be safely used even if bleeding risks are high. As temperatures of 33-35°C affect platelet function only if surgical procedures are performed under hypothermic conditions, platelet transfusion may be considered.
Side effects of mild hypothermia
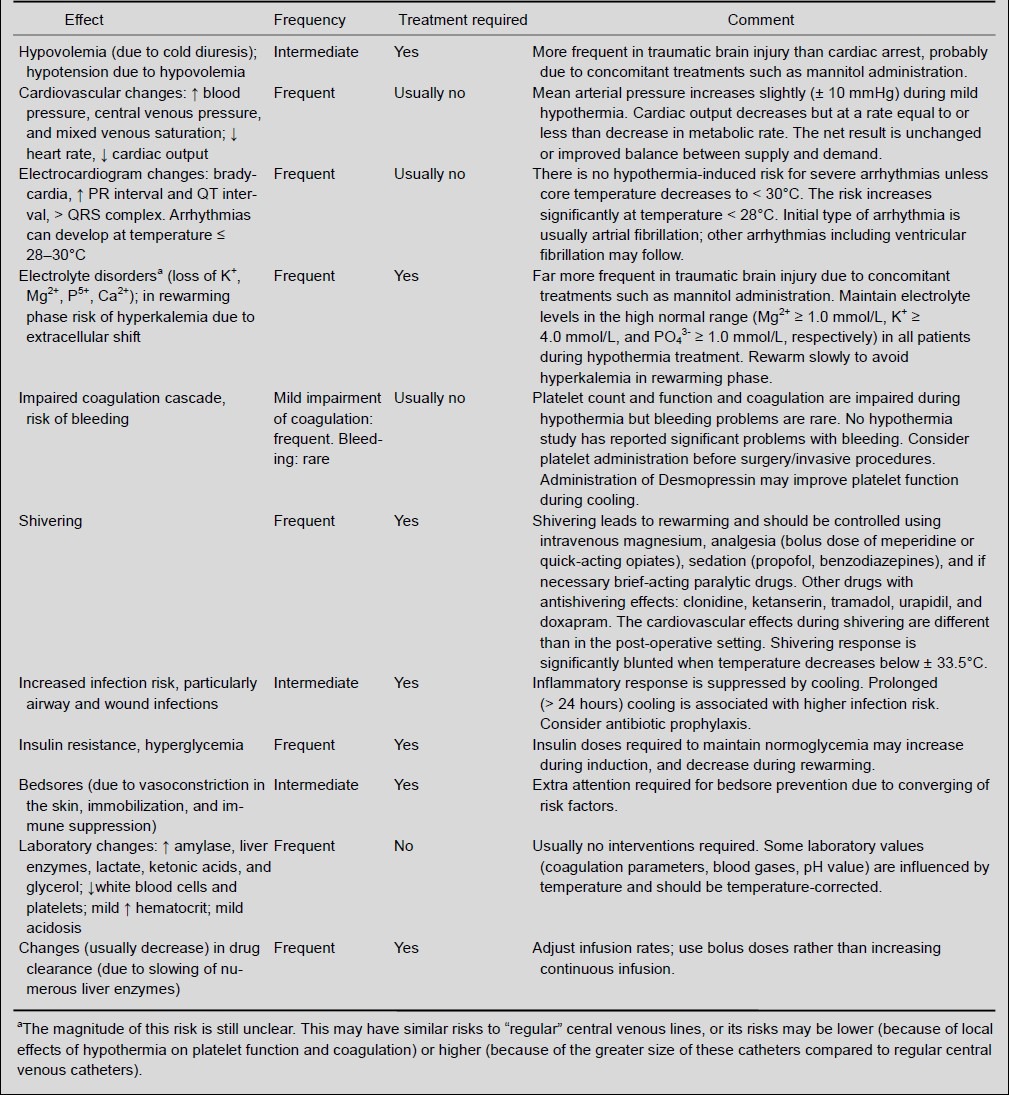
Therapeutic hypothermia is a promising treatment, but the side effects of this treatment need to be managed, especially if extended treatment periods are desired. Understanding its mechanisms, realization of physiological changes correlated with cooling, and prevention of possible side effects are all key factors for the efficient clinical use of hypothermia[30]. Quick induction reduce the risks and consequences of short-term side effects, such as metabolic disorders and shivering[56]. A significant breakthrough was the understanding that neurological outcomes could be improved using mild to moderate hypothermia (31–35°C) rather than deep hypothermia (≤ 30°C), with far fewer and less severe side effects[30] (Table 1).
