Abstract
The distal end of the spinal cord and neuromuscular junction may develop secondary degeneration and damage following spinal cord injury because of the loss of neural connections. In this study, a rat model of spinal cord injury, established using a modified Allen's method, was injected with basic fibroblast growth factor solution via subarachnoid catheter. After injection, rats with spinal cord injury displayed higher scores on the Basso, Beattie and Bresnahan locomotor scale. Motor function was also well recovered and hematoxylin-eosin staining showed that spinal glial scar hyperplasia was not apparent. Additionally, anterior tibial muscle fibers slowly, but progressively, atrophied. nohistochemical staining showed that the absorbance values of calcitonin gene related peptide and acetylcholinesterase in anterior tibial muscle and spinal cord were similar, and injection of basic broblast growth factor increased this absorbance. Results showed that after spinal cord injury, the distal motor neurons and motor endplate degenerated. Changes in calcitonin gene related peptide and acetylcholinesterase in the spinal cord anterior horn motor neurons and motor endplate then occurred that were consistent with this regeneration. Our findings indicate that basic fibroblast growth factor can protect the endplate through attenuating the decreased expression of calcitonin gene related peptide and acetylcholinesterase in anterior horn motor neurons of the injured spinal cord.
Keywords: neural regeneration, spinal cord injury, motor endplate, basic fibroblast growth factor, calcitonin gene related peptide, acetylcholinesterase, subarachnoid catheter, grants-supported paper, neu-roregeneration
Research Highlights
-
(1)
Previous treatment of spinal cord injury does not include protection of the motor endplate because the recovery of muscle motor function depends on the regeneration and reconstruction of the neuromuscular junction.
-
(2)
No studies have reported the effect of basic fibroblast growth factor on anterior horn motor neurons and target organ neuromuscular junctions after spinal cord injury. Results showed that sic fibroblast growth factor can protect the endplate through increasing the expression of calcitonin gene related peptide and acetylcholinesterase in anterior horn motor neurons.
-
(3)
This study also explored the characteristics of subarachnoid cavity administration, which may reduce side effects of systemic administration and directly transfer drugs to the injury site.
INTRODUCTION
The pathological and physiological process of spinal cord injury, mainly caused by spinal trauma, occurs in two phases, namely primary and secondary injury[1,2]. Spinal cord contusion injury and its secondary impairment may lead to neuronal cell death, loss of oligodendrocytes, inflammation, astrocyte glial scar hyperplasia and permanent neurological deficits. Secondary injury may further exacerbate primary lesions, thus aggravating spinal cord injury and neurological deficits. The secondary injury process also provides many targets for the treatment of spinal cord injury. Current studies mainly explore secondary damage following spinal cord injury, pathophysiological changes in the injured spinal cord and neural regeneration at the local injury site[3]; however the degeneration and protection measures in the distal end of the injured spinal cord and target organ muscle effector have scarcely been investigated.
Under normal physiological conditions, the upper and lower motor neurons constantly transfer signals to each other. Anterior horn alpha motor neurons are in contact with skeletal muscle fibers via the neuromuscular junction (motor endplate). They concomitantly regulate a variety of physiological functions, and provide nutrition, in the skeletal muscle. When the distal reflex arc is in good condition, the injured spinal cord may develop secondary degeneration and impairment due to the loss of nerve connection in the distal spinal cord and neuromuscular junction[4,5,6,7,8,9,10]. The current treatment of spinal cord injury pays little attention to the protection of the motor endplate, but this seems counterintuitive as final recovery of neuromuscular function depends on the regeneration and reconstruction of the neuromuscular junction.
The acetylcholinesterase content and activity in motor neurons can reflect its function[11,12,13]. Acetylcholinesterase content in the neuromuscular junction is regarded as an indicator of motor endplate degeneration, recovery, and regeneration[14,15,16,17,18,19]. When injured motor neurons begin to recover, acetylcholinesterase content in the neuromuscular junction gradually returns to normal, and endplate ultrastructure is restored[4]. Calcitonin gene related peptide is widely distributed in the central and peripheral nervous system, and is involved in a variety of physiological and pathological activities[20,21,22,23,24,25,26,27,28,29,30]. Calcitonin gene related peptide can induce the expression and aggregation of the acetylcholine receptor, and also participates in the regulation of acetylcholinesterase through unknown pathways[31,32,33]. Calcitonin gene related peptide, synthesized in alpha motor neurons of spinal cord anterior horn, is associated with persistent excitability of central nerves[34]. In a spinal cord transection model in rats, upper motor neurons were injured and alpha motor neurons lost signal stimulation from higher centers to promote the synthesis of calcitonin gene related peptide. Calcitonin gene related peptide synthesis in cell bodies subsequently decreased, causing a reduction in the release from the axon terminal junction. Cyclic adenosine monophosphate then increased in skeletal muscle cells, thus weakening the ability of calcitonin gene related peptide to regulate acetylcholine receptor phosphorylation and aggregation, leading to endplate degeneration and muscle atrophy[35]. The content of calcitonin gene related peptide at the neuromuscular junction can reflect endplate degeneration and restoration. In fact, calcitonin gene related peptide is a more sensitive marker than acetylcholinesterase for the detection of motor endplate changes[10].
Basic fibroblast growth factor is involved in the protection of nerves and contributes to the nutrition of damaged neurons following spinal cord injury[36,37,38,39,40,41,42,43,44]. In a peripheral nerve injury model, basic fibroblast growth factor can prevent the degeneration and death of motor neurons in the anterior horn of the spinal cord caused by the attenuation of effector feedback[45]. Basic fibroblast growth factor also promotes the regeneration of the muscle motor endplate and the recovery of motor function after denervation[46]. However, the effect of basic fibroblast growth factor on motor neurons in the anterior horn of the injured spinal cord, and on the number of neuromuscular junctions in target organs, remains elusive.
Hematoxylin-eosin staining revealed that white matter residue of varying thickness was visible in the injured spinal cord of rats. Residual motor neurons were also seen in the anterior horn of the spinal cord[36]. Local drug administration via subarachnoid catheter shows good effectiveness, because of compensatory effects in residual motor neurons[36,37].
The present study explored the expression of calcitonin gene related peptide and acetylcholinesterase in spinal cord anterior horn motor neurons of rats with spinal cord injury, following the injection of basic fibroblast growth factor using a subarachnoid catheter. These studies provide evidence for the protection of motor endplate degeneration after spinal cord injury and the possible mechanism of these protective effects.