Table 1.
The physiological changes and potential side effects of hypothermia[95]
CONCLUSION
Therapeutic hypothermia is a highly promising treatment. During the last several decades, a significant amount of experimental and clinical work has established therapeutic hypothermia as one of the few effective treatment strategies for brain and spinal cord injuries. Indeed, temperature, before and after an injury, seems to be a critical factor in irreversible neuronal damage and subsequent neuronal dysfunction.
Understanding the underlying mechanisms, awareness of physiological changes associated with cooling, and prevention of potential side effects are all key factors in the effective clinical use of hypothermia. However, there are numerous pitfalls in using therapeutic cooling to maximum effect, particularly if prolonged treatments are required to improve outcomes. Nevertheless, more work needs to be conducted to determine appropriate mild hypothermia and temperature management strategies and which patient populations benefit most from these treatment procedures.
This review provides clear mechanisms by which therapeutic hypothermia protects against central nervous system injuries. Understanding the positive and negative aspects of hypothermia can optimize protective effects.
Acknowledgments:
We would like to thank Department of Neurosurgery, the First Affiliated Hospital of Chongqing Medical University for providing useful advice and relevant assistance, and Liu Q from the Department of Neurosurgery, the First Affiliated Hospital of Chongqing Medical University for helpful editorial assistance.
Footnotes
Conflicts of interest: None declared.
(Reviewed by Murnane K, Norman C, Yu JQ, Pan J)
(Edited by Yu J, Yang Y, Li CH, Song LP, Liu WJ, Zhao M)
REFERENCES
- [1].Horner PJ, Gage FH. Regenerating the damaged central nervous system. Nature. 2000;407(6807):963–970. doi: 10.1038/35039559. [DOI] [PubMed] [Google Scholar]
- [2].Dietrich WD, Bramlett HM. The evidence for hypothermia as a neuroprotectant in traumatic brain injury. Neurotherapeutics. 2010;7(1):43–50. doi: 10.1016/j.nurt.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Huang T, Solano J, He D, et al. Traumatic injury activates MAP kinases in astrocytes: mechanisms of hypothermia and hyperthermia. J Neurotrauma. 2009;26(9):1535–1545. doi: 10.1089/neu.2008.0743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Dietrich WD, Atkins CM, Bramlett HM. Protection in animal models of brain and spinal cord injury with mild to moderate hypothermia. J Neurotrauma. 2009;26(3):301–312. doi: 10.1089/neu.2008.0806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Dietrich WD, Levi AD, Wang M, et al. Hypothermic treatment for acute spinal cord injury. Neurotherapeutics. 2011;8(2):229–239. doi: 10.1007/s13311-011-0035-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wikimedia Foundation, Inc; 2013. Therapeutic Hypothermia. http://en.wikipedia.org/wiki/Therapeutic_hypothermia . [Google Scholar]
- [7].Winslow R. How Ice Can Save Your Life. The Wall Street Journal. 2009. Oct 06, http://online.wsj.com/article/SB1000 1424052748703298004574455011023363866.html .
- [8].Sessler D. Thermoregulation and Heat Balance. In: Stephen M, Sessler D, editors. Therapeutic Hypothermia. New York: Marcel Decker; 2005. [Google Scholar]
- [9].Calver P, Braungardt T, Kupchik N, et al. The big chill: Improving the odds after cardiac arrest. Modern Medicine. 2005. May 01, http://rn.modernmedicine.com/rnweb/article/article Detail.jsp?id=158219 . [PubMed]
- [10].Kammersgaard LP, Jørgensen HS, Rungby JA, et al. Admission body temperature predicts long-term mortality after acute stroke: the Copenhagen Stroke Study. Stroke. 2002;33(7):1759–1762. doi: 10.1161/01.str.0000019910.90280.f1. [DOI] [PubMed] [Google Scholar]
- [11].Schwab S, Georgiadis D, Berrouschot J, et al. Feasibility and safety of moderate hypothermia after massive hemispheric infarction. Stroke. 2001;32(9):2033–2035. doi: 10.1161/hs0901.095394. [DOI] [PubMed] [Google Scholar]
- [12].Schubert A. Side effects of mild hypothermia. J Neurosurg Anesthesiol. 1995;7(2):139–147. doi: 10.1097/00008506-199504000-00021. [DOI] [PubMed] [Google Scholar]
- [13].Dietrich WD, Busto R, Alonso O, et al. Intraischemic but not postischemic brain hypothermia protects chronically following global forebrain ischemia in rats. J Cereb Blood Flow Metab. 1993;13(4):541–549. doi: 10.1038/jcbfm.1993.71. [DOI] [PubMed] [Google Scholar]
- [14].Colbourne F, Corbett D. Delayed postischemic hypothermia: a six month survival study using behavioral and histological assessments of neuroprotection. J Neurosci. 1995;15(11):7250–7260. doi: 10.1523/JNEUROSCI.15-11-07250.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lo TP, Jr, Cho KS, Garg MS, et al. Systemic hypothermia improves histological and functional outcome after cervical spinal cord contusion in rats. J Comp Neurol. 2009;514(5):433–448. doi: 10.1002/cne.22014. [DOI] [PubMed] [Google Scholar]
- [16].Hansebout RR, Kuchner EF, Romero-Sierra C. Effects of local hypothermia and of steroids upon recovery from experimental spinal cord compression injury. Surg Neurol. 1975;4(6):531–536. [PubMed] [Google Scholar]
- [17].Casas CE, Herrera LP, Prusmack C, et al. Effects of epidural hypothermic saline infusion on locomotor outcome and tissue preservation after moderate thoracic spinal cord contusion in rats. J Neurosurg Spine. 2005;2(3):308–318. doi: 10.3171/spi.2005.2.3.0308. [DOI] [PubMed] [Google Scholar]
- [18].Hansebout RR, Tanner JA, Romero-Sierra C. Current status of spinal cord cooling in the treatment of acute spinal cord injury. Spine (Phila Pa 1976) 1984;9(5):508–511. doi: 10.1097/00007632-198407000-00020. [DOI] [PubMed] [Google Scholar]
- [19].Marsala M, Vanicky I, Yaksh TL. Effect of graded hypothermia (27 degrees to 34 degrees C) on behavioral function, histopathology, and spinal blood flow after spinal ischemia in rat. Stroke. 1994;25(10):2038–2046. doi: 10.1161/01.str.25.10.2038. [DOI] [PubMed] [Google Scholar]
- [20].Robertson CS, Foltz R, Grossman RG, et al. Protection against experimental ischemic spinal cord injury. J Neurosurg. 1986;64(4):633–642. doi: 10.3171/jns.1986.64.4.0633. [DOI] [PubMed] [Google Scholar]
- [21].Kakinohana M, Taira Y, Marsala M. The effect of graded postischemic spinal cord hypothermia on neurological outcome and histopathology after transient spinal ischemia in rat. Anesthesiology. 1999;90(3):789–798. doi: 10.1097/00000542-199903000-00022. [DOI] [PubMed] [Google Scholar]