RESULTS
Quantitative analysis of experimental animals
Forty-five Sprague-Dawley rats were randomly divided into three groups: sham operated group (n = 5), basic fibroblast growth factor group (n = 20), and saline group (n = 20). A spinal cord injury model was produced in the basic fibroblast growth factor and saline groups. In the basic fibroblast growth factor group, rats were injected with basic fibroblast growth factor solution via subarachnoid catheter into the injured spinal cord at 0.5, 1, 2, 3, 4, 6, 12, 24, 48 hours post-injury, and then once per week. In the saline group, rats were injected with an equal volume of physiological saline at the same time. The sham operated control group received no treatment. There were six deaths in the basic fibroblast growth factor group and four deaths in the saline group, due to paraplegia and complications. In addition, five rats in the basic fibroblast growth factor group and six rats in the saline group were excluded due to catheter prolapse, which was the result of rat self-discomfort or poor fixation. Two rats in the basic fibroblast growth factor group and three rats in the saline group failed model establishment. All the excluded rats were supplemented in time. Overall, 45 rats were involved in the results analysis. Five rats in the basic fibroblast growth factor group and saline group were selected at 1, 2, 4, and 8 weeks, while 5 rats in the sham operated group were used at 1 week after model establishment, for the detection of hindlimb function using the Basso, Beattie and Bresnahan (BBB) locomotor scale. Tissue at the injured spinal cord and anterior tibial muscle were observed using hematoxylin-eosin staining, and changes in calcitonin gene related peptide and acetylcholinesterase were determined using immunohistochemistry.
Basic fibroblast growth factor promoted the recovery of hindlimb locomotor function in rats with spinal cord injury
Prior to the establishment of spinal cord injury, the baseline BBB score was 21 points. After the model was established, rats in the sham operated group showed normal function, with 21 points. BBB scores in the basic fibroblast growth factor and saline groups increased gradually, and hindlimb motor function recovered to different degrees. At 1 week after modeling, there was no significant difference in BBB scores between the basic fibroblast growth factor group and saline group (P > 0.05). At 2 weeks, the basic fibroblast growth factor group showed significantly higher BBB scores and better recovery of motor function than the saline group (P < 0.05; Table 1).
Table 1.
Rat hindlimb motor function after spinal cord injury (BBB score, mean ± SD)
Basic fibroblast growth factor improved the pathological changes of injured spinal cord and anterior tibial muscle in rats
Hematoxylin-eosin staining in spinal cord
At 1 week post-injury, neurons and glial cells in the sham group showed a dense structure, apparently visible nuclei and deeply stained nucleolus, with no infiltration of inflammatory cells (Figure 1).
Figure 1.
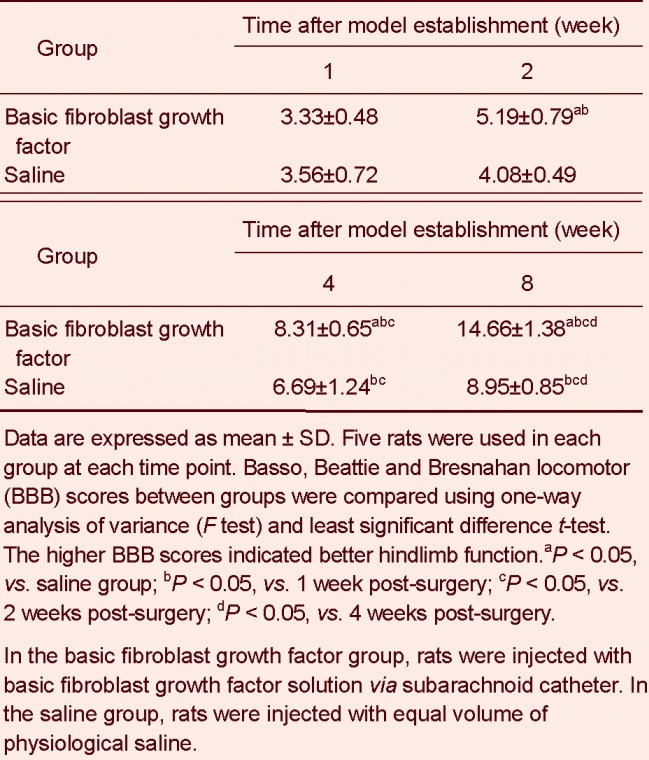
Hematoxylin-eosin staining in rats from the sham operated group (× 200).
(A) Spinal cord; (B) anterior tibial muscle. Under an XSP8CE optical microscope, spinal cord neurons and glial cells displayed a compact structure with clearly visible nuclei. The nucleoli were also stained and no inflammatory cell infiltration was observed. Anterior tibial muscle fibers were arranged in an orderly manner and were uniformly stained. The number of nuclei were increased and strongly stained. Arrow shows the stained neurons.
In the basic fibroblast growth factor and saline groups at 1 week, anterior horn motor neurons were pyknotic and lightly stained because of cell body edema. Cystic changes were present in the gray matter, and the number of Nissl bodies decreased. Nucleoli also disappeared, and inflammatory cell infiltration and glial cell hypertrophy were observed. These findings were consistent with the motor neuron damage after spinal cord injury, but the signal in the basic fibroblast growth factor group was lighter than the saline group (Figure 2). At 2 weeks, anterior horn motor neurons in the saline group began to shrink, nuclei disappeared, and projections and Nissl bodies decreased. Necrotic cavities were observed, glial cell proliferation and inflammatory reaction were visible. By contrast, glial cell proliferation was not obvious, and nuclei were visible in motor neurons in the basic fibroblast growth factor group. At 4 weeks, neural protrusions and Nissl bodies increased in the saline group, but cells were still lightly stained, and glial cell hyperplasia was apparent. The number of spinal cord anterior horn neurons increased in the basic fibroblast growth factor group, and displayed normal morphology and staining for the cell nucleus and nucleolus. At 8 weeks, the number of spinal cord anterior horn motor neurons was higher in the basic fibroblast growth factor group, and some cells became larger and recovered normal morphology. Nuclei were clearly visible, and hyperplasia of the glial scar was not obvious. In the saline group, motor neurons were unchanged when compared with results seen at 4 weeks, and a glial scar had formed (Figure 3).
Figure 2.
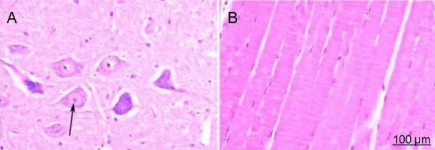
Hematoxylin-eosin staining of rats at 1 week after spinal cord injury (× 200).
(A) Spinal cord and (B) anterior tibial muscle from the saline group. (C) Spinal cord and (D) anterior tibial muscle from the basic fibroblast growth factor (bFGF) group. After spinal cord injury, rats in the bFGF group were injected with bFGF solution via subarachnoid catheter, while the saline group received an equal volume of saline. At 1 week after injury, spinal cord and anterior tibial muscle specimens were stained with hematoxylin and eosin, and then observed under an XSP8CE optical microscope. The anterior horn motor neurons were pyknotic, and cystic changes were present in the gray matter. The number of Nissl bodies decreased, and nucleoli disappeared. Inflammatory cell infiltration and glial cell hypertrophy were observed in the saline and bFGF groups. However, the staining in the bFGF group was lighter than the saline group. In addition, anterior tibial muscle appeared to progressively atrophy and hyperplasia in connective tissues was observed more readily in the saline group compared with the bFGF group.
Figure 3.

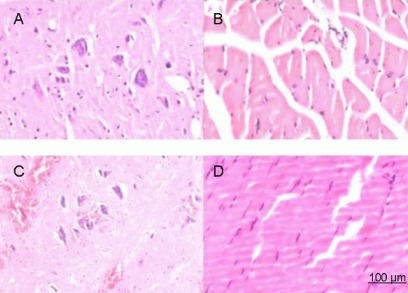
Hematoxylin-eosin staining of rats at 8 weeks after spinal cord injury (× 200).