- [22].Wakamatsu H, Matsumoto M, Nakakimura K, et al. The effects of moderate hypothermia and intrathecal tetracaine on glutamate concentrations of intrathecal dialysate and neurologic and histopathologic outcome in transient spinal cord ischemia in rabbits. Anesth Analg. 1999;88(1):56–62. doi: 10.1097/00000539-199901000-00011. [DOI] [PubMed] [Google Scholar]
- [23].Westergren H, Holtz A, Farooque M, et al. Systemic hypothermia after spinal cord compression injury in the rat: does recorded temperature in accessible organs reflect the intramedullary temperature in the spinal cord? J Neurotrauma. 1998;15(11):943–954. doi: 10.1089/neu.1998.15.943. [DOI] [PubMed] [Google Scholar]
- [24].Ha KY, Kim YH. Neuroprotective effect of moderate epidural hypothermia after spinal cord injury in rats. Spine (Phila Pa 1976) 2008;33(19):2059–2065. doi: 10.1097/BRS.0b013e31818018f6. [DOI] [PubMed] [Google Scholar]
- [25].Kwon BK, Mann C, Sohn HM, et al. Hypothermia for spinal cord injury. Spine J. 2008;8(6):859–874. doi: 10.1016/j.spinee.2007.12.006. [DOI] [PubMed] [Google Scholar]
- [26].Clifton GL, Jiang JY, Lyeth BG, et al. Marked protection by moderate hypothermia after experimental traumatic brain injury. J Cereb Blood Flow Metab. 1991;11(1):114–121. doi: 10.1038/jcbfm.1991.13. [DOI] [PubMed] [Google Scholar]
- [27].Lyeth BG, Jiang JY, Liu S. Behavioral protection by moderate hypothermia initiated after experimental traumatic brain injury. J Neurotrauma. 1993;10(1):57–64. doi: 10.1089/neu.1993.10.57. [DOI] [PubMed] [Google Scholar]
- [28].Koizumi H, Povlishock JT. Posttraumatic hypothermia in the treatment of axonal damage in an animal model of traumatic axonal injury. J Neurosurg. 1998;89(2):303–309. doi: 10.3171/jns.1998.89.2.0303. [DOI] [PubMed] [Google Scholar]
- [29].Marion DW, White MJ. Treatment of experimental brain injury with moderate hypothermia and 21-aminosteroids. J Neurotrauma. 1996;13(3):139–147. doi: 10.1089/neu.1996.13.139. [DOI] [PubMed] [Google Scholar]
- [30].Maxwell WL, Donnelly S, Sun X, et al. Axonal cytoskeletal responses to nondisruptive axonal injury and the short-term effects of posttraumatic hypothermia. J Neurotrauma. 1999;16(12):1225–1234. doi: 10.1089/neu.1999.16.1225. [DOI] [PubMed] [Google Scholar]
- [31].Marion DW, Obrist WD, Carlier PM, et al. The use of moderate therapeutic hypothermia for patients with severe head injuries: a preliminary report. J Neurosurg. 1993;79(3):354–362. doi: 10.3171/jns.1993.79.3.0354. [DOI] [PubMed] [Google Scholar]
- [32].Clifton GL, Allen S, Barrodale P, et al. A phase II study of moderate hypothermia in severe brain injury. J Neurotrauma. 1993;10(3):263–271. doi: 10.1089/neu.1993.10.263. 273. [DOI] [PubMed] [Google Scholar]
- [33].Brain Trauma Foundation, American Association of Neu-rological Surgeons, Congress of Neurological Surgeons, et al. Guidelines for the management of severe traumatic brain injury. III. Prophylactic hypothermia. J Neurotrauma. 2007;24(Suppl 1):S21–25. doi: 10.1089/neu.2007.9993. [DOI] [PubMed] [Google Scholar]
- [34].Aibiki M, Maekawa S, Yokono S. Moderate hypothermia improves imbalances of thromboxane A2 and prostaglandin I2 production after traumatic brain injury in humans. Crit Care Med. 2000;28(12):3902–3906. doi: 10.1097/00003246-200012000-00029. [DOI] [PubMed] [Google Scholar]
- [35].Qiu W, Zhang Y, Sheng H, et al. Effects of therapeutic mild hypothermia on patients with severe traumatic brain injury after craniotomy. J Crit Care. 2007;22(3):229–235. doi: 10.1016/j.jcrc.2006.06.011. [DOI] [PubMed] [Google Scholar]
- [36].Minamisawa H, Nordström CH, Smith ML, et al. The influence of mild body and brain hypothermia on ischemic brain damage. J Cereb Blood Flow Metab. 1990;10(3):365–374. doi: 10.1038/jcbfm.1990.66. [DOI] [PubMed] [Google Scholar]
- [37].Welsh FA, Sims RE, Harris VA. Mild hypothermia prevents ischemic injury in gerbil hippocampus. J Cereb Blood Flow Metab. 1990;10(4):557–563. doi: 10.1038/jcbfm.1990.98. [DOI] [PubMed] [Google Scholar]
- [38].Corbett D, Nurse S, Colbourne F. Hypothermic neuroprotection. A global ischemia study using 18- to 20-month-old gerbils. Stroke. 1997;28(11):2238–2243. doi: 10.1161/01.str.28.11.2238. [DOI] [PubMed] [Google Scholar]
- [39].Dong H, Moody-Corbett F, Colbourne F, et al. Electrophysiological properties of CA1 neurons protected by postischemic hypothermia in gerbils. Stroke. 2001;32(3):788–795. doi: 10.1161/01.str.32.3.788. [DOI] [PubMed] [Google Scholar]
- [40].Zhao H, Wang JQ, Shimohata T, et al. Conditions of protection by hypothermia and effects on apoptotic pathways in a rat model of permanent middle cerebral artery occlusion. J Neurosurg. 2007;107(3):636–641. doi: 10.3171/JNS-07/09/0636. [DOI] [PubMed] [Google Scholar]
- [41].Yanamoto H, Nagata I, Niitsu Y, et al. Prolonged mild hypothermia therapy protects the brain against permanent focal ischemia. Stroke. 2001;32(1):232–239. doi: 10.1161/01.str.32.1.232. [DOI] [PubMed] [Google Scholar]
- [42].Clark DL, Penner M, Orellana-Jordan IM, et al. Comparison of 12, 24 and 48 h of systemic hypothermia on outcome after permanent focal ischemia in rat. Exp Neurol. 2008;212(2):386–392. doi: 10.1016/j.expneurol.2008.04.016. [DOI] [PubMed] [Google Scholar]
- [43].Horn M, Schlote W, Henrich HA. Global cerebral ischemia and subsequent selective hypothermia. A neuropathological and morphometrical study on ischemic neuronal damage in cat. Acta Neuropathol. 1991;81(4):443–449. doi: 10.1007/BF00293466. [DOI] [PubMed] [Google Scholar]
- [44].Jia X, Koenig MA, Nickl R, et al. Early electrophysiologic markers predict functional outcome associated with temperature manipulation after cardiac arrest in rats. Crit Care Med. 2008;36(6):1909–1916. doi: 10.1097/CCM.0b013e3181760eb5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Krieger DW, Yenari MA. Therapeutic hypothermia for acute ischemic stroke: what do laboratory studies teach us? Stroke. 2004;35(6):1482–1489. doi: 10.1161/01.STR.0000126118.44249.5c. [DOI] [PubMed] [Google Scholar]
- [46].Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346(8):557–563. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- [47].Seupaul RA, Wilbur LG. Evidence-based emergency medicine. Does therapeutic hypothermia benefit survivors of cardiac arrest? Ann Emerg Med. 2011;58(3):282–283. doi: 10.1016/j.annemergmed.2011.02.002. [DOI] [PubMed] [Google Scholar]