(A) Spinal cord and (B) anterior tibial muscle from the saline group. (C) Spinal cord and (D) anterior tibial muscle from the basic fibroblast growth factor (bFGF) group. After spinal cord injury, rats in the bFGF group were injected with bFGF solution via subarachnoid catheter, while the saline group received an equal volume of saline. At 8 weeks, spinal cord and anterior tibial muscle specimens were stained with hematoxylin and eosin and then observed under an XSP8CE optical microscope. Spinal cord anterior horn motor neurons increased in the bFGF group, with some cells becoming larger and recovering normal morphology. Nuclei were clearly visible and hyperplasia was not obvious. By contrast, a glial scar formed in the saline group. The anterior tibial muscle in the saline group showed progressive atrophy and hyperplasia of connective tissues, while muscle fibers in the bFGF group fully recovered.
Hematoxylin-eosin staining in anterior tibial muscle
In the sham group, muscle fibers were arranged in an orderly manner, with uniform and deep staining. The number of muscle cells increased (Figure 1). At 1 week post-injury, muscle fibers in the saline group showed progressive atrophy and hyperplasia in connective tissue was observed. Atrophy of muscle fibers occurred to a lesser extent in the basic fibroblast growth factor group compared with the saline group at each time point, and were completely recovered at 8 weeks (Figures 2, 3).
Basic fibroblast growth factor increased calcitonin gene related peptide expression in spinal cord and anterior tibial muscle of rats with spinal cord injury
Positive expression of calcitonin gene related peptide in anterior horn motor neurons of the spinal cord in the sham group stained as pale yellow, yellow, and brown (Figure 4). At 1 week post-injury, absorbance in the basic fibroblast growth factor and saline groups decreased significantly, and the distribution of staining reduced significantly. Staining also became lighter compared with the sham group (Figure 5). After spinal cord injury, absorbance in the basic fibroblast growth factor and saline groups gradually increased, but was still lower than the sham group. At 2 weeks, the absorbance in the basic fibroblast growth factor group was higher than the saline group (Figures 6, 7). Immunohistochemical staining showed that anterior tibial muscle fibers in the sham group were stained as pale yellow and yellow spots or patches (Figure 4). The time-dependent absorbance change of anterior tibialis muscle was consistent with immunohistochemical staining results of calcitonin gene related peptide in spinal cord.
Figure 4.
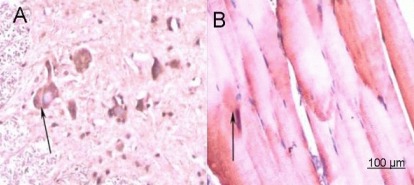
Immunohistochemical staining for calcitonin gene related peptide (CGRP) in the sham operated group (× 200).
(A) Spinal cord; (B) anterior tibial muscle. No treatment was given in sham operated group after injury. At 1 week post-surgery, spinal cord and anterior tibial muscle specimens were subjected to CGRP immunohistochemical staining, and observed under an XSP8CE optical microscope. Positive staining for CGRP in anterior horn motor neurons was pale yellow, yellow, and brown. Anterior tibial muscle fibers were arranged in order, with uniform and deep staining. The number of nuclei in muscle cells increased. Anterior tibial muscle fibers were stained as light yellow or yellow spots or patches. Arrows represent cells positively stained for CGRP.
Figure 5.
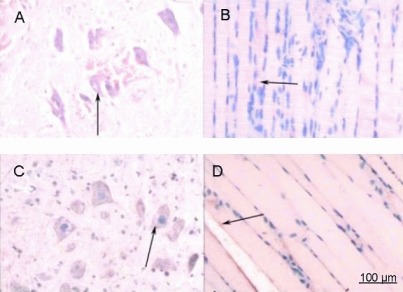
Immunohistochemical staining for calcitonin gene related peptide (CGRP) in rats at 1 week after spinal cord injury (× 200).
(A) Spinal cord and (B) anterior tibial muscle from the saline group.(C) Spinal cord and (D) anterior tibial muscle from the basic fibroblast growth factor (bFGF) group. After spinal cord injury, rats in the bFGF group were injected with bFGF solution via subarachnoid catheter, while the saline group received an equal volume of saline. At 1 week, spinal cord and anterior tibial muscle specimens were subjected to immunohistochemical staining for CGRP and observed under an XSP8CE optical microscope. Compared with the sham operated group, immunoreactive cells in the spinal cord and anterior tibial muscle were significantly reduced and lightly stained in the bFGF and saline groups. There was no significant difference between the bFGF and saline groups. Arrows represent cells positively stained for CGRP.
Figure 6.

Immunohistochemical staining for calcitonin gene related peptide (CGRP) in rats at 8 weeks after spinal cord injury (× 200).
(A) Spinal cord and (B) anterior tibial muscle from the saline group. (C) Spinal cord and (D) anterior tibial muscle from the basic fibroblast growth factor (bFGF) group. After spinal cord injury, rats in the bFGF group were injected with bFGF solution via subarachnoid catheter, while the saline group received an equal volume of saline. At 8 weeks, spinal cord and anterior tibial muscle specimens were subjected to immunohistochemical staining for CGRP, and observed under an XSP8CE optical microscope. Compared with the saline group, immunoreactive cells in the spinal cord and anterior tibial muscle were significantly increased and deeply stained in the bFGF group. Arrows represent cells positively stained for CGRP.
Figure 7.
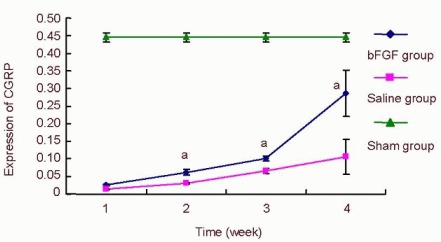
Effect of basic fibroblast growth factor (bFGF) on the expression of calcitonin gene related peptide (CGRP) in the rat spinal cord.
After spinal cord injury, rats from the bFGF group were injected with bFGF solution via a subarachnoid catheter, while the saline group received an equal volume of saline. Five rats in the bFGF group and saline group were selected at 1, 2, 4, and 8 weeks, while five rats at 1 week from the sham operated (sham) group were used. CGRP staining in the spinal cord and specimens was observed under an XSP8CE optical microscope. The absorbance of positively expressed cells was measured with an Image-Pro Plus 6 image analysis system.
Data were expressed as mean ± SD and were compared using one-way analysis of variance (F test) and least significant difference t-test. After spinal cord injury, the absorbance in the bFGF group and saline group gradually increased. aP < 0.05, vs. saline group.
Basic fibroblast growth factor increased acetylcholinesterase expression in spinal cord and anterior tibial muscle of rats with spinal cord injury
Positive expression of acetylcholinesterase in anterior horn motor neurons of the spinal cord in the sham group stained as pale yellow, yellow, and brown (Figure 8). At 1 week post-injury, the absorbance in the basic fibroblast growth factor and saline groups decreased significantly compared with the sham group (Figure 9). At 2 weeks, absorbance in the two groups remained unchanged and gradually increased at 4 and 8 weeks. Absorbance in the basic fibroblast growth factor group was higher than the saline group (Figures 10, 11). Immunohistochemical staining showed that anterior tibial muscle fibers in the sham group were stained as pale yellow and yellow spots or patches (Figure 8). The time-dependent absorbance change of anterior tibialis muscle was consistent with immunohistochemical staining results for acetylcholinesterase in the spinal cord.