- [48].Shankaran S, Laptook AR, Ehrenkranz RA, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353(15):1574–1584. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- [49].Guest JD, Dietrich WD. Spinal cord ischemia and trauma. In: Tisherman SA, Sterz F, editors. Therapeutic Hypothermia. New York: Springer; 2005. [Google Scholar]
- [50].Atkins CM, Oliva AA, Jr, Alonso OF, et al. Hypothermia treatment potentiates ERK1/2 activation after traumatic brain injury. Eur J Neurosci. 2007;26(4):810–819. doi: 10.1111/j.1460-9568.2007.05720.x. [DOI] [PubMed] [Google Scholar]
- [51].Chatzipanteli K, Yanagawa Y, Marcillo AE, et al. Posttraumatic hypothermia reduces polymorphonuclear leukocyte accumulation following spinal cord injury in rats. J Neurotrauma. 2000;17(4):321–332. doi: 10.1089/neu.2000.17.321. [DOI] [PubMed] [Google Scholar]
- [52].Ishikawa T, Marsala M. Hypothermia prevents biphasic glutamate release and corresponding neuronal degeneration after transient spinal cord ischemia in the rat. Cell Mol Neurobiol. 1999;19(2):199–208. doi: 10.1023/a:1006973026514. [DOI] [PubMed] [Google Scholar]
- [53].Lotocki G, de Rivero Vaccari JP, Perez ER, et al. Alterations in blood-brain barrier permeability to large and small molecules and leukocyte accumulation after traumatic brain injury: effects of post-traumatic hypothermia. J Neurotrauma. 2009;26(7):1123–1134. doi: 10.1089/neu.2008.0802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Morino T, Ogata T, Takeba J, et al. Microglia inhibition is a target of mild hypothermic treatment after the spinal cord injury. Spinal Cord. 2008;46(6):425–431. doi: 10.1038/sj.sc.3102163. [DOI] [PubMed] [Google Scholar]
- [55].Tator CH. Update on the pathophysiology and pathology of acute spinal cord injury. Brain Pathol. 1995;5(4):407–413. doi: 10.1111/j.1750-3639.1995.tb00619.x. [DOI] [PubMed] [Google Scholar]
- [56].Small DL, Morley P, Buchan AM. Biology of ischemic cerebral cell death. Prog Cardiovasc Dis. 1999;42(3):185–207. doi: 10.1016/s0033-0620(99)70002-2. [DOI] [PubMed] [Google Scholar]
- [57].Milde LN. Clinical use of mild hypothermia for brain protection: a dream revisited. J Neurosurg Anesthesiol. 1992;4(3):211–215. doi: 10.1097/00008506-199207000-00012. [DOI] [PubMed] [Google Scholar]
- [58].Hägerdal M, Harp J, Nilsson L, et al. The effect of induced hypothermia upon oxygen consumption in the rat brain. J Neurochem. 1975;24(2):311–316. doi: 10.1111/j.1471-4159.1975.tb11881.x. [DOI] [PubMed] [Google Scholar]
- [59].Tabayashi K, Niibori K, Konno H, et al. Protection from postischemic spinal cord injury by perfusion cooling of the epidural space. Ann Thorac Surg. 1993;56(3):494–498. doi: 10.1016/0003-4975(93)90885-l. [DOI] [PubMed] [Google Scholar]
- [60].Busto R, Dietrich WD, Globus MY, et al. Small differences in intraischemic brain temperature critically determine the extent of ischemic neuronal injury. J Cereb Blood Flow Metab. 1987;7(6):729–738. doi: 10.1038/jcbfm.1987.127. [DOI] [PubMed] [Google Scholar]
- [61].Palmer C, Vannucci RC, Christensen MA, et al. Regional cerebral blood flow and glucose utilization during hypothermia in newborn dogs. Anesthesiology. 1989;71(5):730–737. doi: 10.1097/00000542-198911000-00017. [DOI] [PubMed] [Google Scholar]
- [62].Aoki M, Nomura F, Stromski ME, et al. Effects of pH on brain energetics after hypothermic circulatory arrest. Ann Thorac Surg. 1993;55(5):1093–1103. doi: 10.1016/0003-4975(93)90014-9. [DOI] [PubMed] [Google Scholar]