Figure 8.
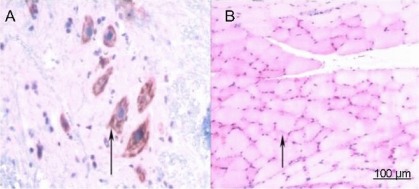
Immunohistochemical staining for acetylcholinesterase in the sham operated group (× 200).
(A) Spinal cord; (B) anterior tibial muscle. No treatment was given to the sham group after injury. At 1 week post-surgery, spinal cord and anterior tibial muscle specimens were subjected to immunohistochemical staining for acetylcholinesterase and observed under an XSP8CE optical microscope. Positive staining for acetylcholinesterase in anterior horn motor neurons was pale yellow, yellow, and brown. Anterior tibial muscle fibers were stained as light yellow or yellow spots or patches. Arrows represent positively staining for acetylcholinesterase.
Figure 9.
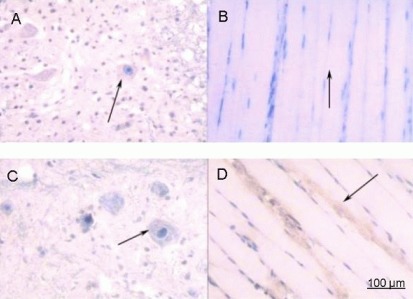
Immunohistochemical staining for acetylcholinesterase in rats 1 week after spinal cord injury (× 200).
(A) Spinal cord and (B) anterior tibial muscle from the saline group.(C) Spinal cord and (D) anterior tibial muscle from the basic fibroblast growth factor (bFGF) group. After spinal cord injury, rats in the bFGF group were injected with bFGF solution via subarachnoid catheter, while the saline group received an equal volume of saline. At 1 week post-injury, spinal cord and anterior tibial muscle specimens were subjected to immunohistochemical staining for acetylcholinesterase and were observed under an XSP8CE optical microscope. Compared with the sham operated group, immunoreactive cells in the spinal cord and anterior tibial muscle were significantly reduced and lightly stained in the bFGF and saline groups. More immunoreactive cells were seen in the bFGF group than in the saline group. Arrows represent cells positively expressing acetylcholinesterase.
Figure 10.
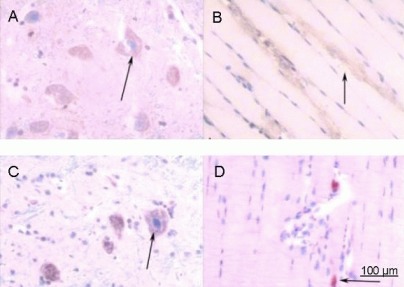
Immunohistochemical staining for acetylcholinesterase in rats at 8 weeks after spinal cord injury (× 200).
(A) Spinal cord and (B) anterior tibial muscle from the saline group. (C) Spinal cord and (D) anterior tibial muscle from the basic fibroblast growth factor (bFGF) group. After spinal cord injury, rats in the bFGF group were injected with bFGF solution via subarachnoid catheter, while the saline group received an equal volume of saline. At 8 weeks, spinal cord and anterior tibial muscle specimens were subjected to immunohistochemical staining for acetylcholinesterase, and observed under an XSP8CE optical microscope. Compared with the saline group, immunoreactive cells in spinal cord and anterior tibial muscle were significantly increased and deeply stained in the bFGF. Arrows represent cells positively expressing acetylcholinesterase.
Figure 11.
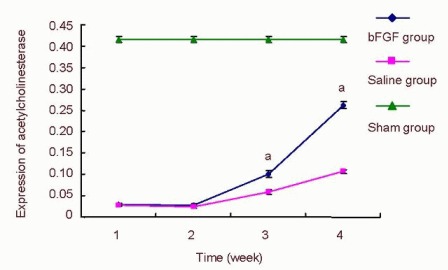
Effect of basic fibroblast growth factor (bFGF) on the expression of acetylcholinesterase in the spinal cord of rats.
After spinal cord injury, rats in the bFGF group were injected with bFGF solution via subarachnoid catheter, while the saline group received an equal volume of saline. Five rats in the bFGF group and saline group were selected at 1, 2, 4, and 8 weeks, while five rats at 1 week post injury were used in the sham operated (sham) group for immunohistochemical staining. Specimens were observed under an XSP8CE optical microscope. The absorbance of positively expressed cells was measured using an Image-Pro Plus 6 image analysis system.
Data were expressed as mean ± SD and were compared using one-way analysis of variance (F test) and least significant difference t-test. After spinal cord injury, the absorbance in the bFGF group and saline group was significantly lower than that of the sham group. At 2 weeks, the absorbance was unchanged. At 4 and 8 weeks, the absorbance gradually increased. aP < 0.05, vs. saline group.
DISCUSSION
Our findings have confirmed that anterior horn motor neurons and motor endplates degenerated following spinal cord injury. Basic fibroblast growth factor could protect motor endplates from degeneration, through protecting motor neurons and mediating the expression of calcitonin gene related peptide and acetylcholinesterase, accordingly promoting the recovery of motor function in rats. The activity, distribution and quantity of acetylcholinesterase and calcitonin gene related peptide can reflect the degeneration and regeneration of motor neurons[4] and the endplates[15]. Calcitonin gene related peptide, synthesized in spinal cord anterior horn motor neurons, may reach the neuromuscular junction via anterograde axoplasmic transport, and then co-exist with acetylcholine in the motor endplate. Calcitonin gene related peptide also contributes to the regulation of the acetylcholinesterase dimer and performs other functions in the end plate through as-yet unknown pathways[31]. Our findings suggest that calcitonin gene related peptide levels increase earlier during the recovery phase compared with acetylcholinesterase, which is consistent with a previous report[47].
Within 8 weeks post-injury, the expression of acetylcholinesterase and calcitonin gene related peptide in rat injured spinal cord from the saline group was lower than the sham rats. Furthermore, anterior horn motor neurons of the spinal cord T9 segment were injured and displayed local inflammation, as well as hindlimb muscle atrophy. BBB score was also significantly lower than the sham group, indicating motor neuron degeneration after spinal cord injury, as previously described[48]. The degeneration mechanism may depend on two factors: one is rupture of nerve fibers at the primary injury site, resulting in loss of nutrition and signal transmission support in the distal motor neurons, ultimately leading to neuronal degeneration[34]. The other factor is the decrease in neurotrophic factor feedback provided for motor neurons, resulting in a weakened ability for muscle movement[49].
Similarly, our study also confirmed motor endplates below the injury level also degenerated after spinal cord injury[49]. Within 8 weeks after spinal cord injury, acetylcholinesterase and calcitonin gene related peptide expression levels in anterior tibial muscle motor endplate from the saline treated rats were still lower than in the sham operated group.