- [63].Ehrlich MP, McCullough JN, Zhang N, et al. Effect of hypothermia on cerebral blood flow and metabolism in the pig. Ann Thorac Surg. 2002;73(1):191–197. doi: 10.1016/s0003-4975(01)03273-8. [DOI] [PubMed] [Google Scholar]
- [64].Yan Y, Tang W, Deng Z, et al. Cerebral oxygen metabolism and neuroelectrophysiology in a clinical study of severe brain injury and mild hypothermia. J Clin Neurosci. 2010;17(2):196–200. doi: 10.1016/j.jocn.2009.05.022. [DOI] [PubMed] [Google Scholar]
- [65].Povlishock JT, Buki A, Koiziumi H, et al. Initiating mechanisms involved in the pathobiology of traumatically induced axonal injury and interventions targeted at blunting their progression. Acta Neurochir Suppl. 1999;73:15–20. doi: 10.1007/978-3-7091-6391-7_3. [DOI] [PubMed] [Google Scholar]
- [66].Xu L, Yenari MA, Steinberg GK, et al. Mild hypothermia reduces apoptosis of mouse neurons in vitro early in the cascade. J Cereb Blood Flow Metab. 2002;22(1):21–28. doi: 10.1097/00004647-200201000-00003. [DOI] [PubMed] [Google Scholar]
- [67].Adachi M, Sohma O, Tsuneishi S, et al. Combination effect of systemic hypothermia and caspase inhibitor administration against hypoxic-ischemic brain damage in neonatal rats. Pediatr Res. 2001;50(5):590–595. doi: 10.1203/00006450-200111000-00010. [DOI] [PubMed] [Google Scholar]
- [68].Kammersgaard LP, Rasmussen BH, Jørgensen HS, et al. Feasibility and safety of inducing modest hypothermia in awake patients with acute stroke through surface cooling: A case-control study: the Copenhagen Stroke Study. Stroke. 2000;31(9):2251–2256. doi: 10.1161/01.str.31.9.2251. [DOI] [PubMed] [Google Scholar]
- [69].Ning XH, Chen SH, Xu CS, et al. Hypothermic protection of the ischemic heart via alterations in apoptotic pathways as assessed by gene array analysis. J Appl Physiol. 2002;92(5):2200–2207. doi: 10.1152/japplphysiol.01035.2001. [DOI] [PubMed] [Google Scholar]
- [70].Polderman KH. Mechanisms of action, physiological effects, and complications of hypothermia. Crit Care Med. 2009;37(7 Suppl):S186–202. doi: 10.1097/CCM.0b013e3181aa5241. [DOI] [PubMed] [Google Scholar]
- [71].Baker AJ, Zornow MH, Grafe MR, et al. Hypothermia prevents ischemia-induced increases in hippocampal glycine concentrations in rabbits. Stroke. 1991;22(5):666–673. doi: 10.1161/01.str.22.5.666. [DOI] [PubMed] [Google Scholar]
- [72].Leker RR, Shohami E. Cerebral ischemia and trauma-different etiologies yet similar mechanisms: neuroprotective opportunities. Brain Res Brain Res Rev. 2002;39(1):55–73. doi: 10.1016/s0165-0173(02)00157-1. [DOI] [PubMed] [Google Scholar]
- [73].Auer RN. Non-pharmacologic (physiologic) neuroprotection in the treatment of brain ischemia. Ann N Y Acad Sci. 2001;939:271–282. doi: 10.1111/j.1749-6632.2001.tb03635.x. [DOI] [PubMed] [Google Scholar]
- [74].Raghupathi R, Graham DI, McIntosh TK. Apoptosis after traumatic brain injury. J Neurotrauma. 2000;17(10):927–938. doi: 10.1089/neu.2000.17.927. [DOI] [PubMed] [Google Scholar]
- [75].Yang D, Guo S, Zhang T, et al. Hypothermia attenuates ischemia/reperfusion-induced endothelial cell apoptosis via alterations in apoptotic pathways and JNK signaling. FEBS Lett. 2009;583(15):2500–2506. doi: 10.1016/j.febslet.2009.07.006. [DOI] [PubMed] [Google Scholar]