In addition, anterior tibial muscle in the hindlimb showed atrophy to varying degrees, and motor function was significantly reduced. Muscle fiber atrophy and connective tissue were visible and then gradually recovered. This evidence suggests that the quantity, morphology and distribution of muscle fiber neuromuscular junctions changed or degenerated[10,50]. After spinal cord injury, calcitonin gene related peptide degeneration and recovery were the same in spinal cord anterior horn motor neurons and motor endplate. Acetylcholinesterase expression level changes in motor neurons and neuromuscular junction were also consistent, but peripheral nerves did not degenerate[5]. We conclude that after spinal cord injury, although the lower motor neurons and neural circuits were intact, anterior horn motor neurons below the injury level degenerated, thus reducing calcitonin gene related peptide synthesis and release from nerve endings. Calcitonin gene related peptide and acetylcholinesterase content in the endplate also decreased, and motor endplates accordingly degenerated and muscle fiber atrophy occurred.
Methylprednisolone is generally considered the most effective drug in the treatment of acute spinal cord injury, as it can significantly reduce secondary injury, prevent apoptosis of nerve cells, and promote nerve cell recovery[51]. The suggested regime for methylprednisolone in this situation is administration within 8 hours via shock therapy; however high-dose chemotherapy may produce serious complications[51,52]. Nerve growth factor also has some protective effects and the muscle extract, motoneuronotrophic factor 1 or 2, can promote motor neuron growth[52]; however it is difficult to obtain these extractions[53]. After spinal cord injury, glial cell-derived neurotrophic factor increased the expression of calcitonin gene related peptide in spinal cord, and promoted motor recovery, but also increased positive expression of calcitonin gene related peptide in pain neurons, and significantly decreased pain threshold[53]. At present, protection of motor neurons following traumatic injury still lacks an effective factor. Basic fibroblast growth factor has been shown to have a protective effect on injured spinal cord, such as reducing neural cell death and promoting the recovery of neurological function[38,39,54,55]. Follesa and colleagues[56] and Moccheti et al[57] found that basic fibroblast growth factor mRNA and protein levels in the injured spinal cord were increased significantly during the early stages of injury. High-dose methylprednisolone may reduce secondary injury and increase the expression of endogenous basic fibroblast growth factor in the spinal cord, and also provide nutrition for nerve cells and protect injured spinal cord[58]. Following spinal cord injury, local damage factor c-fos mRNA transcription increased, but exogenous basic fibroblast growth factor can inhibit the c-fos gene at the injury site[59,60]. After an injured peripheral nerve is repaired following treatment with basic fibroblast growth factor or physiological saline, basic fibroblast growth factor could promote motor endplate regeneration and motor functional recovery at 12 weeks after denervation[24].
Therefore, we used recombinant bovine basic fibroblast growth factor for local delivery to explore the morphology of motor neurons and motor endplate. Changes in calcitonin gene related peptide and acetylcholinesterase following spinal cord injury were also investigated. We conclude that subarachnoid injection of basic fibroblast growth factor can protect motor neurons in the spinal cord anterior horn, and promote motor neuron synthesis and release motor endplate protective factors (calcitonin gene related peptide and acetylcholinesterase), thus preventing motor endplate degeneration and promoting the recovery of motor function in rats. We speculate that basic fibroblast growth factor has four effects on the injured spinal cord: it protects damaged neurons; reduces glial cell hypertrophy; provides nutrition for the regeneration of nerve fibers and promote angiogenesis to improve microcirculation[61]. The quantity, ultrastructure, and changes at a molecular level of motor endplates and neurons require further study.
In summary, the repair of spinal cord injury should not be restricted to functions of the local spinal cord and neural regeneration through nerve stump. More consideration focusing on the functions of the distal spinal cord after spinal cord injury and the degeneration of target organ effector structure function should be made because eventual recovery of muscle motor function depends on neuromuscular junction regeneration and reconstruction. This evidence will guide research into the repair mechanisms and clinical treatment of spinal cord injury.
MATERIALS AND METHODS
Design
An in vivo animal experiment.
Time and setting
Experiments were completed in the Third Xiangya Hospital of Central South University, China from April to December in 2011. Experimental animal feeding and model establishment were performed in the Experimental Animal Center of the Third Xiangya Hospital of Central South University in China. Specimen collection, slicing and immunohistochemistry were conducted in the Central Laboratory of the Third Xiangya Hospital of Central South University, China.
Materials
Animals
Forty-five healthy adult Sprague-Dawley male rats, weighing 200–250 g, and of clean grade, were provided by the Experimental Animal Center of the Third Xiangya Hospital of Central South University, China [license No. SCXK (Xiang) 2009-0004]. Rats were housed in separate cages (Suzhou Fengshi Laboratory Animal Equipment Co., Ltd., Suzhou, Jiangsu Province, China), and allowed free access to food and water. Rats lived under 14-hour lighting conditions in 40–60% humidity at 20–22°C.
Drugs
Recombinant bovine basic fibroblast growth factor (Yisheng Biological Pharmaceutical Co., Ltd., Zhuhai, Guangdong Province, China), at analytically pure grade (1 mg powder is equivalent to 1 890 000 IU) was used in this study. Basic fibroblast growth factor (1 mg) was dissolved in sterile saline and stock solution volume was made up to 189 mL. The stock solution was then diluted to 10 IU/1 μL, so each 20 μL aliquot contained 0.106 μg of basic fibroblast growth factor.
Methods
Establishment of spinal cord injury model
A spinal cord injury model was established in the basic fibroblast growth factor and saline groups. Briefly, rats were intraperitoneally anesthetized with 5% chloral hydrate (0.4 mL/100 g), and fixed in the prone position. T8-10 segments of the spinal cord were exposed for surgery. A sterilized metal pad was placed on the T9 dorsal side, serving as the hit plate. According to the modified Allen's method[62], a 10-g weight fell onto the hit plate from a 4-cm height to produce incomplete spinal cord injury in rats. At the damage zone, a PE10 thin catheter was always implanted via the subarachnoid cavity to 1 cm from the distal end of the injured spinal cord. The incision was sutured with 1-0 silk thread and the catheter was firmly fixed. The model was considered successful upon animal appearance, for example on observation of a spastic swing of the rat tail and dragging of the lower limbs. In the sham operation group, rats were anesthetized and the vertebral plate was opened and the spinal cord was exposed, but animals did not experience trauma or catheter placement.
Administration of basic fibroblast growth factor
Rats in the basic fibroblast growth factor group, were injected with 20 μL basic fibroblast growth factor (containing 0.106 μg recombinant bovine basic fibroblast growth factor) via a microsyringe (Pengshen Medical Devices Co., Ltd., Shanghai, China) at 0.5, 1, 2, 3, 4, 6, 12, 24, 48 hours after injury and then once per week. The saline group was injected with an equal volume of saline and the sham operation group received no treatment. After surgery, rats in the basic fibroblast growth factor group and saline group underwent bladder massage to assist urination until the voiding function recovered.
BBB scale assessment
Movement function of the hind limbs was evaluated by two testers using the BBB scale[63] in a double-blind independent manner. Higher scores indicated better function. Complete paralysis = 0 point, completely functional: 21 points.
Specimen collection and sectioning
After BBB scale assessment, rats were killed under intraperitoneal anesthesia and fixed in a supine position with 4% paraformaldehyde and PBS via cardiac perfusion. T8-11 segments of the spinal cord and anterior tibialis muscle in the right hind limb were collected and sliced into continuous sections at 4–6 μm thickness using a paraffin slicing machine (Shandon Finesse ME+, Edinburgh, UK). Spinal cord cross-sections 5 mm below the injury level were observed to detect the degeneration at the distal end of the injured spinal cord, and anterior tibial muscle longitudinal slices (4–6 μm thick) were also observed. Spinal cord and anterior tibial muscle tissue was stained with hematoxylin-eosin and underwent immunohistochemical staining for calcitonin gene related peptide and acetylcholinesterase.
Hematoxylin-eosin staining
Specimens were dewaxed, hydrated, and stained with hematoxylin solution for 20 minutes. Sections were then separated with 0.5% hydrochloric acid solution for 3 seconds, immersed in water for 20 minutes and then in 0.5% eosin for 2 minutes. After sections were rinsed with distilled water, specimens were dried, dehydrated in gradient alcohol, and sealed. Images were collected using a XSP8CE light microscope (Shanghai Changfang Optical Instrument Co., Ltd., Shanghai, China).
Immunohistochemical staining for calcitonin gene related peptide
Glass slides were treated with poly-L-lysine, dewaxed in paraffin and hydrated. After endogenous enzymes were cleared with 30% hydrogen peroxide and pure methanol at 1:9 (v/v), sections were immersed in 80°C antigen repair solution for 10 minutes. Tissues were then cooled and rinsed with PBS (0.10 mol/L for 10 minutes), and subsequently incubated and blocked with goat serum for 10 minutes at room temperature. Tissues were then exposed to rabbit anti-rat calcitonin gene related peptide solution (1:2 000; Boosen Biological Technology Co., Ltd., Beijing, China) for 18 hours, rinsed with PBS for 10 minutes. Sections were then incubated with biotin labeled goat anti-rabbit IgG (1:300; Beijing CoWin Bioscience Co., Ltd., Beijing, China) at 37°C for 10 minutes, rinsed with PBS and incubated with horseradish peroxidase conjugated streptavidin (1:300; Boosen Biological Technology Co., Ltd.) for 10 minutes at room temperature, and rinsed with PBS. Signal was developed using 3,3’-diaminobenzidine, and sections were lightly counterstained with hematoxylin, dehydrated, and mounted onto slides. Staining images were also obtained under an XSP8CE optical microscope and observed using an Image-Pro Plus 6 image analysis system (Media Cybernetics Inc, Bethesda Maryland, USA) to measure the absorbance of calcitonin gene related peptide positive cells. Absorbance = integrated absorbance/positive expression area. Three images in each section were applied to calculate the average value of primitive absorbance.
Immunohistochemical staining for acetylcholinesterase
The absorbance of acetylcholinesterase positive cells was determined using the same methods as for calcitonin gene related peptide immunohistochemical staining. Specimens were incubated with rabbit anti-rat acetylcholinesterase solution (1:2 000; Boosen Biological Technology Co., Ltd.).
All other antibodies and methodology were the same as calcitonin gene related peptide immunohistochemical staining.
Statistical analysis
Data were expressed as mean ± SD using SPSS 11.6 software (SPSS, Chicago, IL, USA). One-way analysis of variance (F test) and least significant difference t-test were performed. P < 0.05 was considered significant.
Acknowledgments:
We would like to thank Zhou J, Tian Y, Ding ZG and Hu Y from Central Laboratory, Third Xiangya Hospital, Central South University, China for their great help and unremitting support during the study design and experimental procedures.
Footnotes
Funding: This study was supported by a grant from the Hunan Provincial Science and Technology Ministry in China, No. 2012SK3222 and Funding for New Teachers by the Ministry of Education in China, No. 200805331166.
Conflicts of interest: None declared.
Ethical approval: Experimental animals were treated according to the Animal Ethnical Criteria of Central South University in China.
(Reviewed by Apricò B, Xu WM, Zeng XN)
(Edited by Mu WJ, Yang Y, Li CH, Song LP, Liu WJ, Zhao M)
REFERENCES
- [1].Young W. Secondary injury mechanisms in acute spinal cord injury. J Emerg Med. 1993;11:13–22. [PubMed] [Google Scholar]
- [2].Xu ST. Primary and secondary spinal cord injury. Zhonghua Guke Zazhi. 2005;25(9):575–576. [Google Scholar]
- [3].Raisman G. Repair of spinal cord injury: ripples of incoming tide, or how I spent my first 40 years in reserch. Spinal Cord. 2006;44(7):406–413. doi: 10.1038/sj.sc.3101948. [DOI] [PubMed] [Google Scholar]
- [4].Yin Q, Lu YP, Hu WY, et al. Experimental research of motor endplate in the early degeneration at muscle loss of nerve. Zhongguo Xiufu Chongjian Waike. 1995;9:35–37. [Google Scholar]
- [5].Zhang SC, Zheng XD, Xiu XL, et al. The preliminary research on the peripheral nerve pathological changes after spinal cord injury and clinical observation. Zhongguo Xianwei Waike Zazhi. 2000;23(3):215–216. [Google Scholar]
- [6].Moon L, Bunge MB. From animal models to humans: strategies for promoting CNS axon regeneration and recovery of limb function after spinal cord injury. Neurol Phys Ther. 2005;29:55–69. doi: 10.1097/01.npt.0000282512.16964.94. [DOI] [PubMed] [Google Scholar]
- [7].Diets V, Muller R. Degradation of neuronal function following a spinal cord injury:mechanisms and countermeasures. Brain. 2004;127:2221–2231. doi: 10.1093/brain/awh255. [DOI] [PubMed] [Google Scholar]
- [8].Lin CS, Macefield VG, Elam M, et al. Axonal changes in spinal cord injured patients distal to the site of injury. Brain. 2007;130:985–994. doi: 10.1093/brain/awl339. [DOI] [PubMed] [Google Scholar]
- [9].Nogajski JH, Engel S, Kiernan MC. Focal and generalized nerve dysfunction in spinal cord--injured patients. J Clin Neurophysiol. 2006;23:273–279. doi: 10.1097/01.wnp.0000201062.99671.27. [DOI] [PubMed] [Google Scholar]
- [10].Zhang QM, Guan H, Hong Y. Relationship of the change of calcitonin gene-related peptide and degeneration of the motor endplate after spinal cord injury in the rat. Zhongguo Kangfu Lilun yu Shijian. 2005;11(2):89–90. [Google Scholar]
- [11].Fernandez HL, Smith A, Dennis JS, et al. Calcitonin receptor-like receptor expression in rat skeletal muscle fibers. Brain Res. 2011;1371:1–6. doi: 10.1016/j.brainres.2010.11.034. [DOI] [PubMed] [Google Scholar]
- [12].Legay C. Why so many forms of acetylcholinesterase. Microsc Res Tech. 2000;49(1):56–72. doi: 10.1002/(SICI)1097-0029(20000401)49:1<56::AID-JEMT7>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- [13].Sun YX, Liu N, Xu AH, et al. The effect of chondroitinase ABC combined with hyperbaric oxygen preconditioningtreatment on the motor function of hindlimbs in spinal cord injury rats. Jiepou Kexue Jinzhan. 2012;18(6):526–529. [Google Scholar]
- [14].Gao J, Ba F, Li HP. Effects of methylprednisolone on acetylcholinesterase in motor endplate after spinal cord semi-transected in rats. Zhongguo Yike Daxue Xuebao. 2012;41(3):197–200. [Google Scholar]
- [15].Xu XS, Shen JY. Motor endplate and the dyeing method of nerve endings. Zhongguo Renmin Jiefangjun Junshi Yixue Kexueyuan Yuankan. 1980;9:105–105. [Google Scholar]
- [16].Li HP, Bai XD, Gao J, et al. Effects of chondroitinase ABC injection on motor and acetylcholinestarase in gastrocnemius of spinal cord injuried adult rats. Zhongguo Kangfu Lilun yu Shijian. 2012;18(3):219–221. [Google Scholar]
- [17].Zhou Y, Wang B. Effects of tail suspension and angiotensin II on motor end plate of rat skeletal muscle. Tianjin Tiyu Xueyuan Xuebao. 2012;27(2):158–161. [Google Scholar]
- [18].Zhang AF, Su ZY, Yang ZY, et al. Repair of sciatic nerve gap by transplanting chitosan tube in rats. Shenjing Jiepouxue Zazhi. 2010;26(5):507–512. [Google Scholar]
- [19].Su ZY, Yan ZY, Li XG. Adjustment and maintenance of spinal cord reconstruction tubes on hindlimb motor end-plates about spinal cord injury at T8 in rats. Zhongguo Kangfu Lilun yu Shijian. 2010;16(3):205–208. [Google Scholar]
- [20].Amara SG, Jonas V, Rosenfeld MG, et al. Alternative RNA processing in calcitonin gene expression generates mRNAs encoding different polypeptide products. Nature. 1982;298(5871):240–244. doi: 10.1038/298240a0. [DOI] [PubMed] [Google Scholar]
- [21].Buffeli M, Pasino E, Cangiano A. In vivo acetylcholine expression induced by calcitonin gene-related peptide in rat soleus muscle. Neuroscience. 2001;104:561–567. doi: 10.1016/s0306-4522(01)00090-2. [DOI] [PubMed] [Google Scholar]
- [22].Du ZX, Zhou Z, Wang XJ, et al. The influences of brain injury on the expressions of CGRP and PDGF in femoral fracture callus of rats. Linchuang Yixue Gongcheng. 2011;18(2):186–188. [Google Scholar]
- [23].Zhou B, Han ZM. Calcitonin gene-related peptide in bone repair and bony remodeling. Wujing Houqin Xueyuan Xuebao: Yixue Ban. 2012;21(2):137–143. [Google Scholar]
- [24].Gong ZG, Song CH, Shi XQ. Effects of Puerarin on calcitonin gene-related peptide in DN rats. Zhongguo Dangdai Yixue. 2013;20(7):7–13. [Google Scholar]
- [25].Wang YG, Zhou HY, Wang SK, et al. Distribution and co-expression of neuropeptide Y/calcitonin gene-related peptide in the lumbarintervertebral disc. Zhongguo Zuzhi Gongcheng Yanjiu. 2012;16(48):9039–9043. [Google Scholar]
- [26].Luo D, Yin HK, Li QX. The change regularities of plasma endothelin and calcitonin gene-related peptide in patients with brain surgery. Xiandai Shengwu Yixue Fazhan. 2012;12(26):5140–5146. [Google Scholar]
- [27].Zhao LF, Jiang R, Ji P. Effect of calcitonin gene-related peptide receptor on expression of nf-κb in rat lung after ischemia reperfusion injury. Sichuan Daxue Xuebao: Yixue Ban. 2012;43(5):666–669. [PubMed] [Google Scholar]
- [28].Wang YC, Guo QL, Wang MD, et al. Effect of intrathecal sufentanil and protein kinase C inhibitoron pain threshold and the expression of NMDA receptor/CGRP in spinal dorsal horn in rats with neuropathic pain. Zhongnan Daxue Xuebao: Yixue Ban. 2012;37(8):783–789. doi: 10.3969/j.issn.1672-7347.2012.08.005. [DOI] [PubMed] [Google Scholar]
- [29].Wang X, Ma LM, Jia SJ, et al. Effects of hormone on heart function protection and the levels of serum calcitonin-gene-related peptide and endothelin-1 in brain-dead pigs. Zhongguo Zuzhi Gongcheng Yanjiu. 2012;16(18):3263–3266. [Google Scholar]
- [30].Tuo YH, Guo XL, Zhang XX, et al. Effect of calcitonin gene-related peptide on proliferation and migration of human umbilical vein endothelial cells. Zhongguo Xiufu Chongjian Waike Zazhi. 2012;26(4):495–500. [PubMed] [Google Scholar]
- [31].Fernandez HL, Ross GS, Nadelhaft I. Neurogenic calcitonin gene-related peptide: a neurot rophic factor in t he main tenance of acetylcholinesterase molecular forms in adult skeletal muscles. Brain Res. 1999;844(1-2):83–97. doi: 10.1016/s0006-8993(99)01891-0. [DOI] [PubMed] [Google Scholar]
- [32].Tsim KW, Choi RC, Xie HQ, et al. Transcriptional control of different subunits of AChE in muscles: signals triggered by the motor nerve-derived factors. Chem Biol Interact. 2008;175(1-3):58–63. doi: 10.1016/j.cbi.2008.04.014. [DOI] [PubMed] [Google Scholar]
- [33].Mao YA, Li JA, Li B, et al. Morphological study and clinical significance of skeletal muscle's motor end plates and motor points in rabbits. Zhongguo Kangfu Yixue Zazhi. 2011;26(11):1014–1019. [Google Scholar]
- [34].Stedler H, Kiene ML. Synaptic vesicles in electromotoneurons II. Heterogeneity of populations is expressed in uptake properties: exocytosis and insertion of a core proteoglycan into the extracellular matri. EMBO. 1987;6:2217–2221. doi: 10.1002/j.1460-2075.1987.tb02493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Changeux J. Molecular biology of acetylcholine receptor: Functional architecture and development regulation. Eur J Neurosci. 1991;4:1–10. [Google Scholar]
- [36].Chi LT, Pei FX, Wang GL, et al. Experimental study on dynamic changes of expression of basic fibroblast growth factor mRNA after peripheral nerve injury. Zhonghua Chuangshang Zazhi. 1999;15:407. [Google Scholar]
- [37].Deng L, Ling M. Expression change of basic fibroblast growth factor and its significance after experimental spinal cord injury in rat. Xiandai Yiyao Weisheng. 2011;27(15):2243–2244. [Google Scholar]
- [38].Yang XG, Jiang LB. Alterations and roles of basic FGF expression following experimental spinal cord injury. Zhongguo Linchuang Yixue. 2002;9(1):55–57. [Google Scholar]
- [39].Liu WG, Wang F. Effect of basic fibroblast growth factor on the expression of bcl-2 and bax gene in spinal cord injury. Zhongguo Linchuang Kangfu. 2003;7(29):3956–3957. [Google Scholar]
- [40].Yang YL. Effect of adenovirus-mediated basic fibroblast growth factor gene transfer in vivo on oligodendrocyte cell numbers throughout ventrolateral white matter following spinal cord injury in rats. Acta Academiae Medicinae Sinicae. 2012;34(4):348–352. doi: 10.3881/j.issn.1000-503X.2012.04.007. [DOI] [PubMed] [Google Scholar]
- [41].Yang YL. Adenovirus-mediated FGF-2 gene promotes motor function of rats with spinal cord injury. Dier Junyi Daxue Xuebao. 2011;10(32):1150–1152. [Google Scholar]
- [42].Wang F, Chu GJ, Zhu Y. The effect of bFGF on glial differentiation of neural stem cells. Zhongguo Yike Daxue Xuebao. 2012;3(41):193–196. [Google Scholar]
- [43].Delgado-Rivera R, Harris SL, Ahmed I, et al. Increased FGF-2 secretion and ability to support neurite outgrowth by astrocytes cultured on polyamide nanofibrillar matrices. Matrix Biol. 2009;28(3):137–147. doi: 10.1016/j.matbio.2009.02.001. [DOI] [PubMed] [Google Scholar]
- [44].Chen H, Fu WX, Du T, et al. Transplantation of microencapsulated rabbit schwann cells in rats after spinal cord injury: Basic fibroblast growth factor expression and hindlimb movement function changes. Zhongguo Zuzhi Gongcheng Yanjiu yu Linchaung Kangfu. 2010;14(8):1372–1376. [Google Scholar]
- [45].Liu JH, Li ZH, Du YH, et al. Protective effect of bFGF on motoneuron death of the spinal cord in rat. Jinan Daxue Xuebao: Ziran Kexue yu Yixue Ban. 1998;9(4):1–6. [Google Scholar]
- [46].Liang GL, Chen ZH, Li GT, et al. Effect of exogenous basic fibroblast growth factor in the regeneration of motor endplate in peripheral nerve injury. Zhongguo Linchuang Kangfu. 2006;0(5):58–60. [Google Scholar]
- [47].Zhang KF, Ou XC, Yang ZY. Changes of calcitonin gene-related peptide and acetylcholine esterase in motor end plates after spinal cord injury in adult rats. Zhongguo Kangfu Lilun yu Shijian. 2008;14(11):1030–1032. [Google Scholar]
- [48].Wrigley PJ, Gustin SM, Macey PM, et al. Anatomical changes in human motor cortex and motor path ways following complete thoracic spinal cord injury. Cereb Cortex. 2009;19:224–232. doi: 10.1093/cercor/bhn072. [DOI] [PubMed] [Google Scholar]
- [49].Sleek GC, Mantilla CB. Role of neurotrophins in recovery of phrenic motor function folloWing spinal cord injury. Respir Physiol Nenrobiol. 2009;169:219–225. doi: 10.1016/j.resp.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Ying SW, Wu XM, Wang G. Motor neuron injury after spinal cord on calcitonin gene related peptide and the changes of skeletal muscle acetylcholine receptors. Beijing Daxue Xuebao: Yixue Ban. 2002;34(3):281–285. [Google Scholar]
- [51].Nasn WB, Perot PL, Jr, Cox RD. The neuro protect effect of high dose Methylpredniso lone in rat spinal cord heuisection. Neurosci Lett. 1995;189(3):176–178. doi: 10.1016/0304-3940(95)11473-a. [DOI] [PubMed] [Google Scholar]
- [52].Zhou MH, Wu XY, Ren F, et al. the effect of 22 and 35 KD protein extracted from skeletal muscle on the cultivated lumbar spinal cord anterior horn motor neurons in rat. Kexue Tongbao. 1992;37:841. [Google Scholar]
- [53].Ramer MS, Bradbury EJ, Michael GJ, et al. Glial cell line-derived neurotrophic factor increases Calcitonin gene-related peptide immunoreactivity in sensory and motoneurons in vivo. Eur J Neurosci. 2003;18:2713–2721. doi: 10.1111/j.1460-9568.2003.03012.x. [DOI] [PubMed] [Google Scholar]
- [54].Lee TT, Green BA, Dietrich WD, et al. Neuroprotective effects of baisc fibroblast growth factor following spinal cord contusion injury in the rat. J Neurotrauma. 1999;16:347–356. doi: 10.1089/neu.1999.16.347. [DOI] [PubMed] [Google Scholar]
- [55].Rabahevsky AG, Fugaccia I, Turner AF, et al. Basic fibroblast growth factor (bFGF) enhances tissue sparing and functional recovery following moderate spinal cord injury. J Neurotrauma. 1999;16:817–830. doi: 10.1089/neu.1999.16.817. [DOI] [PubMed] [Google Scholar]
- [56].Follesa P, Wrathall JR, Mocchetti I. Increased basic fibroblast growth factor mRNA following contusive spinal cord injury. Mol Brain Res. 1994;22:1–8. doi: 10.1016/0169-328x(94)90026-4. [DOI] [PubMed] [Google Scholar]
- [57].Moccheti I, Rabin SJ, Colangelo AM, et al. Increased basic fibroblast growth factor expression following contusive spinal cord injury. Exp Neurol. 1996;141:154–164. doi: 10.1006/exnr.1996.0149. [DOI] [PubMed] [Google Scholar]
- [58].Pan L, Lu GH, Zhang PF. Effect of large dose methylprednisolone on the change of endogenous basic fibroblast growth factor in rats after spinal cord injury. Hunan Yixue. 2002;19(11):411–414. [Google Scholar]
- [59].Liu WG, Song JR, Lin JJ. Effect of bFGF on c-fos mRNA expression in rats after spinal cord injury. Zhongguo Jiaoxing Waike Zazhi. 2000;12:1178–1180. [Google Scholar]
- [60].Liu WG, Wang ZY, Huang ZS. Bone marrow-derived mesenchymal stem cells expressing the bFGF transgene promote axon regeneration and functional recovery after spinal cord injury in rats. Neurol Res. 2011;33(7):686–693. doi: 10.1179/1743132810Y.0000000031. [DOI] [PubMed] [Google Scholar]
- [61].Dow JK, DeVere White RW. Fibroblast growth factor 2: its structure and property, paracrine function, tumor angiogenesis, and prostate-related mitogenic and, oncogenic functions. Urology. 2000;55:800. doi: 10.1016/s0090-4295(00)00457-x. [DOI] [PubMed] [Google Scholar]
- [62].Allen AR. Surgery of experimental lesion of spinal cord equivalent to crush injury of fr acture dislocaion of spinalcolum. JAMA. 1911;57(4):878–880. [Google Scholar]
- [63].Basso DM, Beattie MS, Bresnahan JC, et al. Evaluation of open field locomotorscores: effects of experience and teamwork on reliability. Multicenter Animal Spinal Cord Injury Study. J Neurotrauma. 1996;13(7):343–359. doi: 10.1089/neu.1996.13.343. [DOI] [PubMed] [Google Scholar]


